Abstract
Intramyocellular lipid (IMCL) is predominantly stored as intramuscular triglyceride (IMTG) in lipid droplets and is utilized as metabolic fuel during physical exercise. IMTG is also implicated in muscle insulin resistance (IR) in type 2 diabetes. However, it has become apparent that lipid moieties such as ceramide and diacylglycerol are the likely culprits of IR. This article reviews current knowledge of IMCL-mediated IR and important areas of investigation, including myocellular lipid transport and lipid droplet proteins. Several crucial questions remain unanswered, such as the identity of specific ceramide and diacylglycerol species that mediate IR in human muscle and their subcellular location. Quantitative lipidomics and proteomics of targeted subcellular organelles will help to better define the mechanisms underlying pathological IMCL accumulation and IR.
Keywords: adipose triglyceride lipase, hormone sensitive lipase, insulin resistance, fatty acids, lipidomics, intramyocellular lipid, ceramide, diacylglycerol
Skeletal muscle fatty acid uptake, oxidation and storage
The prevalence of obesity worldwide has now reached 500 million and is a serious healthcare problem. Obesity is associated with many co-morbidities including insulin resistance (IR), which predisposes the onset of type 2 diabetes (T2D) [1]. In simplest terms, obesity is the result of excess caloric intake and lack of physical activity or energy expenditure [2]. This energetic imbalance results in adipose tissue hypertrophy and hyperplasia, which in turn can lead to elevated inflammation and oxidative stress [3]. Dysfunctional adipocytes have an inappropriately higher rate of lipolysis, releasing fatty acids (FAs) into the circulation bound to albumin. One contemporary hypothesis to explain skeletal muscle IR in obesity is that of ectopic accumulation in lipid droplets, that is, excess lipid storage in non-adipose tissue. Skeletal muscle takes up plasma free fatty acids (FFAs) by passive diffusion and by fatty acid translocase/cluster of differentiation 36 (FAT/CD36) or plasma membrane fatty acid binding protein (FABPpm), and then partitions them between mitochondrial oxidation and triglyceride (TG) synthesis and lipid droplet storage [4]. Muscle also takes up FAs liberated from core triglycerides in chylomicrons and very low-density lipoproteins (VLDL) via the lypolytic action of lipoprotein lipase (LPL). The vast majority of FAs taken up by muscle are either oxidized or stored (up to 90% in soleus) [5], with the remainder incorporated into phospholipid, monoacylglcyerol, and diacylglycerol (DAG; see Glossary) pools. Once FAs are inside myocytes, the enzyme long chain acyl-CoA synthetase (LC-FACS) catalyzes their binding to acetyl CoA. If destined for oxidation, the fatty acyl CoA is then transported into mitochondria via the action of carnitine palmitoyl transferase I (CPTI), which is expressed on the outer mitochondrial membrane [6]. It has been reported that FAT/CD36 is also expressed on mitochondrial membranes and it has been hypothesized that it influences mitochondrial FA transport and oxidation [7]. This role, however, remains controversial. Once inside mitochondria, fatty acyl CoA enters β-oxidation, which generates acetyl CoA for the tricarboxylic acid cycle and reducing equivalents for the electron transport system (ETS). The mechanisms of muscle FA uptake, oxidation, and storage have been studied in two discrete yet related metabolic contexts: fuel metabolism and lipotoxicity-induced IR in obesity [8]. This article briefly reviews the history of intramyocellular lipid (IMCL) research and discusses the current state of the art in the field. We also address unanswered questions and future research directions related to the role of muscle lipids in human health.
A brief history of research on intramuscular lipid
We have long known that lipid is used as a metabolic fuel source for muscle. The earliest studies using calorimetry from 1900 to 1930 indicated that both fat and carbohydrate are used as fuel sources during mechanical work [9]. In 1927, Himwich and Rose examined muscular fuel utilization by measuring arteriovenous differences in oxygen and carbon dioxide across skeletal muscle in dogs during rest and exercise, and in fed and fasted states [10]. These studies provided the first clues that muscle could switch fuel source depending on the metabolic context. Studies by Fritz et al. provided irrefutable evidence that muscle oxidizes lipid to support contraction [11]. The presence of intramuscular triglyceride (IMTG) was first described by Denton and Randle in 1967 [12]. Soon after, studies indicated that IMTG could be used as a fuel source during exercise [13]. By the 1990s, it was recognized that IMTG accumulation was associated with IR in human studies [14]. The development of analytical techniques to specifically quantify IMCL (composed mostly of TG) in lipid droplets (LDs), including histological staining with oil red O and 1H magnetic resonance spectroscopy (MRS) [15] facilitated examination of IMCL and spurred a resurgence of interest in both its role as metabolic fuel and its relationship with IR.
IMTG as a fuel source during exercise
Data from early studies with biochemical quantification of total TG extracted from muscle biopsy specimens demonstrated that muscle TG is depleted following prolonged exercise [16]. Conversely, many studies indicated that the IMTG content was not depleted [17]. This dichotomy of results was likely due to methodological factors, including contamination of muscle biopsy samples by intermuscular adipocytes, and interbiopsy variation, resulting in low non-significant decreases in IMTG content following exercise [18]. Elevated plasma FFAs following exercise may replenish IMTG stores and thus the timing of muscle IMTG measurement after exercise is an important experimental consideration. More recent studies using isotopic tracer methodologies [19] and 1H MRS [20] support the concept that IMTG is utilized as a fuel source by exercising muscle. Furthermore, semi-quantitative histology has revealed that acute exercise causes IMTG depletion predominantly in type I muscle fibers [21]. It is also known that skeletal muscle hormone-sensitive lipase (HSL) can be activated by catecholamines [22] and muscle contraction [23], and that adipose triglyceride lipase (ATGL) expression is upregulated by training [24], providing further support for the hypothesis that (LD)-derived FAs may be utilized as a fuel source by exercising muscle (Figure 1).
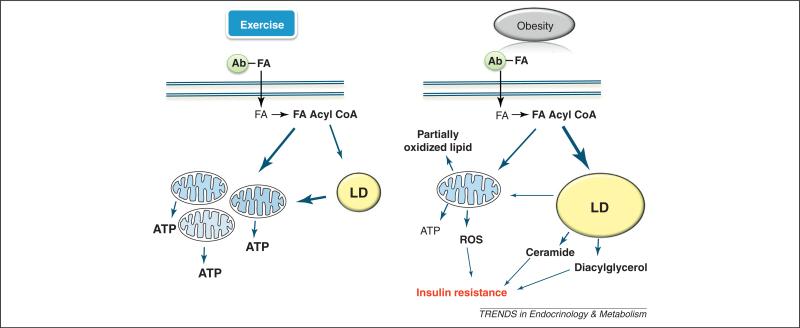
Figure 1.
Role of intramyocelluar lipid (IMCL) during exercise and in obesity. This schematic depicts the fate of the major IMCL lipid species within the context of exercise and obesity. During exercise, fatty acid (FA) acyl CoA is oxidized by mitochondria to synthesize ATP. FA acyl CoA is also partitioned to lipid droplets (LDs), where it is esterified to triglyceride (TG). TG can subsequently be lyophilized to release FAs for mitochondrial oxidation. In obesity, because of lower energetic demand, most FA acyl CoA is partitioned to LDs. IMCL in LDs can then act as a surrogate for ceramide and DAG. FA CoA oversupply to mitochondria during low energetic demand results in incomplete β oxidation and reactive oxygen species (ROS) production. The size of the arrows represents the rate of flux. Ab, albumin.
Although informative, these descriptive studies do not reveal how IMTG use during exercise, or its regulation, may be linked to the etiology of IR or diabetes. There are, however, some important clues emanating from acute exercise studies that IMTG use and TG resynthesis may play a role. For example, Schenk et al. reported that previous exercise channeled FAs toward IMTG synthesis, reduced the accumulation of FA metabolites, and prevented lipid-induced IR [25]. Additional similar studies are needed to develop a much better understanding of how IMTG use during exercise is regulated, including the role for lipases and LD proteins, as well as the exercise intensity and volume required for IMTG utilization. This could lead to better insight into the links between IMTG utilization and lipid-induced IR in obesity and T2D. The role of ATGL, HSL, and the PAT family of proteins in regulating FA flux in LDs is presented in Box 1.
Box 1. The dynamic lipid droplet.
Lipid droplets (LDs) are highly dynamic organelles with many roles, including cell signaling, vesicle trafficking, and a fuel source for mitochondria. LD are located proximal to muscle mitochondria and sarcoplasmic reticulum and are more abundant in type I compared to type II fibers, concomitant with the greater oxidative capacity of type I fibers [34]. LDs contain predominantly TG, but also DAGs, cholesterol esters, and free cholesterol, surrounded by a phospholipid monolayer [71]. LD function is mediated by a complement of proteins that coat the phospholipid monolayer and mediate TG synthesis and breakdown, vesicle trafficking, intracellular signaling, and LD fusion and fission events. Such proteins include the PAT family of proteins (PLIN2–5) [72]. A functional role for PLIN2 (ADRP) in skeletal muscle has yet to be described; however, studies on other cell types suggest that PLIN2 may act as a scaffold protein and its expression seems to be reciprocal to TG content [73]. In skeletal muscle of endurance cyclists, PLIN2 content is twofold greater in type I fibers compared to type II fibers and only ~60% of LDs contain PLIN2 [74], suggesting heterogeneity in the LD population. PLIN3 (TIP47) is expressed in skeletal muscle and although a functional role in muscle has not been confirmed, PLIN3 might be involved in LD synthesis [75]. PLIN4 (S3–12) is expressed in skeletal muscle [76], but its function has not been elucidated. PLIN5 (OXPAT), the best-characterized of the PAT proteins, is thought to be an important scaffold protein for lipolysis and it has been shown that it interacts with HSL, comparative gene identificantion-58 (CGI-58), and ATGL [77,78]. PLIN5 is highly expressed in muscle, is localized to mitochondria and LDs, and is closely associated with IMTG content [79]. PLIN5 and PLIN2 are co-localized with LDs in a heterogeneous manner, and muscle contraction does not affect recruitment to LDs [80]. Collectively, these studies suggest an important role for PLIN5 in regulating FA oxidation. However, further studies are needed to delineate the role of PAT proteins in lipid metabolism in the context of exercise and skeletal muscle IR.
Some 50–60% of FAs taken up by muscle are esterified to TG and act as an oxidative fuel reserve [81]. In skeletal muscle, sequential mobilization of FAs from IMTG stores in LDs is mediated by HSL, ATGL, and monoacylglycerol (MAG) lipase. Hydrolysis of TG by ATGL releases FA and DAG [82,83]. HSL acts predominantly on DAG, but can also hydrolyze TAG, MAG, and cholesterol esters. HSL activity during exercise is regulated by protein kinase K (PKA), extracellular signal-regulated kinase (ERK), and AMKP [84]. Muscle contraction also results in HSL translocation to LDs to facilitate lipolysis. HSL and ATGL work in concert to regulate lipolysis in skeletal muscle. Discordant activity of HSL and ATGL is responsible for impaired lipolysis in skeletal muscle of obese individuals [85]. Badin et al. observed altered expression of ATGL and HSL protein expression in obese diabetics compared to controls [86] and elevated muscle DAG content which was associated with the ratio of DAG to TAG hydrolase activity and IR. The final step in IMTG hydrolysis is conversion of MAG to FA and glycerol by MAG. Currently, little is known about MAG activity in skeletal muscle. Further studies are warranted to gain an understanding of the role of lipase activity and impaired lipolysis in muscle IR.
Although the roles of these LD proteins have been described, there are many more that have not. It was recently reported that the LD proteome of mouse skeletal muscle contains 324 proteins including heat shock proteins and proteins from the Rab family [87]. A large number of these proteins are also expressed in mitochondria, underlining a close functional relationship between the two organelles. A role for most of these proteins during lipid oversupply and IR remains unknown and represents an important direction for future research.
IMCL accumulation is implicated in muscle IR
Early human studies demonstrated that accumulation of IMCL, presumed to be mostly IMTG, is associated with IR [14]. The use of non-invasive 1H MRS also helped in defining the relationship between IMCL and skeletal muscle IR by reducing or eliminating the confounding influence of extramyocellular lipid. For example, it was observed that IMCL was elevated in first-degree relatives of T2D subjects and was associated with impaired insulin-stimulated glucose uptake by muscle [26]. Subsequent studies using oil red O staining in human biopsies confirmed the association between IMCL and IR in obesity and T2D [27].
Studies of early weight loss interventions also seemed to support the relationship between IMCL and muscle IR. Diet-induced weight loss decreased IMCL in concert with improved insulin sensitivity [28]. Moreover, lipid infusion and a high-fat diet increased IMCL and impaired insulin sensitivity [29]. However, the positive correlation between IMCL content and skeletal muscle IR was not a universal finding; it was also shown that IMTG content is elevated in highly insulin-sensitive athletes, a phenomenon termed the athlete's paradox [30]. It is now thought that IMTG does not cause IR within muscle, but rather increases as part of an orchestrated upregulation of fuel supply (TG) and energetic capacity in muscle with exercise training [30,31]. The athlete's paradox represents a convergence of two separate areas of research, one linking IMTG to fuel metabolism and the other linking IMTG to IR. This also supported the model concept that IMTG was more likely – under specific conditions – to act as a precursor for lipotoxic intermediates, including long-chain acyl CoA, DAG, and ceramide, that mediate IR (Figure 1). The current paradigm is that regular exercise increases turnover and content of the IMTG pool, which prevents accumulation of lipotoxic intermediates [8]. However, the basis of the athlete's paradox may not be exclusive to effects on lipid. Recent studies have identified the production of mitochondrial reactive oxygen species (ROS) and oxidant stress as important contributors to muscle IR [32]. Studies have shown that regular exercise can improve mitochondrial function and antioxidant capacity [33] and alleviate IR in muscle. A reduction in oxidant stress with regular exercise may also underlie the athlete's paradox. This is supported by the fact that not all studies indicate a role for DAG [34] and ceramide [35] in muscle IR. However, for the purpose of this review we concentrate on the role of lipid mediators on muscle IR.
Intramyocellular FA transport
It is now recognized that several mechanisms exist to facilitate the transport of FAs (up to 90%) into myocytes, including pmFABP, CD36/FAT, and the six fatty acid transport proteins FATP1–6, also known as solute carrier family 27A1–6. The regulation of cellular uptake of FAs is analogous to uptake of glucose. Translocation of CD36 from intracellular vesicles to the sarcolemma membrane to facilitate FA uptake is stimulated by insulin (PI3K–PKB–Akt) and muscle contraction (AMPK) in a manner similar to that for glucose transporter type 4 (GLUT4) translocation [4]. IMTG accumulation is potentiated by dysregulation of this mechanism in T2D. Studies in diabetic animals and humans with T2D indicate that rates of FA transport into muscle markedly increase due to permanent relocation of CD36, and to a lesser degree FATP4, to the sarcolemma membrane [36]. The increase in sarco-lemma CD36 content correlated well with rates of FA transport in lean, obese and T2D muscle (R = 0.93) [36].
Exercise training shifts resting and exercising fuel selection preference to FA oxidation and esterification in muscle. Given these adaptations, it seems feasible that the capacity for FA transport would also be enhanced. Studies in animal models suggested that chronic low-frequency stimulation of the peroneal nerve increases both CD36 and pmFABP protein expression and their membrane contents, concomitant with elevated sarcolemmal FA transport [37,38]. Training studies in humans have indicated that training at lower VO2 peak will elicit a greater increase in CD36 and pmFABP [39,40] compared to training at 100% VO2 peak, which induces upregulation of proteins involved in glycogen utilization, with no change in CD36 or pmFABP content [41]. The dietary state in which training is conducted also seems to play a role. Exercise training in the fasted state elicits a greater increase in CD36 and pmFABP expression compared to training following consumption of a high-carbohydrate meal [42]. Future studies should focus on subcellular relocation of FATPs with acute and chronic exercise independent of total changes in expression.
IMCL could act as a surrogate for specific lipid species that mediate IR
Sphingolipids
Ceramides, which are part of the sphingolipid family, were initially attributed a role as inert structural components of biological membranes. We now appreciate a far more diverse role for ceramides as intracellular messengers. In particular, ceramide accumulates in conjunction with a number of cellular stresses, such as ROS accumulation, inflammation, and hypoxia, as part of a highly conserved stress response [43]. A profound alteration in tissue sphingolipid pools is also a consequence of nutritional stress due to FA oversupply [44]. In muscle, ceramide has been identified as a key mediator of IR via inhibition of the serine/threonine-specific protein kinase Akt/protein kinase B (PKB) [45], an important intermediate linking insulin signaling to GLUT4 translocation to the sarcolemma, potentially via protein phosphatase 2 (PP2)- and protein kinase C zeta (PKCζ)-dependent pathways [46]. Other sphingolipid species and mechanisms may be involved in IR. A recent study showed that direct interaction of the ganglioside GM3 with insulin receptor results in disassociation from the scaffolding protein caveolin-1 (Cav-1) and subsequent IR [47]. Ceramides are also linked to mitochondrial dysfunction, which in turn is implicated in IMCL accumulation and IR (Box 2). Several groups have reported a variety of sphingolipid species present in highly purified mitochondria free of contaminating membranes [48], including saturated and unsaturated long-chain ceramide species. This may be significant, because it has been shown that ceramide inhibits the ETS and induces ROS production. It was recently shown that mitochondria contain ceramide-producing (ceramide synthases and sphingomyelinases) and ceramide-degrading (ceramidases) enzymes [49], as well as the major ceramide transporter goodpasture binding protein (GPBP)/ceramide transporter (CERT) [50]. These findings are in line with an emerging hypothesis that ceramide species-specific functions are regulated by local pathways situated in distinct subcellular compartments (e.g. mitochondria, lysosomes) [51]. In mitochondria, ceramides interfere with mitochondrial bioenergetics by inhibiting electron transport at complex I and complex III [52] and by elevating ROS production [53]. Interestingly, in a cerebral ischemia–reperfusion model, c-Jun N-terminal kinase (JNK) 3 signaling is associated with stimulation of mitochondrial ceramide synthesis, including C16:0, C18:0, and C18:1, and inhibition of mitochondrial ETS. Conversely, JNK3 deficiency prevents ceramide synthesis in mitochondria and restores ETS function following ischemia– reperfusion [48]. Further studies are needed to evaluate the potential importance of these mechanisms to the pathophysiology of muscle IR.
Box 2. Mitochondrial dysfunction and IMCL accumulation.
Another line of evidence to help elucidate IR was that reduced FA oxidation, resulting from mitochondrial dysfunction, predisposed obese individuals to elevated IMCL. An impaired ability to oxidize FAs has been observed in obese individuals at the whole body level [88] and in muscle homogenates [89]. It was thought that impaired FA oxidation is primarily caused by a reduction in mitochondrial content and/or oxidative function [90]. Indeed, experimental models of increased mitochondrial fatty acid uptake and β-oxidation in muscle demonstrate protection against IR induced by lipid overload [91]. Similar protective effects are elicited by acute exercise and increased mitochondrial FA flux induced by muscle contraction [92].
In stark contrast, recent data challenge this consensus and indicate that mitochondria may be engaged in overconsumption of lipids and elevated β-oxidation during the early stages of diet-induced obesity. The result is accumulation of impartially oxidized lipids, as evidenced by elevated acylcarnitine levels in muscle and plasma [93]. The exact identity and nature of these partially oxidized metabolites are still unknown, but a recent study by Harper et al. using unbiased metabolomics on isolated mitochondria shows promise as an experimental approach [94]. Another aspect of mitochondrial lipid stress is elevated production of ROS, concomitant with lower antioxidant capacity [32]. A result of high-fat feeding is elevated β-oxidation, which provides an abundance of FADH2 to feed electrons to the Q-cycle of the electron transport system (ETS). The resulting high inner membrane potential, in concert with low ATP consumption and production, results in superoxide generation at complex I and III of the ETS. Elevated ROS in turn activates serine kinase pathways (including JNK), which negatively regulate insulin signaling [95].
Lipolysis of TG from LDs might be necessary for maintenance of oxidative gene expression and FA oxidation. ATGL deficiency in mouse heart decreases expression of PPAR-α, PPAR-δ, PGC-1α, and PGC-1β, concomitant with disrupted mitochondrial substrate oxidation [96]. Knockdown of hepatic ATGL in mice resulted in decreased lipolysis of TG, decreased expression of PGC-1α, and reduced FA oxidation [97]. The opposite was true for overexpression of hepatic ATGL. These studies suggest a novel mechanism by which defects in LD lipolysis result in TG accumulation and reduced mitochondrial content or function. However, these mechanisms have not been examined in human muscle IR.
In summary, contemporary understanding of the term mitochondrial dysfunction should not be restricted to ‘reduced oxidative capacity’, but can now be further defined as an imbalance between β-oxidation and oxidative phosphorylation or TCA cycle activity and elevated oxidant stress. Taken together, these studies suggest a chronology of mitochondrial dysfunction from early high-fat feeding-induced dysfunction (elevated β-oxidation) to the well-established severely obese phenotype (reduced oxidative capacity, reduced mitochondrial content). The net result of mitochondrial dysfunction is lipid partitioning and ectopic storage of IMCL, resulting in accumulation of lipid species that mediate IR.
Inhibition of ceramide synthesis prevents IR induce by lipid oversupply and glucocorticoids [54]. Inhibition of serine palmitoyl transferase-1 by the potent inhibitor myriocin also prevented IR induce by a high-fat diet [55]. By contrast, some studies did not observe increases in muscle ceramide content, with lipid emulsions consisting primarily of unsaturated FAs [56,57]. These studies and others suggest that lipid overload with saturated FAs preferentially induces ceramide-mediated IR, whereas overload with unsaturated lipid may result in DAG-mediated IR (Box 3, Figure 2).
Box 3. FFA saturation may determine the nature of lipid-induced IR.
DAG and ceramide have both been implicated in mediation of lipid-induced IR. However, it is not clear to what extent DAG and ceramide are involved in muscle IR in human obesity and T2D. The degree of saturation of plasma FAs may also play a role in the relative contribution of DAG and ceramide to muscle IR. Studies using FFA oversupply in myocyte cell cultures, isolated rodent muscle and lipid-infused rodents suggest that DAG may not contribute to saturated lipid-induced IR to a great extent. FA oversupply result in elevations in both ceramide and DAG, concomitant with IR [98]. However, treatment with myriocin, a ceramide synthesis inhibitor, alleviated saturated FA-induced IR, despite elevated muscle DAG content [45,54]. Conversely, unsaturated FA-induced IR may act through a ceramide-independent mechanism. Studies that used infusions of lipid cocktails enriched with linoleate observed IR and muscle DAG elevation, with no change in ceramide content [54,56,57]. Interestingly, rats fed a high-fat PUFA diet (safflower oil; linoleic acid, 18:2, n-6) showed improved insulin sensitivity and increased muscle DAG with no change in muscle ceramide compared to animals fed standard chow [99]. Together, these studies suggest that the degree of FA saturation in plasma FFAs in obesity and T2D may be important factors in distinguishing DAG- and ceramide-mediated IR (Figure 2). However, human studies thus far have yielded equivocal results. Kien et al. fed healthy individuals with a high-palmitate or low-palmitate and high-oleate diet for 7 days and did not observe any changes in muscle DAG content [100]. Further dietary intervention studies are needed to explore impact of dietary FA saturation on human muscle ceramide- and DAG-mediated IR.
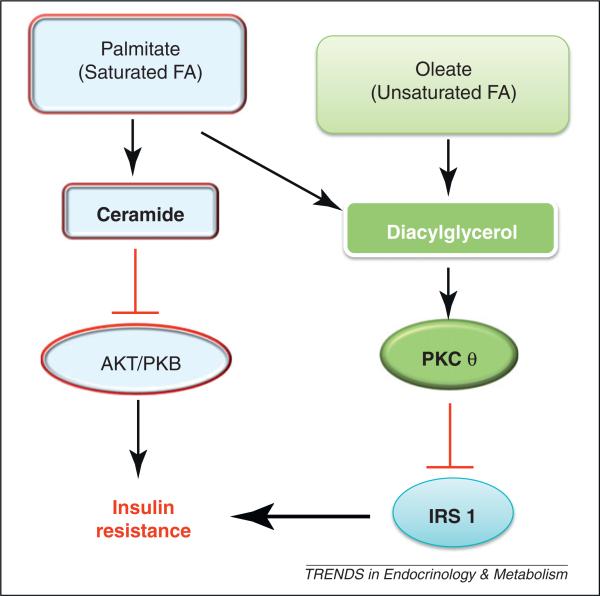
Figure 2.
Putative role of saturated and unsaturated fatty acids (FAs) in mediating insulin resistance (IR). In obesity, saturated FAs may result in preferential accumulation of ceramide, which mediates IR by inhibiting phosphorylation and activation of protein kinase B (PKB). Conversely, unsaturated FAs may preferentially result in diacylglycerol accumulation and subsequent activation of protein kinase C theta (PKCθ), leading to serine phosphorylation of insulin receptor substrate-1 (IRS-1) and IR.
Human studies largely support the relationship between muscle ceramide content and IR. Muscle ceramide content is elevated in obese compared to lean individuals [58]. Muscle ceramide content has also been associated with muscle IR in obese individuals [34]. Furthermore, exercise training can decrease ceramide content concomitant with improved insulin sensitivity [31]. In contrast, it has been reported that total muscle ceramide content is not different between subjects with a wide range of insulin sensitivity [35]. Also, Itani et al. reported that acute lipid-induced IR did not alter muscle ceramide content [56]. However, the lipid infusion used in this study contained primarily unsaturated lipids, potentially explaining the lack of ceramide accumulation. Other members of the sphingolipid family, such as sphingosine and sphingosine 1-phosphate, have been linked to human obesity and IR [34,59]. However, a mechanism for their role in muscle IR has yet to be reported. A number of other important questions regarding the role of ceramide and sphingolipids in IR still need to be addressed and are outlined in the Box 4.
Box 4. Outstanding questions.
Which ceramide species and in which subcellular locations (mitochondria? sarcolemma membrane?) impact human skeletal muscle IR?
What is the normal physiological function of ceramides in insulin-sensitive muscle?
Which stereospecific isoforms of DAG (1,3-DAG, 2,3-DAG, and 1-2-DAG) are more specifically implicated in muscle IR? The subcellular location (cytosol or sarcolemmal membrane) important for DAG and the degree of saturation of DAG FAs are factors that need to be clarified in DAG-induced human muscle IR.
What are the specific enzyme pathways and isoforms responsible for DAG and ceramide production in human muscle IR?
What are the incompletely oxidized lipid moieties within dysfunctional mitochondria of IR muscle? Do these lipid species mediate IR?
Does mitochondrial dysfunction occur first and thus lead to IMCL accumulation? Or does IMCL accumulation induce mitochondrial dysfunction?
Which LD proteins are important mediators of lipid partitioning and accumulation of lipotoxic mediators in IR? Does exercise training induce a change in the expression of LD proteins to facilitate increased or more efficient use as a metabolic fuel during exercise?
Diacylglycerols
DAGs are intracellular second messengers at the level of the sarcolemma and an intermediate in TG synthesis and breakdown in LDs. DAGs have also been identified as lipotoxic mediators of IR. Elevated DAG is capable of increasing the activity of protein kinase C (PKC) ε and θ [60]. Activated PKCs then serine phosphorylate insulin receptor substrate 1 (IRS-1), inhibiting kinase activity and subsequent activation of PI3-kinase and PKB/Akt [60]. As a result, insulin stimulated GLUT4 translocation is impaired, resulting in IR. A number of animal studies have demonstrated links between DAG and muscle IR in obesity [61]. However, others have shown dissociation between DAG accumulation and muscle IR. Overexpression of the enzyme diglyceride acyltransferase 1 (DGAT-1) in muscle resulted in DAG accumulation and improvements in IR induced by a high-fat diet [62]. In human studies, the extent to which muscle DAG content relates to IR is not clear. Muscle DAG content is elevated in obese and T2D subjects [63] and is increased following acute IR induced by lipid infusion [56]. Weight loss and exercise both decrease muscle DAG concomitant with improvements in insulin sensitivity [64]. By contrast, other studies have reported that DAG content in muscle was not elevated in obesity [65], with IR [66], or in obese IR compared to insulin-sensitive obese subjects [34].
It is difficult to reconcile the divergent results describing the relationship between DAG and IR in human muscle. An important factor may be the degree of FA saturation in DAG. Bergman et al. demonstrated that athletes have a lower degree of DAG saturation compared to sedentary controls. In line with this finding, Van Hees et al. showed that a higher degree of DAG saturation was associated with IR in men with metabolic syndrome [66]. Others have shown no such association [34] or even the inverse relationship [59]. Another factor that may be at play is the subcellular location of DAG accumulation. The vast majority of human studies examine only whole-muscle DAG concentrations. DAG is present in the sarcolemma membrane, sarcoplasmic reticulum, LDs, and mitochondrial membrane; this could certainly obscure the relationship between whole-muscle DAG quantification and IR. However, Bergman et al. recently showed that membrane DAG was associated with PKC activation and insulin sensitivity in obese T2D subjects and lean athletes [63]. Finally, and perhaps most importantly, there are three distinct DAG stereoisomers (1,3-DAG, 2,3-DAG, and 1-2-DAG), which may influence muscle IR to different degrees. To date, only 1,2-DAG has been associated with insulin signaling [61]. Only the 1,2-DAG stereoisomer can activate PKC; 1,3-DAG and 2,3-DAG lack this ability [67]. The classical mechanism describes 1,2-DAG formation from hydrolysis of phosphatidylinositol-4,5-phosphate by phospholipase C (PLC) in the plasma membrane. It is at this location that 1,2-DAG activates PKC isoforms. It has also been proposed that 1,2-DAG may be synthesized at the endoplasmic reticulum. This is not, however, a viable subcellular location for activation of plasma membrane PKC. A third potential source of DAG is from lipolysis of IMTG from LDs by ATGL and HSL. However, it has been suggested that both ATGL and HSL do not have the ability to generate the 1,2-DAG stereoisomer [68]. It also remains questionable whether LD-derived 1,2-DAG could associate with and activate membrane-bound PKC. Determination of 1,2-DAG concentrations specifically in the plasma membrane may reveal a clearer picture of the relationship between DAG and muscle IR. To date, no human studies have examined the different stereoisomers of DAG and their relationship to IR.
Long-chain acyl CoA
Long-chain acyl-CoA is formed when FAs taken up into myocytes are conjugated to a CoA group. This commits the FA to either lipid synthesis or β-oxidation. Long-chain acyl CoAs are IMCL species that have been associated with IR, but have received less attention than other species. In the context of lipid oversupply and physical inactivity, acyl CoA accumulates in myocytes. Obesity is associated with elevations in muscle long-chain acyl CoA and reduced muscle oxidative capacity [69]. Houmard et al. showed that weight loss reduces muscle long-chain CoA content in obesity, concomitant with improvements in insulin sensitivity [70]. Lipid infusion studies also showed acute elevations in acyl CoA content concomitant with PKC activation and IR [57]. Although associations with IR have been described, no mechanism by which acyl CoA causes IR has been identified. It is possible that acyl CoA acts as a precursor for DAG or ceramide synthesis, resulting in IR.
Concluding remarks
We have come a long way in our understanding of intramuscular lipids in human health since the earliest studies on muscle lipid metabolism in the early 1900s. We have gained considerable insight into the role of IMCL in fuel metabolism, but have a long way to go to firmly establish whether or not IMCL actually causes muscle IR or T2D. Important questions that still need to be addressed center on DAGs and ceramides and their roles in human muscle IR. Although cell culture and animal studies have elegantly unveiled roles for both of these lipid intermediates in inhibition of insulin signaling, the relative contribution of each in human IR still needs to be elucidated. Human studies to date have reported that several lipid species within skeletal muscle may contribute to lipid-induced IR. Advances in analytical lipidomics such as tandem mass spectrometry and lipid imaging could provide crucial information regarding the role of specific DAG and ceramide species, as well as other lipid intermediates, in the etiology of human IR and T2D. Determination of specific characteristics of FAs, including degree of saturation, chain length, and stereoisomer specificity, will also be important, as will identification of the specific subcellular locations where lipid species elicit their effects. Sphingolipids in particular mediate a very diverse array of signaling events in the context of normal muscle physiology and in IR, of which we know very little about. The coming years promise further exciting insights into the metabolic role of IMCL in human health and disease.
Glossary
- Athlete's paradox
phenomenon observed in endurance-trained athletes, who possess higher intramyocellular lipid content, coupled to high oxidative capacity and enhanced insulin sensitivity.
- Ceramide
sphingolipid molecule composed of sphingosine and a fatty acid.
- Diacylgylcerol (DAG)
glyceride consisting of two fatty acid chains bound to a glycerol molecule.
- Ectopic lipid
ectopic means displaced or out of place. Ectopic lipid refers to TG deposition within non-adipose tissue that normally only contains small amounts of lipid.
- Electron transport system (ETS)
system that mediates electron transfer between electron donors (NADH and FADH2) and an electron acceptor (oxygen) and concomitantly transfers H+ ions across the inner mitochondrial membrane.
- Ganglioside
component of the cell plasma membrane that modulates cell signal transduction events and concentrates in lipid rafts. The molecule is composed of a glycosphingolipid (ceramide and oligosaccharide) with one or more sialic acids linked to the sugar chain.
- Indirect calorimetry
method for estimating energy expenditure by measuring oxygen consumption and carbon dioxide release.
- Intralipid
lipid emulsion containing soybean oil, egg phospholipid, and glycerin.
- Intramyocellular lipid (IMCL)
any lipid species that resides within myocytes. IMCL is predominantly TG but also includes diacylglycerols, sphingolipids and membrane phospholipids.
- Liposyn II
lipid emulsion containing safflower oil, soybean oil, egg phospholipid, and glycerin.
- Myriocin
also known as antibiotic ISP-1 and thermozymocidin; a very potent inhibitor of serine palmitoyltransferase, the first step in sphingosine biosynthesis. It is an atypical amino acid and an antibiotic derived from certain thermophilic fungi.
- Oil red O
lysochrome diazo dye used for staining of neutral triglycerides and lipids in frozen muscle sections.
- Sarcolemma
cell membrane of a muscle cell (skeletal, cardiac, and smooth muscle). It consists of a plasma membrane and an outer coat that is made up of a thin layer of polysaccharide material containing numerous thin collagen fibrils.
- Sphingolipids
class of lipids containing a sphingoid base backbone. Sphingoid bases are a group of aliphatic amino alcohols that include sphingosine.
References
- 1.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol. Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 2.Webber J. Energy balance in obesity. Proc. Nutr. Soc. 2003;62:539–543. doi: 10.1079/pns2003256. [DOI] [PubMed] [Google Scholar]
- 3.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 4.Glatz JF, et al. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 5.Dyck DJ, et al. Functional differences in lipid metabolism in resting skeletal muscle of various fiber types. Am. J. Physiol. 1997;272:E340–E351. doi: 10.1152/ajpendo.1997.272.3.E340. [DOI] [PubMed] [Google Scholar]
- 6.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 7.Holloway GP, et al. Contribution of FAT/CD36 to the regulation of skeletal muscle fatty acid oxidation: an overview. Acta Physiol. (Oxf.) 2008;194:293–309. doi: 10.1111/j.1748-1716.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- 8.van Loon LJ, Goodpaster BH. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflugers Arch. 2006;451:606–616. doi: 10.1007/s00424-005-1509-0. [DOI] [PubMed] [Google Scholar]
- 9.Krogh A, Lindhard J. The relative value of fat and carbohydrate as sources of muscular energy: with appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochem. J. 1920;14:290–363. doi: 10.1042/bj0140290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himwich HE, Rose MI. The respiratory quotient of exercising muscle. Am. J. Physiol. 1927;81:485–486. [Google Scholar]
- 11.Fritz IB, et al. Fatty acid oxidation by skeletal muscle during rest and activity. Am. J. Physiol. 1958;194:379–386. doi: 10.1152/ajplegacy.1958.194.2.379. [DOI] [PubMed] [Google Scholar]
- 12.Denton RM, Randle PJ. Concentrations of glycerides and phospholipids in rat heart and gastrocnemius muscles. Effects of alloxan-diabetes and perfusion. Biochem. J. 1967;104:416–422. doi: 10.1042/bj1040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson LA, et al. Concentration of triglycerides, phospholipids and glycogen in skeletal muscle and of free fatty acids and beta-hydroxybutyric acid in blood in man in response to exercise. Eur. J. Clin. Invest. 1971;1:248–254. doi: 10.1111/eci.1971.1.4.248. [DOI] [PubMed] [Google Scholar]
- 14.Pan DA, et al. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J. Clin. Invest. 1995;96:2802–2808. doi: 10.1172/JCI118350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dube J, Goodpaster BH. Assessment of intramuscular triglycerides: contribution to metabolic abnormalities. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:553–559. doi: 10.1097/01.mco.0000241664.38385.12. [DOI] [PubMed] [Google Scholar]
- 16.Essen B, et al. Utilization of blood-borne and intramuscular substrates during continuous and intermittent exercise in man. J. Physiol. 1977;265:489–506. doi: 10.1113/jphysiol.1977.sp011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergman BC, et al. Evaluation of exercise and training on muscle lipid metabolism. Am. J. Physiol. 1999;276:E106–E117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- 18.Watt MJ, et al. Intramuscular triacylglycerol utilization in human skeletal muscle during exercise: is there a controversy? J. Appl. Physiol. 2002;93:1185–1195. doi: 10.1152/japplphysiol.00197.2002. [DOI] [PubMed] [Google Scholar]
- 19.Guo Z, et al. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J. Appl. Physiol. 2000;89:2057–2064. doi: 10.1152/jappl.2000.89.5.2057. [DOI] [PubMed] [Google Scholar]
- 20.White LJ, et al. Intramyocellular lipid changes in men and women during aerobic exercise: a 1H-magnetic resonance spectroscopy study. J. Clin. Endocrinol. Metab. 2003;88:5638–5643. doi: 10.1210/jc.2003-031006. [DOI] [PubMed] [Google Scholar]
- 21.van Loon LJ, et al. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J. Physiol. 2003;553:611–625. doi: 10.1113/jphysiol.2003.052431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watt MJ, et al. Effects of plasma adrenaline on hormone-sensitive lipase at rest and during moderate exercise in human skeletal muscle. J. Physiol. 2003;550:325–332. doi: 10.1113/jphysiol.2003.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langfort J, et al. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem. J. 2000;351:207–214. doi: 10.1042/0264-6021:3510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsted TJ, et al. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am. J. Physiol. Endocrinol. Metab. 2009;296:E445–E453. doi: 10.1152/ajpendo.90912.2008. [DOI] [PubMed] [Google Scholar]
- 25.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J. Clin. Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob S, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–1119. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- 27.Goodpaster BH, et al. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 28.Toledo FG, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–994. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann OP, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster BH, et al. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 31.Dube JJ, et al. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson EJ, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leeuwenburgh C, et al. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;272:R363–R369. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- 34.Coen PM, et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes. 2010;59:80–88. doi: 10.2337/db09-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skovbro M, et al. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia. 2008;51:1253–1260. doi: 10.1007/s00125-008-1014-z. [DOI] [PubMed] [Google Scholar]
- 36.Bonen A, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18:1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 37.Bonen A, et al. Muscle contractile activity increases fatty acid metabolism, transport, FAT/CD36. Am. J. Physiol. Endocrinol. Metab. 1999;276:E642–E649. doi: 10.1152/ajpendo.1999.276.4.E642. [DOI] [PubMed] [Google Scholar]
- 38.Koonen DP, et al. Different mechanisms can alter fatty acid transport when muscle contractile activity is chronically altered. Am. J. Physiol. Endocrinol. Metab. 2004;286:E1042–E1049. doi: 10.1152/ajpendo.00531.2003. [DOI] [PubMed] [Google Scholar]
- 39.Tunstall RJ, et al. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002;283:E66–E72. doi: 10.1152/ajpendo.00475.2001. [DOI] [PubMed] [Google Scholar]
- 40.Talanian JL, et al. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J. Appl. Physiol. 2007;102:1439–1447. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- 41.Burgomaster KA, et al. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1970–R1976. doi: 10.1152/ajpregu.00503.2006. [DOI] [PubMed] [Google Scholar]
- 42.De Bock K, et al. Effect of training in the fasted state on metabolic responses during exercise with carbohydrate intake. J Appl. Physiol. 2008;104:1045–1055. doi: 10.1152/japplphysiol.01195.2007. [DOI] [PubMed] [Google Scholar]
- 43.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 44.Deevska GM, Nikolova-Karakashian MN. The twists and turns of sphingolipid pathway in glucose regulation. Biochimie. 2011;93:32–38. doi: 10.1016/j.biochi.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chavez JA, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 46.Stratford S, et al. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 47.Kabayama K, et al. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13678–13683. doi: 10.1073/pnas.0703650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J, et al. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J. Biol. Chem. 2007;282:25940–25949. doi: 10.1074/jbc.M701812200. [DOI] [PubMed] [Google Scholar]
- 49.Novgorodov SA, et al. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J. Biol. Chem. 2011;286:25352–25362. doi: 10.1074/jbc.M110.214866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mencarelli C, et al. The ceramide transporter and the Goodpasture antigen binding protein: one protein–one function? J. Neurochem. 2010;113:1369–1386. doi: 10.1111/j.1471-4159.2010.06673.x. [DOI] [PubMed] [Google Scholar]
- 51.Hannun YA, Obeid LM. Many ceramides. J. Biol. Chem. 2011;286:27855–27862. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Paola M, et al. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39:6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Ruiz C, et al. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 54.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Ussher JR, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59:2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itani SI, et al. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 57.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 58.Adams JM, 2nd, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 59.Amati F, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Timmers S, et al. Muscular diacylglycerol metabolism and insulin resistance. Physiol. Behav. 2008;94:242–251. doi: 10.1016/j.physbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Turinsky J, et al. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J. Biol. Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- 62.Timmers S, et al. Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS ONE. 2011;6:e14503. doi: 10.1371/journal.pone.0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergman BC, et al. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia. 2012;55:1140–1150. doi: 10.1007/s00125-011-2419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dube JJ, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54:1147–1156. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anastasiou CA, et al. Diabetes mellitus is associated with increased intramyocellular triglyceride, but not diglyceride, content in obese humans. Metabolism. 2009;58:1636–1642. doi: 10.1016/j.metabol.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 66.van Hees AM, et al. Skeletal muscle fatty acid handling in insulin resistant men. Obesity (Silver Spring) 2011;19:1350–1359. doi: 10.1038/oby.2011.10. [DOI] [PubMed] [Google Scholar]
- 67.Boni LT, Rando RR. The nature of protein kinase C activation by physically defined phospholipid vesicles and diacylglycerols. J. Biol. Chem. 1985;260:10819–10825. [PubMed] [Google Scholar]
- 68.Zechner R, et al. Fat signals – lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hulver MW, et al. Skeletal muscle lipid metabolism with obesity. Am. J. Physiol. Endocrinol. Metab. 2003;284:E741–E747. doi: 10.1152/ajpendo.00514.2002. [DOI] [PubMed] [Google Scholar]
- 70.Houmard JA, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes. 2002;51:2959–2963. doi: 10.2337/diabetes.51.10.2959. [DOI] [PubMed] [Google Scholar]
- 71.Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 2001;40:325–438. doi: 10.1016/s0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 72.Bickel PE, et al. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Varela GM, et al. Inhibition of ADRP prevents diet-induced insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G621–G628. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw CS, et al. Adipophilin distribution and colocalization with lipid droplets in skeletal muscle. Histochem. Cell Biol. 2009;131:575–581. doi: 10.1007/s00418-009-0558-4. [DOI] [PubMed] [Google Scholar]
- 75.Bulankina AV, et al. TIP47 functions in the biogenesis of lipid droplets. J. Cell Biol. 2009;185:641–655. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dalen KT, et al. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes. 2004;53:1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 77.Granneman JG, et al. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J. Biol. Chem. 2011;286:5126–5135. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, et al. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minnaard R, et al. Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2009;94:4077–4085. doi: 10.1210/jc.2009-0352. [DOI] [PubMed] [Google Scholar]
- 80.MacPherson RE, et al. Subcellular localization of skeletal muscle lipid droplets and PLIN family proteins OXPAT and ADRP at rest and following contraction in rat soleus muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R29–R36. doi: 10.1152/ajpregu.00163.2011. [DOI] [PubMed] [Google Scholar]
- 81.Sacchetti M, et al. High triacylglycerol turnover rate in human skeletal muscle. J. Physiol. 2004;561:883–891. doi: 10.1113/jphysiol.2004.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 83.Yang X, et al. The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watt MJ, et al. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2006;290:E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 85.Blaak EE, et al. Impaired beta-adrenergically mediated lipolysis in skeletal muscle of obese subjects. Diabetologia. 2004;47:1462–1468. doi: 10.1007/s00125-004-1471-y. [DOI] [PubMed] [Google Scholar]
- 86.Badin PM, et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes. 2011;60:1734–1742. doi: 10.2337/db10-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H, et al. Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein a-I. J. Proteome Res. 2011;10:4757–4768. doi: 10.1021/pr200553c. [DOI] [PubMed] [Google Scholar]
- 88.Thyfault JP, et al. Impaired plasma fatty acid oxidation in extremely obese women. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1076–E1081. doi: 10.1152/ajpendo.00177.2004. [DOI] [PubMed] [Google Scholar]
- 89.Berggren JR, et al. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am. J. Physiol. Endocrinol. Metab. 2008;294:E726–E732. doi: 10.1152/ajpendo.00354.2007. [DOI] [PubMed] [Google Scholar]
- 90.Kelley DE, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 91.Bruce CR, et al. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thrush AB, et al. A single prior bout of exercise protects against palmitate-induced insulin resistance despite an increase in total ceramide content. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R1200–R1208. doi: 10.1152/ajpregu.00091.2010. [DOI] [PubMed] [Google Scholar]
- 93.Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 94.Seifert EL, et al. Long-chain fatty acid combustion rate is associated with unique metabolite profiles in skeletal muscle mitochondria. PLoS ONE. 2010;5:e9834. doi: 10.1371/journal.pone.0009834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid. Redox Signal. 2005;7:1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 96.Haemmerle G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ong KT, et al. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montell E, et al. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am. J. Physiol. Endocrinol. Metab. 2001;280:E229–E237. doi: 10.1152/ajpendo.2001.280.2.E229. [DOI] [PubMed] [Google Scholar]
- 99.Lee JS, et al. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J. Appl. Physiol. 2006;100:1467–1474. doi: 10.1152/japplphysiol.01438.2005. [DOI] [PubMed] [Google Scholar]
- 100.Kien CL, et al. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring) 2011;19:305–311. doi: 10.1038/oby.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]