Abstract
Background
The epidemiology of AF without comorbidities, known as ‘lone AF’, is uncertain. Although it has been considered a benign condition, we hypothesized that it confers a worse prognosis compared with a matched sample without AF.
Methods
We described the proportion of AF without comorbidities (clinical, subclinical cardiovascular disease and triggers) among the entire AF sample in Framingham Heart Study (FHS). We compared AF without comorbidities with typical AF, and age-, sex- and cohort-matched individuals without AF, using Cox proportional hazards analysis in relation to combined cardiovascular events (stroke, heart failure, myocardial infarction), and mortality.
Results
Of 10,311 FHS participants, 1,961 were diagnosed with incident AF, among which 173 individuals had AF without comorbidities (47% women, mean age 71±12 years). AF without comorbidities had a prevalence of 1.7% of the entire cohort, and an annual incidence of 0.5 per 1000 person-years. During a median follow-up of 9.7 years after initial AF, 137 individuals with AF without comorbidities (79.2%) died and 141 individuals developed cardiovascular events (81.5%). AF without comorbidities had significantly lower mortality (HR 0.67, 95%CI 0.55-0.81, P<0.001) and total cardiovascular events (HR 0.66, 95% CI 0.55-0.80, P<0.001) compared with typical AF. However, mortality (HR1.43, 95% CI 1.18-1.75, P<0.001) and risk of total cardiovascular events (HR 1.73, 95% CI 1.39-2.16, P<0.001) were higher than age-, sex-, and cohort-matched individuals without AF.
Conclusions
The risk of cardiovascular outcomes and mortality among individuals with AF without comorbidities is lower than typical AF, but is significantly elevated compared with matched individuals without AF.
Keywords: Lone atrial fibrillation, comorbidities, prevalence, incidence, prognosis
‘Lone atrial fibrillation (AF)’ was initially described in the 1950s as AF in the absence of other known risk factors such as structural heart disease, thyrotoxicosis, and hypertension.1, 2 More recently, ‘lone AF’ has been defined as AF with no clinical or echocardiographic evidence of concomitant cardiovascular, or pulmonary conditions in younger adults.3 However, guidelines do not specify which concomitant conditions have to be excluded to classify ‘lone AF’ including the age criteria. In part due to the lack of cohesive definition of this condition, the natural history of ‘lone AF’ has remained unclear. ‘lone AF’ was initially thought to be a benign condition especially when it was diagnosed at a younger age or was paroxysmal.3, 4 However, a community-based cohort study suggested increased risk for mortality and thromboembolism in people with ‘lone AF compared with individuals without AF.5
Recently a plethora of emerging associations with AF has been revealed with improved methods of imaging, and better understanding of the pathophysiology of AF.6 More stringent criteria have been suggested for the definition of ‘lone AF’. Some investigators even question its existence.7 Hence, for the present investigation we use the terminology ‘AF without comorbidities’ to refer to ‘lone AF’.
In a large community-based sample, we sought to investigate the epidemiology and AF-related adverse outcomes (such as stroke, heart failure, myocardial infarction, and all-cause mortality) in individuals with AF without recognized comorbidities. We compared this group with typical AF patients who have comorbidities, and also to an age- and sex-matched group of individuals without AF, respectively. We hypothesized that the risk of cardiovascular outcomes in AF without comorbidities would be less than typical AF but greater than the matched group without AF.
Methods
Study sample
The Framingham Heart Study was initiated in 1948 to examine cardiovascular disease and its risk factors. The details of enrollment and participation have been described previously. Briefly, the study enrolled community-dwelling individuals, termed the Original cohort (N=5,209), who underwent examinations every 2 years.8 In 1971, the study enrolled the Original cohort's children and their spouses into the Offspring cohort (n=5,124), who had examinations approximately every 4 or 8 years.9 During each Heart Study visit, a research physician and technicians collected data for cardiovascular risk factors and interim cardiovascular events. Individuals provided written informed consent at each examination. The Boston University Medical Center Institutional Review Board approved the study protocols at each examination cycle.
Atrial fibrillation ascertainment
Atrial fibrillation was diagnosed by the presence of AF or atrial flutter on electrocardiogram or Holter monitoring obtained during a Framingham Heart Study clinic visit, an external clinician visit, hospitalization, or review of medical records. Two Framingham Heart Study cardiologists adjudicated incident AF events.
Definition of atrial fibrillation without comorbidities
Atrial fibrillation without comorbidities was defined as AF in the absence of traditional risk factors including history of coronary heart disease, heart failure, valvular disease by physical exam (≥3/6 systolic murmur or any diastolic murmur) or echocardiographic data if available (≥moderate mitral, tricuspid or aortic regurgitation, any mitral stenosis, and ≥mild aortic stenosis), cardiomyopathy (left ventricular ejection fraction<50%), treated hypertension or blood pressure≥140 mmHg systolic or ≥90 mmHg diastolic, diabetes mellitus (history of diabetes, use of hypoglycemic agents or insulin), prior stroke or transient ischemic attack, ECG evidence of left ventricular hypertrophy, and hyperthyroidism (thyroid stimulating hormone<0.5μU/L). In addition, we used more stringent criteria incorporating additional risk factors and acute precipitants to define AF without comorbidities after excluding class II or higher obesity (body mass index≥35kg/m2), first-degree atrioventricular block (PR interval≥200msec), cardiac and non-cardiac surgery, coronary interventions, acute medical illness, concurrent infection, acute alcohol intoxication, chronic obstructive pulmonary disease, and intense exercise (3 times a week≥1-hour workout, during the year before initial AF).10 We performed a rigorous chart review searching both traditional and additional risk factors and extracted covariates from the latest examination prior to the onset of AF.
Follow-up and cardiovascular outcomes
We followed participants from the AF onset until the earliest AF-related outcome such as stroke, heart failure, myocardial infarction, or all-cause mortality, and last contact. Stroke, heart failure, and myocardial infarction adjudication at the Framingham Heart Study have been described previously.11, 12
Statistical analysis
We described the proportion and clinical outcomes of AF individuals without recognized comorbidities with and without the age criteria (<60 years) among the entire AF sample. We used Cox proportional hazards regression analysis to estimate the association of AF without comorbidities with the risk of combined and individual cardiovascular events including stroke, heart failure, myocardial infarction, and all-cause mortality in comparison with individuals with typical AF and without AF. Due to a small sample size, this analysis was performed on AF without recognized comorbidities without using the age criteria. When evaluating total incident events, the earliest developed cardiovascular event was counted. We utilized the matched cohort design to generate the group without AF for this purpose, i.e., for each AF case without comorbidities, 10 referents free of AF and prevalent cardiovascular events at the time of the case's diagnosis were identified and matched to the case by age (±1 year), sex, and cohort status (Original vs. Offspring at enrollment) only for a more “realistic” interpretation of the outcome. We evaluated the factors associated with AF-related clinical outcomes among AF individuals without comorbidities in stratified proportional hazards models with matching sets within the group as strata. Age-, sex- and cohort-adjusted estimates of the cumulative incidence of cardiovascular events were calculated using the corrected group prognosis method for individuals with AF without comorbidities, typical AF, and without AF.13, 14
We considered a two-sided p<0.05 as statistically significant. SAS version 9.3 (Copyright, SAS Institute Inc., Cary, NC, USA) was used for all analyses.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Among 10,311 individuals with no known prevalent AF at enrollment, we observed 1,961 individuals with AF (49% women, mean age 71±12 years) over 33±12 years of follow-up, among which we observed 173 individuals with AF without recognized comorbidities. We illustrated the process of exclusion among individuals with AF with both hierarchical and non-hierarchical prevalence of individual cardiovascular conditions at time of diagnosis (Supplementary Table 1). The individual characteristics of each group are summarized in Table 1. AF without comorbidities comprised 1.7% of the entire cohort. The annual incidence of AF without clinically recognized comorbidities was 0.5 per 1000 person-years. Supplementary Table 2 illustrates the percentage of AF without comorbidities according to age, sex, and date of AF diagnosis. Thirty-one among 173 individuals with AF without comorbidities (18%) were below 60 years of age as demonstrated in Supplementary Table 3 and annual incidence in this group was 0.1 per 1000 person-years.
Table 1.
Individual characteristics.
| Characteristics | AF without comorbidities (n=173) | Typical AF without prevalent CVD (n=1031) | Matched cohort without prevalent CVD (n=1700) |
|---|---|---|---|
| Age*, yrs | 70.5±11.6 | 75.0±11.2 | 70.0 ± 11.3 |
| Age* <60 yrs | 31(18%) | 85(8%) | 354(19%) |
| Men | 92(53%) | 509(49%) | 983(53%) |
| Systolic blood pressure (mmHg) | 128±15 | 142±23 | 135±21 |
| Hypertension treatment | - | 489(48%) | 280(21%) |
| Myocardial infarction | - | - | - |
| Valvular heart disease | - | 110(12%) | 26 (2%) |
| (auscultation) | |||
| Heart failure | - | - | - |
| Diabetes mellitus | - | 114(13%) | 70(4%) |
| CABG or PTCA | - | 83(9%) | 21(1%) |
| Previous stroke | - | - | - |
| Body mass index (kg/m2) | 26.0±3.8 | 27.9±5.5 | 26.4±4.1 |
| ECG PR interval (ms) | 162±20 | 178±41 | 166±23 |
Data was presented as counts (percentages) or mean±standard deviation.
– signifies that the feature was an exclusion criteria.
Age was at the beginning of the follow-up period the age when first AF occurred.
Abbreviation: AF = atrial fibrillation; CABG = coronary artery bypass grafting; PTCA = percutaneous transluminal coronary angioplasty; ECG = electrocardiogram.
The median follow-up from the AF onset until the first cardiovascular outcome was 9.7 years (interquartile range 5.2, 17.4). Rates of individual and combined cardiovascular events after an initial episode of AF during follow-up among AF individuals without comorbidities (with and without age criteria) vs. typical AF vs. matched cohort were described in Table 2 and Supplementary Table 4. During a follow-up period, 137 individuals with AF without comorbidities (79.2%) died and 141 individuals developed cardiovascular events (81.5%). Among the group younger than 60 years with AF without comorbidities, 19 individuals died (61.3%) and 20 had cardiovascular events (64.5%). Table 3 compares the hazards of total cardiovascular disease outcomes and all-cause mortality among individuals with AF but no apparent comorbidities with typical AF and separately with a matched cohort without AF. Compared with typical AF, the AF without comorbidities group had a 33% lower hazard of all-cause mortality as well as 34% lower hazard of total cardiovascular disease events. Supplementary Table 5 demonstrates the comparison of individual cardiovascular disease outcome between groups. The AF without comorbidities group had lower incidence of heart failure (hazard ratio [HR] 0.52, P<0.001). Compared with individuals with typical AF, the incidence of stroke, and myocardial infarction were not different significantly. In comparison with the matched cohort without AF, however, AF without comorbidities had significantly higher risk for all-cause mortality (HR 1.43, P<0.001), and total incident cardiovascular events (HR 1.73, P<0.001). The AF without comorbidities group had three-fold higher risk for heart failure (HR 2.94, P<0.001) than the group without AF. However, the incidence of stroke, and myocardial infarction were not significantly different.
Table 2.
Development of cardiovascular events in 10 years after initial AF.
| Cardiovascular events | AF without comorbidities (n=173) | Typical AF (n=1031) | Matched cohort (n=1700) | |||
|---|---|---|---|---|---|---|
| Event (n) | Incidence (per 1000 person-years) | Event (n) | Incidence (per 1000 person-years) | Event (n) | Incidence (per 1000 person-years) | |
| Stroke | 10 | 6 | 60 | 9 | 85 | 4 |
| Heart failure | 33 | 21 | 245 | 42 | 189 | 9 |
| Myocardial infarction | 14 | 8 | 69 | 11 | 175 | 9 |
| All-cause mortality | 137 | 77 | 792 | 118 | 1226 | 59 |
| Total cardiovascular events* | 141 | 94 | 828 | 151 | 1253 | 65 |
Typical AFs with prevalent cardiovascular events were excluded from the analysis (e.g. total # typical AF=1664, # typical AF with prevalent events= 633). Follow-up for outcome started from AF onset date or matched cases' AF onset date for the matched cohort.
For each participant, only the outcome that occurred earliest was counted.
Abbreviation: AF = atrial fibrillation.
Table 3.
Comparison of cardiovascular outcomes in AF without comorbidities, typical AF and matching cohort without AF.
| AF without comorbidities vs. typical AF (n=1204) | AF without comorbidities vs. matched no-AF (n=1873) | |||
|---|---|---|---|---|
| Outcome | HR (95% CI) | P value | HR (95% CI) | P value |
| All-cause mortality | 0.67(0.55-0.81) | <0.001 | 1.43(1.18-1.75) | <0.001 |
| Total cardiovascular events | 0.66(0.55-0.80) | <0.001 | 1.73(1.39-2.16) | <0.001 |
Abbreviation: AF = atrial fibrillation; CI = confidence interval; HR = hazard ratio.
We evaluated different factors associated with the AF-related adverse clinical outcome in individuals with AF without comorbidities in Supplementary Table 6. Among all factors tested (e.g. sex, body mass index, systolic blood pressure, warfarin use, antithrombotic use at the time of AF diagnosis), most were not significantly associated with clinical outcomes except for the age of AF onset. Lastly, we developed corrected group prognostic curves for the cumulative incidence of cardiovascular events in individuals with AF without comorbidities, typical AF, and without AF, adjusted by age, sex, and cohort in Figure 1. The typical AF group showed the highest cumulative incidence of cardiovascular outcomes, followed by the AF without comorbidities group, and the matching group without AF.
Figure 1.
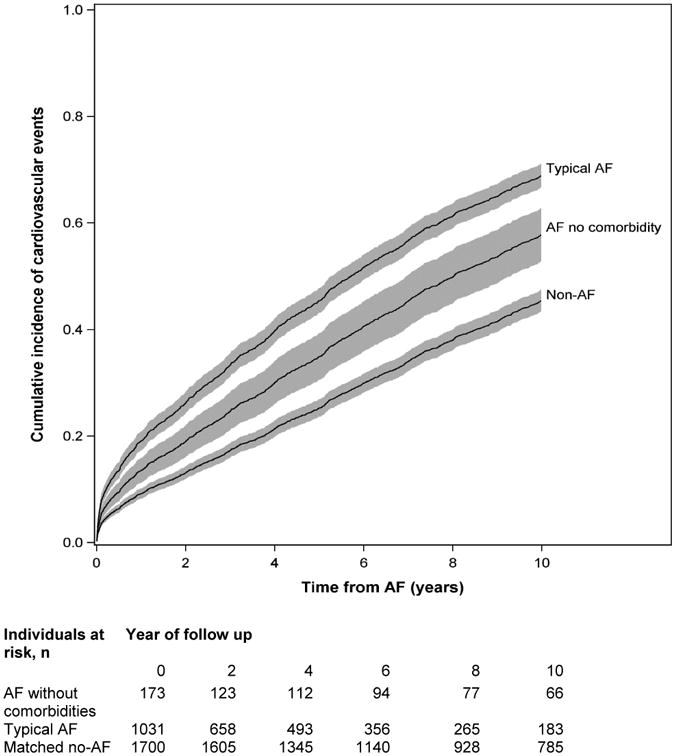
Cumulative incidence of combined cardiovascular events (the earliest of stroke, heart failure, myocardial infarction and death) in individuals with AF without comorbidities, typical AF, and the matching referents without AF adjusted by age, sex, and cohort, accounting for the competing risk of non-cardiovascular death. For the matched non-AF group the follow-up began at the diagnosis date of matched AF without comorbidities.
Discussion
We evaluated the epidemiology and clinical outcomes of AF without recognized comorbidities, which has been referred to as ‘lone AF’. We detected that AF without comorbidities carried significantly higher risk of cardiovascular events and mortality compared with the matching group without AF.
‘lone AF’ receives special attention as it represents a subset of AF without an apparent cause. However, as our knowledge of subclinical cardiovascular disease and pathophysiology of AF has expanded,7 there has been tremendous variation in the definition of ‘lone AF’. Data regarding the characteristics and clinical outcomes of ‘lone AF’ are conflicting and not amenable to a direct comparison due to heterogeneity of study samples.
The prevalence of AF without comorbidities varies from 0.2-11% depending on the definition of AF used.3, 5, 15 In the 1980s, the Framingham Heart Study reported that ‘lone AF’ comprised 11% of all individuals with AF.5 However, the definition used in the prior Framingham analysis was lenient compared with currently accepted definitions and the investigators did not exclude hypertension, or other concurrent medical conditions or triggers. In the same decade, the proportion of ‘lone AF’ over the period of 1950 to 1980, was reported as 2.7% among residents in Olmsted County using a stricter criteria.3 To define AF without comorbidities, the Olmsted investigators additionally excluded individuals with coronary heart disease, valvular heart disease, heart failure, cardiomyopathy, chronic obstructive pulmonary disease, age>60 years at time of diagnosis, and other triggers such as trauma, surgery, or acute medical illness.3
Literature showed conflicting data regarding role of age in lone AF population. It is known that the incidence of AF increases with increasing age, labeling AF “a disease of the elderly”16 Some longitudinal studies incorporated the age <60 years as definition of “lone AF” based on this observation. However, there is no pathophysiological explanation for the age cut-off of 60 yeas. Rather, recent literature questioned the value of age criteria by demonstrating lack of significant atrial enlargement, AF progression or short term prognosis in idiopathic AF patients at advanced age.17 Presumably age limit was used to ensure exclusion of any “unknown” causes of AF or age-related AF onset. But Wyse et al also points out age limit of 60 years seems arbitrary and “excessively conservative in the modern era”.18
In our current work, we performed the analysis without using the age criteria given a small sample size. Compared to the Olmsted county cohort, however, we applied additional criteria such as ECG evidence of left ventricular hypertrophy and prolonged PR interval, obesity, hyperthyroidism, alcohol consumption, and intense exercise via a thorough chart review. We demonstrated that AF without comorbidities had an overall prevalence of 1.7% or 0.3% with age <60 years in our community-based cohort. Also we may speculate that the incidence and prevalence of AF without comorbidities would be even lower if data for comorbidities and echocardiographic data were available for the entire cohort.
The clinical outcomes related to AF without comorbidities conflict in the literature. Initially, it was thought to be a benign condition with normal life expectancy especially if the diagnosis was made at younger age and in a paroxysmal form.1, 3, 4 In contrast, other literature demonstrated that AF, even in the absence of other comorbidities, remains associated with higher mortality and cardiovascular outcomes, with up to four-fold increase in stroke, four-fold increase in cardiovascular death rate, and two-fold higher risk for overall adverse cardiovascular outcomes than the general population without AF.1, 15 Considering that the literature did not suggest a significant risk of heart failure among AF without comorbidities,19, 20 our finding of three-fold increase in heart failure risk in this group, is interesting. The higher risk for heart failure may be due to the older mean age of our individuals with AF without comorbidities compared with other studies as it is suggested in the description of clinical outcomes in AF without comorbidities and age <60 years (Supplementary Table 4). In contrary, the risk for stroke and myocardial infarction were not significantly elevated.
Potpara et al. also suggested that ‘lone AF’ generally has a favorable prognosis but long-term outcome was influenced by the development of cardiovascular disease.19 Our analysis corroborated this notion by showing that advancing age of AF onset was associated with adverse clinical outcomes in AF without comorbidities. It is possible that the cardiovascular outcomes associated with AF without comorbidities is due to development of other cardiovascular risk factors over time, or that AF itself is the first manifestation of subclinical cardiovascular disease, or that it confers an increased risk of cardiovascular events.21
Recently the question was raised if ‘lone AF’ exists at all. With a significant advancement in detecting subclinical cardiovascular disease and numerous emerging risk factors for AF, some literature suggests that ‘lone AF’ is a misnomer; these patients may have subclinical cardiovascular disease that is below the threshold of detection as the development of cardiovascular disease takes place over the years.7, 22, 23 We cannot directly answer the question regarding the existence of ‘lone AF’ in this study. We elucidated by using more contemporary definition, however, that AF without recognized clinical comorbidities, had worse clinical outcomes than individuals without AF. Perhaps, the worse prognosis is due to the possibility that even in the absence of structural heart disease, AF may cause endothelial damage with altered molecules such as E-selectin, von Willebrand factor, and soluble thrombomodulin.24 However, whether AF in itself increases the risk of cardiovascular outcomes or it is a marker of emerging cardiovascular disease still remains unclear. For this reason, ‘lone AF’ term is misleading as it implies a benign condition. As such, if ‘lone AF’ is used, providers should be aware of the potential adverse outcomes of AF of any kind.
The main strengths of our study are the large community-based sample, long follow-up period, and systematic collection of AF, AF risk factors, cardiovascular conditions, and outcomes. Our study has limitations, mainly due to the community-based longitudinal design and modest number of individuals with AF without comorbidities. First, we did not use the age criteria (age<60 years) for our analysis. However we would expect that the age-related impact on the outcomes in our comparative analysis between AF without comorbidities and general population without AF would be nondifferential by using the matched cohort design. Second, we did not investigate different patterns of AF such as transient vs. paroxysmal vs. chronic AF due to imprecise ability to characterize such patterns with FHS data. Therefore, we were also unable to quantify the relation of burden and duration of AF to clinical outcomes. Third, echocardiographic data and chronic obstructive pulmonary disease were available for a subset of the AF individuals without comorbidities (23% and ∼50% respectively). We acknowledge that we may have included individuals with subclinical valvular heart disease, left atrial enlargement, or cardiomyopathy in our AF without comorbidities group. Fourth, we did not match for cardiovascular risk factors in the non-AF population to AF without comorbidities. The non-AF population was matched only for age, sex and cohort in order not to over-interpret our findings. Fifth, we may not have accounted for all possible co-morbidities and triggers despite a vigorous chart review. We could have missed the risk factors that were below the threshold of detection at time of AF diagnosis or not have included all possible co-morbidities that can be observed with AF in a terminal stage of life.21 Sixth, we did not have sufficient data to examine family history or genetic data in relation to our AF classification. Our sample consisted of middle age and older adults predominantly of European ancestry, limiting generalizability to other ethnicities and younger individuals. We had modest power to examine the relation of AF without comorbidities vs. typical AF vs. without AF in relation to specific cardiovascular events, and to examine the risk factors for outcomes in individuals with AF without comorbidities.
Conclusions
‘lone AF’ has been thought to be a benign condition. However, our study confirmed that AF without clinically apparent comorbidities carries a greater risk of total cardiovascular outcomes and mortality than individuals without AF. Thus one should use the term ‘lone AF’ with caution and understand the potential adverse prognosis of AF of any kind. Further research is indicated to address the implication of AF without comorbidities, methodology of follow-up, appropriate prognostication, assessment of individual thromboembolic risk, AF-related symptoms, rate vs. rhythm control, and anticoagulation treatment strategies in this population.
Supplementary Material
Acknowledgments
Funding. 2R01HL092577; 1R01 HL102214; 1RC1HL101056; 6R01-NS 17950; N01-HC 25195; HHSN268201500001I. Dr. M. Rienstra is supported by a grant from the Netherlands Organization for Scientific Research (Veni grant 016.136.055). Dr. Lubitz is supported by a Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105 and NIH/NHLBI K23HL114724 Career Development Award. Dr. McManus is supported NIH grants 1R15HL121761 and KL2RR031981.
Footnotes
Disclosures. All authors have approved the final article. No disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans W, Swann P. Lone auricular fibrillation. Br Heart J. 1954;16(2):189–194. doi: 10.1136/hrt.16.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potpara TS, Lip GY. Lone atrial fibrillation - an overview. Int J Clin Pract. 2014;68(4):418–33. doi: 10.1111/ijcp.12281. [DOI] [PubMed] [Google Scholar]
- 3.Kopecky SL, Gersh BJ, Mcgoon MD, et al. The Natural-History of Lone Atrial-Fibrillation - a Population-Based Study over 3 Decades. New England Journal of Medicine. 1987;317(11):669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 4.Scardi S, Mazzone C, Pandullo C, et al. Lone atrial fibrillation: prognostic differences between paroxysmal and chronic forms after 10 years of follow-up. Am Heart J. 1999;137(4 Pt 1):686–91. doi: 10.1016/s0002-8703(99)70224-3. [DOI] [PubMed] [Google Scholar]
- 5.Brand FN, Abbott RD, Kannel WB, et al. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study. JAMA. 1985;254(24):3449–53. [PubMed] [Google Scholar]
- 6.Kirchhof P, Breithardt G, Aliot E, et al. Personalized management of atrial fibrillation: Proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2013;15(11):1540–56. doi: 10.1093/europace/eut232. [DOI] [PubMed] [Google Scholar]
- 7.Wyse DG, Van Gelder IC, Ellinor PT, et al. Lone atrial fibrillation: does it exist? J Am Coll Cardiol. 2014;63(17):1715–23. doi: 10.1016/j.jacc.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawber T, Meadors G, Moore F. Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health Nations Health. 1951;41:279–86. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannel W, Feinleib M, McNamara P, et al. An investigation of coronary heart disease in families: The Framingham Offspring Study. Am J Epidemiology. 1979;110(3):281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 10.Mont L, Sambola A, Brugada J, et al. Long-lasting sport practice and lone atrial fibrillation. Eur Heart J. 2002;23(6):477–82. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 11.Ho KK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 12.Wolf PA, Abbott RD, Kannel WB. Atrial-Fibrillation as an Independent Risk Factor for Stroke - the Framingham-Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 13.Chang IM, Gelman R, Pagano M. Corrected Group Prognostic Curves and Summary Statistics. Journal of Chronic Diseases. 1982;35(8):669–674. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Loberiza FR, Klein JP, et al. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Computer Methods and Programs in Biomedicine. 2007;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Jouven X, Desnos M, Guerot C, et al. Idiopathic atrial fibrillation as a risk factor for mortality: The Paris Prospective Study I. Euro Heart J. 1999;20:896–899. doi: 10.1053/euhj.1998.1397. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 17.Weijs B, P R, Nieuwlaat R, Breithardt G, Le Heuzey JY, Vardas PE, Limantoro L, Schotten U, Lip GYH, Crijns HJGM. Idiopathic atrial fibrillation revisited in a large longitudinal clinical cohort. Europace. 2012;14:184–90. doi: 10.1093/europace/eur379. [DOI] [PubMed] [Google Scholar]
- 18.Wyse DG. Idiopathic atrial fibrillation: a rose by any other name? Europace. 2012;14:151–2. doi: 10.1093/europace/eur423. [DOI] [PubMed] [Google Scholar]
- 19.Potpara TS, Stankovic GR, Beleslin BD, et al. A 12-Year Follow-up Study of Patients With Newly Diagnosed Lone Atrial Fibrillation Implications of Arrhythmia Progression on Prognosis: The Belgrade Atrial Fibrillation Study. Chest. 2012;141(2):339–347. doi: 10.1378/chest.11-0340. [DOI] [PubMed] [Google Scholar]
- 20.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115(24):3050–6. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of Atrial Fibrillation on the Risk of Death : The Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 22.Potpara TS, Lip GY. Lone atrial fibrillation: where are we now? Hosp Pract (1995) 2011;39(4):17–31. doi: 10.3810/hp.2011.10.919. [DOI] [PubMed] [Google Scholar]
- 23.Weijs B, de Vos CB, Tieleman RG, et al. The occurrence of cardiovascular disease during 5-year follow-up in patients with idiopathic atrial fibrillation. Europace. 2013;15:18–23. doi: 10.1093/europace/eus203. [DOI] [PubMed] [Google Scholar]
- 24.Freestone B, Chong AY, Nuttall S, et al. Soluble E-selectin, von Willebrand Factor, Soluble Thrombomodulin, and Total Body Nitrate/Nitrite Product as Indices of Endothelial Damage/Dysfunction in Paroxysmal, Persistent, and Permanent Atrial Fibrillation. Chest. 2007;132:1253–1258. doi: 10.1378/chest.07-1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


