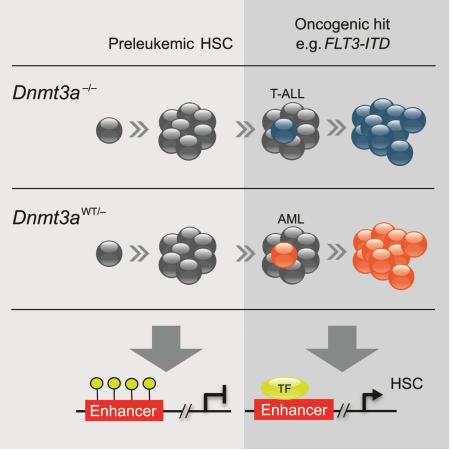
SUMMARY
DNMT3A, the gene encoding the de novo DNA methyltransferase 3A, is among the most frequently mutated genes in hematologic malignancies. However, the mechanisms through which DNMT3A normally suppresses malignancy development are unknown. Here, we show that DNMT3A loss synergizes with the FLT3 internal tandem duplication (ITD) in a dose-influenced fashion to generate rapid lethal lymphoid or myeloid leukemias similar to their human counterparts. Loss of DNMT3A leads to reduced DNA methylation, predominantly at hematopoietic enhancer regions in both mouse and human samples. Myeloid and lymphoid diseases arise from transformed murine hematopoietic stem cells. Broadly, our findings support a role for DNMT3A as a guardian of the epigenetic state at enhancer regions, critical for inhibition of leukemic transformation.
Graphical Abstract
INTRODUCTION
DNA methylation, an epigenetic mark that influences cell fate, has long been recognized to be aberrantly distributed in many cancers (You and Jones, 2012). Recently, mutations in the de novo DNA methyltransferase 3A gene (DNMT3A) have been found in a variety of hematologic malignancies, suggesting a central role in preventing disease development (Reviewed in (Yang et al., 2015). Approximately 25% of human myeloid or lymphoid malignancies, including acute myeloid leukemia (AML) (Ley et al., 2010) and T cell acute lymphoblastic leukemia (T-ALL) (Grossmann et al., 2013; Van Vlierberghe et al., 2013), harbor DNMT3A mutations. In AML, a hotspot mutation at Arginine 882 (R882) is most prevalent, occurring in about 60% of DNMT3Amut cases (Ley et al., 2010). This alteration is thought to act as a dominant-negative, rendering the cell with only about 20% of wild-type DNMT3A activity (Kim et al., 2013; Russler-Germain et al., 2014). In T-ALL, R882 mutations are less prevalent, and about 62% of DNMT3Amut cases harbor homozygous or compound heterozygous mutations (Grossmann et al., 2013). Together, these findings indicate that DNMT3A acts as a tumor suppressor, with a loss of most or all of its function promoting malignancy.
DNMT3A is highly expressed in mouse hematopoietic stem cells (HSCs), where its loss promotes HSC self-renewal at the expense of efficient differentiation (Challen et al., 2012). In AML patients, DNMT3A mutations can be found in non-leukemic and leukemic cells in the peripheral blood (Corces-Zimmerman et al., 2014; Shlush et al., 2014). DNMT3A mutations in humans are associated with increased risk of leukemia, but alone are insufficient for transformation. The presence of DNMT3A mutations in HSCs that can behave relatively normally, and the latency of disease development in individuals that harbor DNMT3Amut HSCs (Xie et al., 2014), suggests that secondary mutations are key in driving the particular type of disease development. In mice transplanted with Dnmt3a-KO HSCs, both lymphoid and myeloid malignancies emerged, and latency was long (3 to 14 months), also supporting the requirement for additional hits (Celik et al., 2015; Mayle et al., 2015).
In AML, 30% of cases with DNMT3A mutations also harbor internal tandem duplications (ITD) in the fms-like tyrosine kinase 3 gene (FLT3-ITD) (Ley et al., 2010). DNMT3A and FLT3-ITD mutations also occur together in early immature T-ALL (Van Vlierberghe et al., 2013). Here we sought to combine Dnmt3a ablation with a specific additional mutation to investigate the mechanisms through which loss of DNMT3A promotes leukemia development.
RESULTS
Dnmt3a loss accelerates FLT3-ITD lymphoid leukemia
We sought to establish a model with both DNMT3A loss and FLT3-ITD expression. Because expression of FLT3-ITD via retrovirus can generate murine T-ALL (Kelly et al., 2002), we first used this strategy in Dnmt3a-KO cells. We deleted Dnmt3a in 8-week-old Mx1-Cre; Dnmt3afl/fl mice, using polyinosinic-polycytidylic acid (pIpC) to generate animals with Dnmt3a−/− bone marrow cells. We transduced 5-fluorouracil-stimulated hematopoietic stem and progenitor cells from these mice with an MSCV retrovirus containing FLT3-ITD-IRES-GFP (3aKO/FLT3-ITD) or IRES-GFP alone (3aKO) and transplanted the cells into lethally irradiated recipients. A separate group of Mx1-Cre; Dnmt3a+/+ mice were transduced with FLT3-ITD-IRES-GFP (FLT3-ITD), or IRES-GFP alone (WT) (Figure 1A). All control mice received pIpC injections.
Figure 1. Dnmt3a deletion potentiates FLT3-ITD-mediated induction of pre T-lymphoblastic leukemia.
(A) Experimental scheme showing induction of Mx1-Cre, FLT3-ITD retroviral transduction, and experimental groups. (B) Kaplan-Meier survival plots comparing WT and 3aKO controls and WT and 3aKO expressing FLT3-ITD n=10, ***p < 0.001 by log-rank test with Bonferroni correction, representative of six independent experiments. (C) Spleen weights of moribund and control mice normalized to body weight (n=9) representative of three independent experiments. (D) Thymus weights normalized to body weights of moribund mice and control mice (n=10 per group) for three independent experiments. (E) Flow cytometry analysis of CD45.2 (donor-derived cells), GFP, CD4 and CD8 in bone marrow (BM). Arrows between graphs indicate gating strategy. Arrows on axes indicate markers used. (F) Histological analysis of peripheral blood (Giemsa-Wright stain), BM (Giemsa-Wright stain), and spleen (H&E stain). Scale bars = 100 m. (G) Ki67 staining of 3aKO/FLT3-ITD and FLT3-ITD (H) Analysis of apoptotic rate of 3aKO FLT3-ITD and FLT3-ITD (n=5). All bars denote mean ± s.e.m values *p < 0.05 and ** p < 0.01 and *** p < 0.001 by one-way ANOVA. See also Figure S1.
Mice transplanted with FLT3-ITD or 3aKO/FLT3-ITD bone marrow cells developed leukemia. Strikingly, 3aKO/FLT3-ITD mice had significantly shorter survival times (79 days vs. 116 days) than FLT3-ITD mice (Figure 1B). Both groups showed weight loss, splenomegaly, and thymomegaly (Figures 1C and 1D) with widespread GFP+ cell infiltration in the bone marrow (Figure 1E). Notably, the 3aKO/FLT3-ITD group had larger spleens and smaller thymuses (Figures 1C and 1D). Immunophenotyping revealed GFP+ T cells that expressed markers of immature thymocytes and progenitors (CD4+CD8+CD25+; Figures 1E and S1A). At this time point, mice transplanted with cells from the 3aKO-alone showed no overt phenotype (Figure 1, S1). Histological examination revealed extensive infiltration of peripheral blood, bone marrow, and spleen (Figure 1F) and nonhematopoietic organs (liver, lung and kidney) by leukemic cells that were cytoplasmic CD3+ and MPO− (Figures S1B and S1C). Consistent with previous reports using the retroviral model (Kelly et al., 2002), we diagnosed the majority of 3aKO/FLT3-ITD and FLT3-ITD mice (90% and 78%, respectively) as having a T cell disease, specifically precursor T cell lymphoblastic lymphoma/leukemia (similar to human T-ALL), based on the Bethesda classification system (Morse et al., 2002). The leukemic cells were capable of self-renewal as demonstrated by transplantation to sublethally irradiated WT recipients (Figure S1D). In addition, 22% of mice transplanted with FLT3-ITD cells and 5% with 3aKO/FLT3-ITD died from myeloproliferative disease and 5% of 3aKO/FLT3-ITD mice died of B-cell ALL (Figure S1E). Compared to the FLT3-ITD T-ALL cells, the 3aKO/FLT3-ITD T-ALL cells were more proliferative and had higher rates of apoptosis by Ki-67 and annexin V staining, respectively (Figures 1G and 1H). These findings indicate that loss of Dnmt3a promotes aggressive T-ALL in hematopoietic cells that express FLT3-ITD.
Dnmt3a loss-related lymphoid leukemia upregulates myeloid programs
To understand how loss of Dnmt3a contributes to lethal lymphoid leukemia in mice, we studied the global gene expression profiles of the 3aKO/FLT3-ITD and FLT3-ITD T-ALLs by RNA-seq. We compared the two sets of leukemic cells (>95% GFP+ and CD4+CD8+) and sorted CD4+CD8+ WT thymocytes from transplanted mice as a control. 3aKO mice were healthy throughout this experiment; CD4+CD8+ cells from these mice had negligible differences compared to the controls (data not shown). Comparison of 3aKO/FLT3-ITD with FLT3-ITD-only cells revealed 696 differentially expressed genes (507 upregulated and 189 downregulated in the 3aKO/FLT3-ITD group) (Figure S2A). Gene ontology (GO) analysis showed that in the FLT3-ITD-only group, upregulated genes were functionally related to the extracellular region, whereas those upregulated in the 3aKO/FLT3-ITD mice included genes related to inflammation and immune response (Figure 2A). Surprisingly, Ingenuity pathway analysis indicated upregulation of myeloid genes in the 3aKO/FLT3-ITD group (Figure 2B), while mature T cell genes were downregulated (Figure 2C). Gene set enrichment analysis revealed genes upregulated in 3aKO/FLT3-ITD were enriched for immature gene sets, including hematopoietic stem cells (e.g. Gata2, H19) and mouse early thymic progenitors. Upregulated genes were also enriched for myeloid, AML and aging gene sets (Figure S2B). The enriched gene sets were highly differentially expressed (Figure 2C).
Figure 2. Deletion of Dnmt3a in T cell acute lymphoblastic leukemia induces aberrant HSC and myeloid gene expression.
(A, B) Gene ontology (DAVID) analysis of pairwise comparisons (A) and Ingenuity pathway analysis (B) of differential gene expression comparing 3aKO/FLT3-ITD and FLT3-ITD leukemic cells, using WT thymocytes as a control (FPKM > 0.5, fold change > 1.5, FDR q-value < 0.05). (C) Heat map of average log-transformed gene scaled FPKM expression values of representative enriched gene sets from GSEA in 3aKO/FLT3-ITD leukemic cells compared to FLT3-ITD leukemic cells and WT thymocytes (CD4+CD8+ cells from each). Up and Down indicate upregulated or downregulated genes in the gene set. (D) Percentage of up- and down-regulated genes in 3aKO/FLT3-ITD murine cells found in the signatures of over- and under-represented genes that characterize human leukemia subtypes (Haferlach et al., 2010). PRE_SUBTYPE, AML_SUBTYPE, ALL_SUBTYPE represent leukemia precursor, AML, and ALL data, respectively. See also Figure S2.
To investigate the extent to which the expression profiles of the mouse model recapitulate those of human disease, we assessed the significance of gene overlap with expression signatures derived from the Microarray Innovations in Leukemia (MILE) patient research study (Haferlach et al., 2010). We compared genes differentially expressed in the 3aKO/FLT3-ITD leukemia model to signatures that distinguish patients with leukemia precursor, ALL, or AML from patients with all other disease in MILE. The mouse model profile was strongly associated with genes characterizing human AML (odds ratio 15.5, p value = 2.23E-16) (Figure 2D and S2C). Interestingly, the strength of association between the model and human ALL was considerably less than expected by chance (odds ratio 0.24, p value = 1.12E-08). These data suggest that in the absence of Dnmt3a, murine lymphoid leukemic cells maintain the expression of T cell surface markers, but activate a myeloid gene signature at the cost of mature T cell genes, which is reminiscent of human early immature T cell leukemias that are enriched in aberrant myeloid genes (Van Vlierberghe et al., 2011).
The T-ALL diagnosed in 3aKO/FLT3-ITD mice is similar to early T cell precursor ALL (ETP-ALL), which is associated with FLT3 and DNMT3A mutations and the expression of stem and myeloid genes (Neumann et al., 2013). The ETP classification is immunophenotypic, and requires absence of the T cell marker CD8 (and others) (Coustan-Smith et al., 2009). T-ALL can alternatively be classified on the basis of gene expression profiles, which reveals two classes of immature vs mature T-ALL with unique survival and mutation profiles (Van Vlierberghe et al., 2013). The immature T-ALL group, associated with dismal survival, is distinguished by a myeloid and stem cell gene expression signature and is enriched for DNMT3A mutations (Van Vlierberghe et al., 2013). While having some overlap with ETP-ALL, the immature classification allows expression of CD4 and CD8 (Coustan-Smith et al., 2009; Van Vlierberghe et al., 2013). Considering the surface marker profile (CD4+CD8+), and myeloid and stem cell-associated gene expression signatures in the leukemia we observe, the 3aKO/FLT3-ITD cells most closely resembles early immature T-ALL. To confirm the immature phenotype, we tested the expression of Notch pathway and early thymic progenitor-related genes by quantitative PCR and found them to be downregulated and upregulated, respectively (Figure S2D), consistent with 3aKO/FLT3-ITD lymphoid leukemia being most similar to human early immature T-ALL, also associated with DNMT3A and FLT3 mutations in patients.
Dnmt3a-loss initiates myeloid and lymphoid Flt3-ITD leukemia
The combination of DNMT3A and FLT3 mutation in patients are more frequently associated with acute myeloid leukemia (AML) (Ley et al., 2010; Neumann et al., 2012). In an attempt to generate myeloid disease, we turned to a Flt3-ITD knock-in model (referred to as Flt3-ITDKI), in which heterozygous mice develop myeloproliferative disease (Lee et al., 2007).
We crossed Flt3-ITDKI mice with Dnmt3afl/fl mice expressing a tamoxifen inducible (ER) Cre (Hinkal et al., 2009). The ERT2-Cre; Dnmt3afl/fl; Flt3+/ITD and controls ERT2-Cre; Dnmt3afl/fland Dnmt3afl/flFlt3+/ITD (lacking Cre) mice were treated with tamoxifen to generate 3aKO/Flt3-ITDKI, 3aKO, and Flt3-ITDKI mice, after which their bone marrow was transplanted into lethally irradiated recipients (Figure 3A). Consistent with previous reports, ablation of Dnmt3a alone led to a spectrum of hematologic diseases (Celik et al., 2015; Mayle et al., 2015), and the Flt3-ITDKI group did not develop lethal disease (Lee et al., 2007). All of the 3aKO/Flt3-ITDKI group died of acute leukemia with a median survival of 225 days, which is not significantly different than the 3aKO alone (Figure 3B), but the disease spectrum was different (Figure 4G, see below), and the median survival was shorter than the Flt3-ITDKI group. The 3aKO/Flt3-ITDKI mice exhibited leukocytosis and myeloproliferation (Figure 3C and 3D), and 8/14 mice were diagnosed with AML, as indicated by myeloid markers and leukemic infiltration in bone marrow and spleen (Figures 3E and S3) (Kogan, 2002). Two mice exhibited mixed lineage leukemia, consisting of a blast-filled bone marrow (Figure 3F) that stained for either myeloid or T cell markers but also had an enlarged thymus infiltrated by donor-derived T-lymphoblasts (Figure 3G). The remaining four mice were diagnosed with T-ALL.
Figure 3. Dnmt3a loss with Flt3-ITDKI initiates acute myeloid and T- lymphoblastic leukemia.
(A) Experimental scheme showing deletion of Dnmt3a by ERT2-Cre and bone marrow transplantation into lethally irradiated recipients to generate 3aKO/Flt3-ITDKI mice and controls (B) Kaplan-Meier survival plot of mice transplanted with cells from 3aKO, Flt3-ITDKI and 3aKO/Flt3-ITDKI mice. (C, D) Peripheral blood analysis showing white blood cell counts (C) and lineage distribution (D) 7 months after transplant as in (B). * p<0.05 and *** p<0.001 by one-way and Two-way ANOVA. (E) Giemsa-Wright staining of 3aKO, 3aKO/Flt3-ITDKI and Flt3-ITDKIbone marrow touch preps. Scale bar = 50 μm. (F) Representative bone marrow touch preps of mice with bone marrow leukemic cell infiltration stained with Giemsa-Wright. Scale bar = 50 μm. (G) Flow cytometry analysis with myeloid markers (Mac-1/Gr-1) (red box) and T cell markers (black box) in the bone marrow (left) and thymus (right). (H) PCR analysis to detect Dnmt3a-floxed vs deleted (Δ) allele in leukemic cells from the bone marrow (BM) and thymus (Th). L=molecular weight ladder. All bars denote mean ± s.e.m values. See also Figure S3.
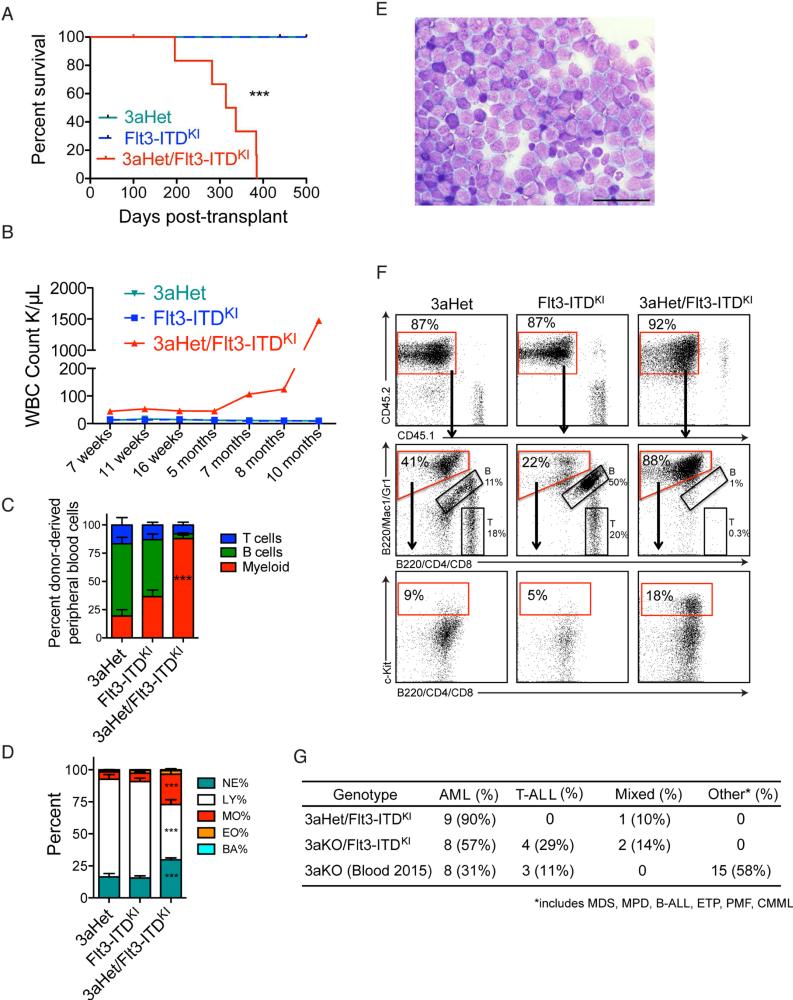
Figure 4. Heterozygous loss of Dnmt3a induces acute myeloid leukemia.
(A) Kaplan-Meier survival plot of mice transplanted with bone marrow of 3aHet/Flt3-ITDKI, 3aHet, or Flt3-ITDKI. (B-D) Peripheral blood WBC count over time with flow cytometry analysis (B), WBC differential (C) and the relative proportions of the indicated populations among the stained cells (D). (E) Bone marrow touch prep stained with Giemsa-wright staining. Scale bar = 50 μm. (F) Flow cytometry analysis of donor-derived CD45.2+ bone marrow cells with myeloid and progenitor surface markers (Mac-1/Gr-1/c-Kit) in 3aHet/Flt3-ITDKI, 3aHet, and Flt3-ITDKI with the red box in the middle panel indicating the myeloid population. Arrows indicate the gating strategy. (G) Table of leukemia incidence in 3aHet/Flt3-ITDKI, 3aKO/Flt3-ITDKI, and 3aKO mice. All bars denote mean ± s.e.m values. See also Figure S4.
To verify complete deletion of Dnmt3a, we tested a subset of AML and T-ALL samples and bone marrow and thymus tissue from mice with mixed-lineage leukemia for Dnmt3a deletion by PCR. Although ERT2-Cre is generally highly efficient, we noted residual floxed allele in most of the AML samples (e.g. Figure 3H). In contrast, Dnmt3a deletion appeared complete in most T-ALL samples. These data suggested a correlation between Dnmt3a dosage and disease type, with incompletely deleted Dnmt3a more common in myeloid than lymphoid leukemia. As discussed above, human AML cases most frequently harbor mono-allelic mutation of DNMT3A at R882, which is thought to retain some DNMT3A activity, while T-ALL cases more commonly harbor biallelic DNMT3A mutations, consistent with more complete loss-of-function.
Heterozygous Dnmt3a loss cooperates with Flt3-ITD to initiate acute myeloid leukemia
To test the hypothesis that heterozygous deletion of Dnmt3a is more likely to result in myeloid disease, we generated Dnmt3a+/−Flt3+/ITD (3aHet/Flt3-ITDKI) mice by crossing ERT2-Cre; Dnmt3afl/fl mice with Dnmt3a+/+Flt3+/ITD mice, and bone marrow from these mice was transplanted into lethally irradiated recipients. Nine out of ten 3aHet/Flt3-ITDKI mice analyzed died from AML with a median survival of 270 days (Figure 4A). These mice developed myelocytosis (Figure 4B and C) with increased monocytes and neutrophils (Figure 4D). Histological examination revealed leukemic blasts in the bone marrow (Figure 4E), peripheral blood (Figure S4A), and extramedullary organs (Figure S4B). The blasts expressed myeloid markers (Mac-1+Gr-1+c-Kit+) (Figures 4F and S4C). The disease was recapitulated in secondary recipients (Figure S4D). Together, these features indicated a diagnosis of AML in the 3aHet/Flt3-ITDKI transplanted mice.
These studies indicate that partial Dnmt3a loss cooperates with Flt3-ITDKI to instigate AML with monocytic and neutrophilic bias similar to the association of DNMT3A mutations with M4/M5 AML (Cancer Genome Atlas Research, 2013; Yan et al., 2011). By transcriptome analysis, genes related to myeloid cell function were enriched in the 3aHet/Flt3-ITDKI leukemic cells compared to Flt3-ITDKI progenitors (Figure S4E-S4G).
Of the cohort of mice transplanted with 3aHet/Flt3-ITDKI cells, 90% developed AML (Figure 4G), in contrast to the 3aKO/Flt3-ITDKI cohort, of which only 57% developed AML with the rest exhibiting T-ALL or mixed lineage leukemia, and the 3aKO cohort, which develop a variety of malignancies (Mayle et al., 2015). Together, these observations indicate a relationship between Dnmt3a-dosage and the type of disease development.
Dnmt3a loss-associated leukemia arises from HSCs
In AML patients, DNMT3A mutations have been found in HSCs, acting as a preleukemic lesion (Corces-Zimmerman et al., 2014; Ding et al., 2012; Shlush et al., 2014). However, whether this DNMT3A mutant cell, or other downstream populations, can serve as the leukemia stem cell (LSC) is not known. Indeed, in some leukemias, LSCs arise from committed progenitors that acquire the ability to self-renew (e.g. MLL-AF9 (Krivtsov et al., 2006)), whereas in others, it arises from HSCs (Rathinam et al., 2010). In our model, all cells carry the Dnmt3a mutation and FLT3-ITD, so we sought to test whether HSCs, their progeny, or both, would transmit the disease.
We sorted Dnmt3a-KO myeloid progenitors (Lin−Sca-1−c-Kit+), lymphoid progenitors (Lin-IL7ra+Sca-1medc-Kitmed), and HSCs (Side population+ Lin−Sca-1+c-Kit+), and transduced them with the FLT3-ITD retrovirus before transplanting them into lethally irradiated recipients. We observed disease only in mice transplanted with HSCs (Figure 5A, B). To determine whether each population could propagate the disease, we also sorted and transplanted various GFP+ populations from the mice after T-ALL development. In this experiment, T-ALL was regenerated in most mice (Figure 5C and 5D), suggesting that committed populations can propagate, but only HSCs can initiate the Dnmt3a-KO FLT3-ITD disease.
Figure 5. Dnmt3a loss-related myeloid leukemia arises from transformed HSCs.
(A) Summary of sorted cell types that were transduced with FLT3-ITD retrovirus and transplanted into lethally irradiated recipients. Representative of three independent experiments. (B) Representative flow cytometry analysis of recipient peripheral blood 10 weeks after transplantation of 3aKO/FLT3-ITD leukemic blast cells. (C) Leukemia incidence after transplantation of various 3aKO/FLT3-ITD leukemic T cell populations into lethally irradiated recipients. (D) Representative flow cytometry analysis of recipient peripheral blood 10 weeks after transplantation of 3aKO/FLT3-ITD leukemic blast cells. (E) Summary of FACS sorted populations from 3aHet/Flt3-ITDKI mice that were transplanted into lethally irradiated recipients and development of leukemia. MPP=multipotent progenitor. LT-HSC= long-term HSC, STHSC= short-term HSC, CMP= common myeloid progenitor. GMP= granulocyte-macrophage progenitor. CLP= common lymphoid progenitor. Disease observation up to 12 months. Representative of two independent experiments. (F) Lineage distribution of donor-derived peripheral blood cells after 3aKO and 3aHet/Flt3-ITDKI LT-HSC transplantation. (G) Donor engraftment of purified HSCs and progenitors from 3aHet/Flt3-ITDKI AML mice at 3, 8, and 12 weeks after transplant, representative of two independent experiments. All bars denote mean ± s.e.m values. ***p<0.001 by two-way ANOVA.
In the Flt3-ITDKI AML model, we tested for leukemia initiation by inducing Dnmt3a deletion by tamoxifen injection and transplanting purified cells one week later. In this short time frame, Dnmt3a-deleted HSCs would likely have only limited contribution to downstream populations. Thus, any contribution of sorted progenitors or differentiated cells to AML development would indicate that Dnmt3a-deletion may exert its effects downstream of the HSC. We sorted LT-HSC, ST-HSC, MPP, CMP, GMP, and CLP and transplanted them into lethally irradiated WT recipients (Figure 5E). Only the LT-HSC population showed long-term engraftment and development of AML, whereas other populations showed only transient blood contribution, indicating limited self-renewal (Figures 5F and 5G). These data establish that in Dnmt3a loss-related AML, the undifferentiated HSCs initiate the leukemia.
Loss of DNMT3A drives enhancer hypomethylation
Recent reports have shown a correlation between DNMT3Amut and specific hypomethylation patterns in cytogenetically normal AML cases (Qu et al., 2014; Russler-Germain et al., 2014). Because we were utilizing a genetically defined mouse model, we could identify specific patterns of DNA methylation changes resulting from loss of DNMT3A. We first sought to assess these changes in the T-ALL model. We generated genome-wide DNA methylation maps of 3aKO/FLT3-ITD and FLT3-ITD leukemic cells, and CD4+CD8+ WT thymocytes by whole genome bisulfite sequencing (WGBS; coverage 8-10x; 7.2 million CpGs with at least 5x coverage across all samples). The overall methylation differences between sample groups were minor, but all comparisons revealed loci with increased and decreased DNA methylation, yet the greatest magnitude of hypomethylation occurred in samples lacking Dnmt3a (Figure 6A).
Figure 6. Dnmt3a loss causes hypomethylation at enhancer sites.
(A) Number of differentially methylated CpGs that were hypo- (blue) and hypermethylated (red) n=3. q-value < 0.05, DM ≥ 25%. (B) Heatmap of percent differentially methylated regions between pairwise comparisons as indicated at genomic regions. CGI, CpG islands. Blue indicates enhancers. Heme, hematopoietic enhancers (Lara-Astiaso et al., 2014). (C) H3K4me1 and H3K27ac signal density across regions differentially enriched in 3aKO/FLT3-ITD cells. Plotted is the subset of significant regions (n = 2,909) located greater than 1 kb away from an annotated Refseq TSS and covered across all experimental WGBS datasets. (D) Violin plot showing CpG methylation distributions at regions differentially enriched with chromatin marks in 3aKO/FLT3-ITD. Plotted are data for 24,409 CpG sites located in the 2,909 significant regions with sufficient CpG coverage and 25,000 randomly selected control CpG sites. (E) Mean CpG methylation ratio of leukemic cells overexpressing DNMT3A or GFP. *p<0.05 by student's t-test. (F) Number of hypermethylated 1 kb regions relative to tumors overexpressing GFP. (G) Number of significant differentially methylated regions between 3aHet/Flt3-ITDKI AML cells and KSL Flt3-ITD progenitor cells at 1 kb tiling windows using RRBS. Analysis compared three biological replicates per condition. (H) Percent of differential methylation of interrogated genomic regions of 3aHet/Flt3-ITDKI AML and 3aKO/FLT3-ITD T-ALL cells. UMR, unmethylated regions in HSC; CGI, CpG island; heme, hematopoietic enhancers (Lara-Astiaso et al., 2014). All bars denote mean ± s.e.m values. See also Figure S5.
We next focused on specific regions such as CpG islands, shores, and regulatory elements such as promoters and enhancers (Hon et al., 2013; Lara-Astiaso et al., 2014). In 3aKO/FLT3-ITD-derived cells, nearly all of these features exhibited marked hypomethylation, with the greatest magnitude of change found in hematopoietic enhancers (bone marrow, spleen, thymus) and at the edges of large under-methylated regions (canyons) (Jeong et al., 2014); >15% of enhancers and canyons were hypomethylated, a proportion ~ 5-fold greater than observed by chance (Figure 6B and Figure S5A). In FLT3-ITD-only leukemic cells, by contrast, hypermethylation of enhancers and canyon edges was much more prominent (Figure 6B).
To confirm that 3aKO hypomethylated enhancers were relevant regulatory elements, we examined them for enrichment of hematopoiesis-related transcription factor-binding sites. We compared our findings to a database of 315 mouse chromatin immunoprecipitation (ChIP)-seq transcription factor studies involving over 400,000 regions (Ruau et al., 2013). The comparison revealed overrepresentation of more than 80 transcription factors at hypomethylated enhancer sites, including master regulators that determine cell fate during stem, myeloid and lymphoid cell differentiation such as FLI1, GFI1B, and PU.1 (Figure S5B). Many of the transcription factors identified have also been implicated in hematopoietic diseases, including FLI1, LMO2, and RUNX1 in T-ALL (Cleveland et al., 2014; Mok et al., 2014; Smeets et al., 2013; Smith et al., 2014), and PU.1 and RUNX1 in AML (Cancer Genome Atlas Research, 2013; Gerloff et al., 2015). These findings suggest that Dnmt3a loss results in the demethylation of previously methylated, functionally relevant enhancers during leukemogenesis, potentially increasing accessibility of these regions to transcription factors.
To further confirm that loss of DNMT3A unveils enhancer sites, we generated maps of putative enhancers (Shen et al., 2013) based on H3K27ac and H3K4me1 histone peaks in leukemic FLT3-ITD and 3aKO/FLT3-ITD cells by ChIP-seq and DNAse1 hotspots from mouse ENCODE database (Consortium et al., 2012). We identified 13,705 regions co-occupied by H3K27ac and H3K4me1 in 3aKO/FLT3-ITD and assessed differential enrichment of H3K27ac and H3K4me1 at these regions. At these co-occupied regions, the interquartile-range distributions of both marks’ signals showed greater intensity in 3aKO/FLT3-ITD cells (Figure S5C). We observed differential enrichment of one or both marks in 3aKO/FTL3-ITD cells at 29.4% (4031 / 13705) of regions tested (Figure S5D). We also observed an overwhelming trend towards increased occupancy of both H3K4me1 and H3K27ac, implying that many regions in 3aKO/FLT3-ITD have acquired the chromatin marks de novo relative to FLT3-ITD alone (Figure 6C). We asked whether these regions with changes in H3K4me1 or H3K27ac also differed in DNA methylation. In regions with differential enrichment of both H3K4me1 and H3K27ac, the proportion of hypomethylated CpG sites was significantly greater in 3aKO/FLT3-ITD than FLT3-ITD (odds ratio 3.16, CI 2.89 – 3.44, p value < 2.2e-16, Fisher's exact test) (Figure 6D). The increase in enrichment of H3K27ac and H3K4me1 peaks at hypomethylated regions in the leukemic cells that lack DNMT3A strongly suggests that DNMT3A regulates active enhancers.
Given the observations that DNA methylation of enhancers was reduced with loss of Dnmt3a, we considered whether exogenous expression of mouse DNMT3A would restore methylation levels at those sites. Leukemic cells derived from 3aKO/FLT3-ITD secondary transplants were transduced with a retrovirus expressing DNMT3A along with a GFP marker (MSCV-DNMT3A-IRES-GFP) or GFP alone (MSCV-GFP) and were transplanted into sublethally irradiated tertiary recipients. Methylome maps of recipient bone marrow cells using RRBS (average of 19 million mapped reads per sample) revealed increased average DNA methylation in tumors with enforced DNMT3A expression (Figure 6E and 6F). Furthermore, enhancers, CGI shores, and canyon edges all showed significant hypermethylation (Figure S5E). These data support our observations that DNMT3A is particularly active at enhancers. Expression of DNMT3A did not affect the survival of the mice, consistent with Dnmt3a being more important for leukemic initiation than maintenance (despite an estimated 4-fold higher DNMT3A expression than that in GFP-transduced samples (data not shown)).
While the 3aKO/FLT3-ITD model showed clear methylation losses, we considered whether the heterozygous Dnmt3a AML model would exhibit similar effects on DNA methylation. We examined the methylome of three 3aHet/Flt3-ITDKI AML samples compared to three sorted stem and progenitor cells (KSL) samples from transplanted Flt3-ITDKI mice by reduced representation bisulfite sequencing (RRBS) (total of 16-37 million reads with at least 13 million aligned reads). Overall mean CpG methylation was not different between the two genotypes (Figure S5F). However, of interrogated CpGs, there were more hypomethylated 1 kb regions than hypermethylated regions (Figure 6G). Further analysis of genomic regions revealed more frequent hypomethylation at enhancer regions defined as hematopoietic (bone marrow, spleen, and thymus) and more frequent hypermethylation of enhancers in lineages such as testes and cerebellum (Figure 6H). Therefore, in the absence of one allele of Dnmt3a, loss of methylation was the most frequent event in the murine model of AML.
To more directly compare methylation changes in the 3aHet/Flt3-ITDKI AML model with the 3aKO/FLT3-ITD T-ALL model, we repeated our methylation analysis on the T-ALL samples using RRBS. Even though RRBS is more biased toward CpG islands, which frequently show hypermethylation, we confirmed enhancers and canyons were dramatically undermethylated in the T-ALL leukemic cells (Figure S5G). Therefore, in both models, RRBS analysis demonstrated that canyon edges were subject to the most frequent methylation changes, with CGIs and promoters having the least frequent methylation changes (Figure 6H). Although we observe more extreme hypomethylation in the T-ALL model (possibly due to the lower dosage of DNMT3A), the shared hypomethylation of enhancers and frequent altered methylation at canyon edges strongly suggest that these regions in particular are regulated by DNMT3A.
Re-expression of DNMT3A inhibits transcription factor binding at enhancers
Having observed significant hypomethylation particularly at enhancer sites in the absence of DNMT3A, we considered whether this had functional consequences on transcription factor binding. We hypothesized that remethylation of enhancers in the presence of enforced DNMT3A expression would reduce the binding of some transcription factors. To test this, we utilized the T-ALL model because of the homogeneity of this leukemia and the complete absence of Dnmt3a expression. In addition to being implicated in T cell development and T-ALL (Smeets et al., 2013; Smeets et al., 2014), FLI1 exhibited robust gene expression across conditions, was not differentially expressed, and the FLI1 consensus motif was enriched in hypomethylated enhancer sequences (p = 3.59e-15, Bonferroni correction). To test the effect of re-methylation on FLI1 binding, we performed ChIP-seq for FLI1 in 3aKO/FLT3-ITD leukemic cells transduced with either the DNMT3A-expressing retrovirus, or that of GFP alone, identifying 6,492 FLI1 binding sites. In the absence of DNMT3A (GFP-transduced cells) compared to the presence of DNMT3A (DNMT3A-transduced cells), FLI1 binding was significantly higher at 472 sites. This suggests re-expression of DNMT3A exerted a mostly disruptive effect on FLI1 binding. Most differential binding occurred outside promoters, frequently in hematopoietic enhancer regions (Figure 7A).
Figure 7. Enhancer hypomethylation is associated with increased recruitment of transcription factor FLI1.
(A) Bar plot summarizing differential binding of FLI1 in 3aKO/FLT3-ITD leukemia cells upon res-expression of Dnmt3a. Data are presented relative to the absence of Dnmt3a. Prom, promoter binding (within 1 kb of annotated TSS); Distal, binding outside of promoter; K4K27 and K27ac, distal regions marked by H3K4me1 and/or H3K27ac in 3aKO/FLT3-ITD leukemia cells; Ref Hemat Enh, reference hematopoietic cell or tissue type enhancer region in Mouse Encode (Bone Marrow, Spleen, Thymus) or Lara-Astiaso datasets. (B) Functional significance of promoter regions differentially bound by FLI1 was predicted by GREAT 2.0. Top, functions associated with increased FLI1. Bottom, functions associated with decreased Fli1. (C) Functional significance of increased FLI1 binding in hematopoietic enhancer regions (consisting of reference datasets and distal regions marked by H3K27Ac and H3K4me1 in 3aKO/FLT3-ITD cells). Horizontal line separates enriched terms from the human Disease Ontology and Mouse Genome Informatics Mouse Phenotype databases. Associations in GREAT based off gene regulatory domain extension of 150 kb up and downstream of TSS.
Next we investigated the genes and biological processes associated with differential occupancy of FLI1. Increased promoter binding was observed at genes involved in regulation of transcription and signal transduction (e.g., Wnt), decreased binding was associated with embryonic lethality, cell cycle, and constituents of the spliceosome (Figure 7B). Remarkably, 76% of distal sites with increased binding in absence of Dnmt3a (255 of 337) occurred in hematopoietic enhancers and were associated with genes involved in human leukemia (including AML) and mouse model phenotypes including abnormal proliferation and differentiation of T cells (Figure 7C). We identified no biological processes or ontologies enriched among the limited number of distal sites with decreased binding (60 of 105). Finally, we asked whether increased binding of FLI1 was concomitant with the hypomethylation we observed in 3aKO/FLT3-ITD leukemia. Sites of increased FLI1 occupancy were significantly enriched for hematopoietic enhancers that are also hypomethylated in the absence of DNMT3A (odds ratio 2.35 p = 5.26e-06, Fisher's test). Whether FLI1 has a role in this particular leukemia is not clear, but it serves to support the concept that DNA methylation changes can affect transcription factor occupancy at enhancer sites
Mutant DNMT3A/FLT3 human AML also exhibits enhancer DNA hypomethylation
Given the findings of DNA methylation changes at enhancer regions when Dnmt3a is lost and ectopically expressed, we considered whether human AML with DNMT3Amut also exhibited DNA methylation loss at hematopoietic enhancer regions. For our analysis, we selected cases from the TCGA AML cohort with the DNMT3AR882 mutation as this group is of sufficient size to allow statistical analysis, and the DNMT3AR882 mutation has been functionally characterized as a dominant negative (Kim et al., 2013; Russler-Germain et al., 2014). Cases with non-R882 DNMT3A mutations were excluded from our analysis due to their uncharacterized nature and small numbers. Leukemic cells with the DNMT3AR882 mutation are thought to retain around 20% of the wild-type DNMT3A activity, so we cannot exclude some effects distinct from the Dnmt3a-KO mouse situation.
We mined the TCGA AML dataset to compare the DNA methylation maps of AML cases with co-mutations of DNMT3AR882 and FLT3 to those with either mutation alone or to those with normal karyotype (WT NK AML) lacking either mutation (Figure 8A) (Cancer Genome Atlas Research, 2013). To control for other epigenetic effects, AML with mutations in IDH1, IDH2, TET1, or TET2 were excluded. We observed a dramatic increase in CpG hypomethylation (n = 35,436 sites) in AML with DNMT3AR882 and FLT3 mutations compared to FLT3 alone (Figure 8B), a unique result (among the group comparisons) in that it completely spanned the inter-quartile range of changes at sites of variable methylation in the four patient groups. The hypomethylated sites were significantly enriched for enhancers and to a lesser extent, CpG island shores (Figure 8C). While limited hypomethylation (n = 3437 sites) was observed in patients with FLT3 and DNMT3AR882 co-mutation compared to DNMT3AR882 alone (Figure 8B), the methylation states we observed in enhancer regions at sites of variable methylation clearly discriminated the two patient groups from one another, though to a lesser extent than either from the WT or FLT3 alone groups (Figure 8D). We mapped the enhancers to gene regulatory domains and assessed what biological functions most distinguished those hypomethylated in DNMT3AR882 + FLT3 mutant patients relative to the larger population of enhancers. They were enriched for transcriptional regulators, corepressors, and other key genes involved in cell fate specification (Figure S6A).
Figure 8. DNMT3A R882 mutation in human AML is associated with enhancer hypomethylation with FLT3 mutations.
(A) Groups of TCGA AML cases organized by mutation status of the indicated gene. (B) Boxplot of differential methylation in indicated comparisons; for each, the distribution of mean β-value differences for 51,776 sites differentially methylated in one or more comparisons are plotted. Hypermethylation (red) and hypomethylation (blue). The box represents the 25th percentile (bottom hinge), median (middle line), and 75th percentile (top hinge) of the data. The whiskers represent data within 1.5 * interquartile range of the third and first quartiles, respectively. Dashed horizontal lines represent β-value difference thresholds. FLT3, FLT3 mutation; R882, DNMT3AR882 mutation; WT, normal karyotype AML lacking mutations in DNMT3A, FLT3, and other epigenetic regulators (and see supplemental methods). Data are represented as individual points are CpG sites meeting the threshold for differential methylation. (C) Percent hypomethylated CpGs (comparing between R882+FLT3 vs FLT3) in indicated regions. Observed and expected proportions of hypomethylated sites in each region expressed as a percentage of total hypomethylated sites. * p < 2.22×10−16 (one-tailed Fisher's exact test). (D) Unsupervised principal coordinate analysis of CpG methylation states at sites of variable enhancer methylation. Analyses were performed on the mean β-values for each patient group at sites with evidence of differential enhancer methylation. (E) Functional significance of differentially expressed genes associated with hypomethylated enhancers in DNMT3AR882 FLT3 mutant patients. A linear model of gene expression contrasted patients with both mutations compared to FLT3 alone. Functional enrichment was assessed by Ingenuity IPA. X-axis, log-transformed p value of enrichment test. Size, fraction of differentially expressed genes associated with the term. HPC, hematopoietic progenitor cells. See also Figure S6.
To investigate the transcriptional profile of genes with hypomethylated enhancers, we generated linear models of RNA-seq counts for 69 AML cases in the TCGA dataset, contrasting the DNMT3A mutation status among those harboring FLT3 mutations. Ingenuity analysis of differentially expressed, hypomethylated-enhancer-associated genes showed over-representation in functions supporting hematopoietic progenitors cells as well as disease states including myeloproliferative disorders and AML (Figure 8E). Applying GSEA we observed a strong negative correlation with targets of MYC and E2F and G2/M checkpoint genes (Figure S6B). We also observed overexpression of homeobox genes, including the HOXB cluster, consistent with other reports (Yan et al., 2011). These data indicate that in human AML with mutated FLT3, the loss of enhancer methylation observed with mutation of DNMT3A may contribute to deregulation of transcriptional programs key to cell identity and normal hematopoietic function, thus promoting leukemogenesis.
DISCUSSION
Here, we show that deletion of Dnmt3a cooperates with FLT3-ITD to initiate both myeloid and lymphoid leukemias, establishing models to investigate the mechanisms of Dnmt3a loss-associated transformation. While loss of Dnmt3a alone resulted in a variety of hematologic diseases (Mayle et al., 2015), Dnmt3a-KO with FLT3-ITD drove specific diseases, with retroviral overexpression of FLT3-ITD leading to rapid early immature-like T cell leukemia, and the Flt3-ITD knock-in leading primarily to myeloid leukemia resembling M4/M5 AML, which is highly associated with DNMT3A mutations (Yan et al., 2011). The disease types were likely influenced by the different promoters driving FLT3-ITD expression and the cells in which FLT3-ITD was expressed (Kelly et al., 2002; Lee et al., 2007). This suggests that loss of DNMT3A establishes a cellular milieu permissive for transformation down myeloid or lymphoid lineages and that the secondary hits play key roles in disease specification and latency.
Importantly, the Dnmt3a/Flt3-ITD knock-in model also generated a number of lymphoid leukemias that were more prevalent with complete absence of Dnmt3a, whereas heterozygous Dnmt3a loss almost exclusively led to myeloid leukemia. These data establish that Dnmt3a dosage also influences disease outcome. This observation is consistent with the distinct mutational spectra of DNMT3A among patients with lymphoid vs. myeloid leukemias (reviewed in (Yang et al., 2015)). The mechanism behind the dosage effect is not clear. During the natural evolution of leukemia, cells will initially have a single mutant allele. We can conjecture that a cell acquiring a dominant-negative DNMT3AR882 mutation would have ~20% of remaining WT DNMT3A activity whereas those with a nonsense mutation in one allele of DNMT3A might have ≥50% activity. Both of these levels may be insufficient for completely normal differentiation but perhaps the lymphoid lineage requires higher levels of DNMT3A activity. After some lag, a second DNMT3A lesion is acquired (likely also in a progenitor), leading to lymphoid (sometimes myeloid) leukemia.
Our data also reinforce the concept that DNMT3A functions primarily at the stem cell level. Both the myeloid and lymphoid leukemias were initiated by HSCs transformed with FLT3-ITD, indicating that Dnmt3a loss in stem cells primes a pre-leukemic clone to promote the impact of secondary hits. These data are consistent with the highly specific effect that Dnmt3a loss has on expansion of the stem cell compartment (Challen et al., 2012). The inability to slow the leukemia by re-expression of DNMT3A suggests that DNMT3A loss is important for leukemia initiation but less so for maintenance. This association with stem cells is also seen in patients, as DNMT3A mutations can be found in non-malignant clones (Corces-Zimmerman et al., 2014; Jan et al., 2012; Shlush et al., 2014). Together, these data support a model in which an HSC acquires a DNMT3A mutation, which expands and remains as a reservoir for clonal expansion until an additional genetic lesion, such as FLT3-ITD, is acquired leading to transformation.
Methylation profiling of Dnmt3amut leukemias showed loss of DNA methylation at hematopoietic enhancer regions, and this hypomethylation could impact transcription factor binding as we showed for FLI1. Similarly, human AML with DNMT3AR882 and FLT3 mutation also exhibited hypomethylation at enhancer regions relative to other non-DNMT3A/FLT3-mutant AML. These observations are consistent with other reports of hypomethylation observed in DNMT3Amut AML (Qu et al., 2014; Russler-Germain et al., 2014). In the retroviral transduction T cell ALL model, global hypomethylation was observed. In the knock-in AML model in which only one copy of Dnmt3a was lost, both hyper- and hypomethylation of enhancers were observed. Together, these results showed that Dnmt3a-KO leads to hypomethylation at distal gene regulatory regions, implicating DNA methylation in their regulation. Our discoveries imply that during leukemogenesis, DNMT3A acts as a tumor suppressor, guarding stem-cell regulatory regions through methylation at enhancers. Overall, these experiments show that Dnmt3a loss drives leukemogenesis in multiple lineages, recapitulating DNMT3Amut AML and T-ALL in a Dnmt3a-dosage dependent manner. These mouse models serves as a valuable tools to study and develop therapeutics for leukemias with DNMT3A mutations.
EXPERIMENTAL PROCEDURES
Detailed methods are described in Supplemental Experimental Procedures.
Vectors, cells, and animals
Animal experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. FLT3-ITD was expressed from MSCV-IRES-GFP. Flt3-ITD mice were obtained from The Jackson Laboratory. Mx1-Cre; Dnmt3afl/fl and ERT2-Cre; Dnmt3afl/fl mice carrying the CD45.2 allele (Challen et al., 2012) on the C57BL/6 background were used. Bone marrow cells were transplanted into lethally irradiated CD45.1 mice. For phenotyping or transplantation, bone marrow or blood was harvested, stained with antibodies and analyzed or sorted (Mayle et al., 2013). For histologic studies, fresh tissues were made into touch preps or fixed and mounted for H&E staining.
RNA/DNA Sequencing
RNA and DNA were isolated and libraries were made for WGBS, RNA-seq or RRBS (Boyle et al., 2012) from 2 to 3 biological replicates. The data are deposited in NCBI with accession number GSE61971 and can be visualized on the UCSC browser via this link: http://dldcc-web.brc.bcm.edu/lilab/Dnmt3aFlt3/index.html
TCGA analysis
AML patient DNA methylation data were obtained from the TCGA Research Network dataset http://cancergenome.nih.gov/. Patient groupings were established according to DNMT3AR882 and FLT3 mutation status (and see detailed methods). Differential methylation between two groups were defined as: FDR < 0.05 and mean (Beta-value) methylation difference > 0.15. Hypomethylated enhancers covered ≥ 3 CpGs. Enhancer probes were assigned by overlap of H3K27ac or H3K4me1 peaks outside of Refseq promoter regions. H3K27ac peaks and H3K4me1 peaks were called by MACS2 (Zhang et al., 2008) with CD34+ primary cells (GSM772885 and GSM706845).
Statistics
All values are means ± s.e.m. Comparisons between groups were made with ANOVA, the log-rank test, or Student's t-test, using Graphpad Prism 5.0b.
Supplementary Material
SIGNIFICANCE.
Epigenetic regulators, including DNMT3A, have emerged as potent tumor suppressors in many hematologic malignancies, but the mechanisms conferring this role are unknown. Here, we show that combined loss of DNMT3A with the leukemia-associated FLT3-internal tandem duplication (FLT3-ITD) in mice induces hematological malignancies that mimic human diseases, with DNMT3A dosage influencing leukemia type. Both mouse and human DNMT3Amut leukemias are characterized by diminished DNA methylation in regulatory regions, particularly enhancers, suggesting a central role for DNMT3A in maintaining epigenetic integrity to prevent transformation. These murine models of DNMT3A AML and early immature T-ALL provide a venue to further study the mechanism of DNMT3A/FLT3-ITD leukemia and to test potential therapies.
HIGHLIGHTS.
Dnmt3a loss and FLT3-ITD expression preferentially initiate early immature-like T-ALL
Heterozygous Dnmt3a loss and Flt3-ITD expression initiate AML in mouse models
Dnmt3a loss-associated myeloid and lymphoid leukemias arise from HSCs
Dnmt3a loss leads to hypomethylation of active hematopoietic enhancers
ACKNOWLEDGEMENTS
We thank J. Licht and F. Rassool for the FLT3-ITD construct, Y. Zheng, A. Rosen, R. Nitsal, M. Landis, K. Lin for technical support, and to J. Gilbert and C. Gillespie for critical reading of the manuscript. L.Y. is funded by the Robert and Janice McNair Foundation as an MD/PhD McNair Scholar. This project was funded by CPRIT (RP110028, RP110471 and RP150292), the NIH (DK092883 and HG007538), and the Samuel Waxman Cancer Research Foundation. We also thank the Cytometry and Cell Sorting and Genomic and RNA Profiling Cores (NCI P30CA125123, P30 AI036211, P30 CA125123, and S10 RR024574) at Baylor College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conceptualization, M.A.G., W.L., L.Y., B.R., A.M., G.A.C., B.G., and R.R.; Methodology M.A.G., L.Y., B.R., A.M., M.L., M.J., J.L., X.Z., G.A.C., V.I.R., T.Z., M.Z., R.R., B.G., and W.L.; Formal Analysis B.R., D.R., H.J.P., X.L., S.K., J.L. and B.G .; Investigation L.Y., B.R., A.M., M.L., M.J., X.Z., C.V.C, X.Z., T.Z., and M.Z.; Writing - Original Draft M.A.G., L.Y., B.R., and A.M.; Writing – Reviewing and Editing L.Y., B.R., A.M., H.J.P., X.L., M.L., M.J., C.V.C., S.K., D.R., X.Z., T.Z., V.I.R., G.A.C., B.G., J.L., R.R., W.L., M.A.G.; Supervision M.A.G., W.L., R.R., J.L., B.G., G.A.C., V.I.R; Funding Acquisition M.A.G and W.L.
Authors declare no conflicts of interest.
REFERENCES
- Boyle P, Clement K, Gu H, Smith ZD, Ziller M, Fostel JL, Holmes L, Meldrim J, Kelley F, Gnirke A, et al. Gel-free multiplexed reduced representation bisulfite sequencing for large-scale DNA methylation profiling. Genome Biol. 2012;13:R92. doi: 10.1186/gb-2012-13-10-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research, N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik H, Mallaney C, Kothari A, Ostrander EL, Eultgen E, Martens A, Miller CA, Hundal J, Klco JM, Challen GA. Enforced differentiation of Dnmt3a-null bone marrow leads to failure with c-Kit mutations driving leukemic transformation. Blood. 2015;125:619–628. doi: 10.1182/blood-2014-08-594564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland SM, Goodings C, Tripathi RM, Elliott N, Thompson MA, Guo Y, Shyr Y, Davé UP. LMO2 induces T-cell leukemia with epigenetic deregulation of CD4. Experimental hematology. 2014;42:581–593. e585. doi: 10.1016/j.exphem.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium ME, Stamatoyannopoulos J, Snyder M, Hardison R, Ren B, Gingeras T, Gilbert D, Groudine M, Bender M, Kaul R, et al. An encyclopedia of mouse DNA elements (Mouse ENCODE). Genome Biology. 2012;13:418. doi: 10.1186/gb-2012-13-8-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci U S A. 2014;111:2548–2553. doi: 10.1073/pnas.1324297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, Cheng C, Su X, Rubnitz JE, Basso G, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncology. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff D, Grundler R, Wurm AA, Bräuer-Hartmann D, Katzerke C, Hartmann J-U, Madan V, Muller-Tidow C, Duyster J, Tenen DG, et al. NF-κB/STAT5/miR-155 network targets PU.1 in FLT3-ITD-driven acute myeloid leukemia. Leukemia. 2015;29:535–547. doi: 10.1038/leu.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann V, Haferlach C, Weissmann S, Roller A, Schindela S, Poetzinger F, Stadler K, Bellos F, Kern W, Haferlach T, et al. The molecular profile of adult T-cell acute lymphoblastic leukemia: mutations in RUNX1 and DNMT3A are associated with poor prognosis in T-ALL. Genes Chromosomes Cancer. 2013;52:410–422. doi: 10.1002/gcc.22039. [DOI] [PubMed] [Google Scholar]
- Haferlach T, Kohlmann A, Wieczorek L, Basso G, Kronnie GT, Bene MC, De Vos J, Hernandez JM, Hofmann WK, Mills KI, et al. Clinical Utility of Microarray-Based Gene Expression Profiling in the Diagnosis and Subclassification of Leukemia: Report From the International Microarray Innovations in Leukemia Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2529–2537. doi: 10.1200/JCO.2009.23.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkal G, Parikh N, Donehower LA. Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression. PLoS One. 2009;4:e6654. doi: 10.1371/journal.pone.0006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon GC, Rajagopal N, Shen Y, McCleary DF, Yue F, Dang MD, Ren B. Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat Genet. 2013;45:1198–1206. doi: 10.1038/ng.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR, Majeti R. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med. 2012;4:149ra118. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M, Sun D, Luo M, Huang Y, Challen GA, Rodriguez B, Zhang X, Chavez L, Wang H, Hannah R, et al. Large conserved domains of low DNA methylation maintained by Dnmt3a. Nat Genet. 2014;46:17–23. doi: 10.1038/ng.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LM, Kutok JL, Williams IR, Boulton CL, Amaral SM, Curley DP, Ley TJ, Gilliland DG. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci U S A. 2002;99:8283–8288. doi: 10.1073/pnas.122233699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Zhao H, Hardikar S, Singh AK, Goodell MA, Chen T. A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood. 2013;122:4086–4089. doi: 10.1182/blood-2013-02-483487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan SC. Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood. 2002;100:238–245. doi: 10.1182/blood.v100.1.238. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Lara-Astiaso D, Weiner A, Lorenzo-Vivas E, Zaretsky I, Jaitin DA, David E, Keren-Shaul H, Mildner A, Winter D, Jung S, et al. Immunogenetics. Chromatin state dynamics during blood formation. Science. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Tothova Z, Levine RL, Anderson K, Buza-Vidas N, Cullen DE, McDowell EP, Adelsperger J, Frohling S, Huntly BJ, et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12:367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363:2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle A, Luo M, Jeong M, Goodell MA. Flow cytometry analysis of murine hematopoietic stem cells. Cytometry A. 2013;83:27–37. doi: 10.1002/cyto.a.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle A, Yang L, Rodriguez B, Zhou T, Chang E, Curry CV, Challen GA, Li W, Wheeler D, Rebel VI, et al. Dnmt3a loss predisposes murine hematopoietic stem cells to malignant transformation. Blood. 2015;125:629–638. doi: 10.1182/blood-2014-08-594648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok MMH, Du L, Wang CQ, Tergaonkar V, Liu TC, Kham SKY, Sanda T, Yeoh AE-J, Osato M. RUNX1 point mutations potentially identify a subset of early immature T-cell acute lymphoblastic leukaemia that may originate from differentiated T-cells. Gene. 2014;545:111–116. doi: 10.1016/j.gene.2014.04.074. [DOI] [PubMed] [Google Scholar]
- Morse HC, 3rd, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Jaffe ES, Kogan SC, MacLennan IC, Pattengale PK, et al. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood. 2002;100:246–258. doi: 10.1182/blood.v100.1.246. [DOI] [PubMed] [Google Scholar]
- Neumann M, Heesch S, kbuget N. G. o., Schwartz S, Schlee C, Benlasfer O, Farhadi-Sartangi N, Thibaut J, Burmeister T, Hoelzer D, et al. Clinical and molecular characterization of early T-cell precursor leukemia: a high-risk subgroup in adult T-ALL with a high frequency of FLT3 mutations. Blood Cancer Journal. 2012;2:e55–57. doi: 10.1038/bcj.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Heesch S, Schlee C, Schwartz S, Gokbuget N, Hoelzer D, Konstandin NP, Ksienzyk B, Vosberg S, Graf A, et al. Whole-exome sequencing in adult ETP ALL reveals a high rate of DNMT3A mutations. Blood. 2013;121:4749–4752. doi: 10.1182/blood-2012-11-465138. [DOI] [PubMed] [Google Scholar]
- Qu Y, Lennartsson A, Gaidzik VI, Deneberg S, Karimi M, Bengtzen S, Hoglund M, Bullinger L, Dohner K, Lehmann S. Differential methylation in CN-AML preferentially targets non-CGI regions and is dictated by DNMT3A mutational status and associated with predominant hypomethylation of HOX genes. Epigenetics. 2014;9:1108–1119. doi: 10.4161/epi.29315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C, Thien CBF, Flavell RA, Langdon WY. Myeloid Leukemia Development in c-Cbl RING Finger Mutant Mice Is Dependent on FLT3 Signaling. Cancer Cell. 2010;18:341–352. doi: 10.1016/j.ccr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Ruau D, Ng FS, Wilson NK, Hannah R, Diamanti E, Lombard P, Woodhouse S, Gottgens B. Building an ENCODE-style data compendium on a shoestring. Nat Methods. 2013;10:926. doi: 10.1038/nmeth.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russler-Germain DA, Spencer DH, Young MA, Lamprecht TL, Miller CA, Fulton R, Meyer MR, Erdmann-Gilmore P, Townsend RR, Wilson RK, et al. The R882H DNMT3A Mutation Associated with AML Dominantly Inhibits Wild-Type DNMT3A by Blocking Its Ability to Form Active Tetramers. Cancer Cell. 2014;25:442–454. doi: 10.1016/j.ccr.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, et al. A map of the cis-regulatory sequences in the mouse genome. Nature. 2013;488:116–120. doi: 10.1038/nature11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets MFMA, Chan AC, Dagger S, Bradley CK, Wei A, Izon DJ. Fli-1 Overexpression in Hematopoietic Progenitors Deregulates T Cell Development and Induces Pre-T Cell Lymphoblastic Leukaemia/Lymphoma. PLoS ONE. 2013;8:e62346. doi: 10.1371/journal.pone.0062346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets MFMA, Wiest DL, Izon DJ. Fli-1 regulates the DN2 to DN3 thymocyte transition and promotes γδ T-cell commitment by enhancing TCR signal strength. European Journal of Immunology. 2014;44:2617–2624. doi: 10.1002/eji.201444442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Tripathi R, Goodings C, Cleveland S, Mathias E, Hardaway JA, Elliott N, Yi Y, Chen X, Downing J, et al. LIM Domain Only-2 (LMO2) Induces T-Cell Leukemia by Two Distinct Pathways. PLoS ONE. 2014;9:e85883. doi: 10.1371/journal.pone.0085883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vlierberghe P, Ambesi-Impiombato A, De Keersmaecker K, Hadler M, Paietta E, Tallman MS, Rowe JM, Forne C, Rue M, Ferrando AA. Prognostic relevance of integrated genetic profiling in adult T-cell acute lymphoblastic leukemia. Blood. 2013;122:74–82. doi: 10.1182/blood-2013-03-491092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vlierberghe P, Ambesi-Impiombato A, Perez-Garcia A, Haydu JE, Rigo I, Hadler M, Tosello V, Della Gatta G, Paietta E, Racevskis J, et al. ETV6 mutations in early immature human T cell leukemias. The Journal of Experimental Medicine. 2011;208:2571–2579. doi: 10.1084/jem.20112239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, Shi JY, Zhu YM, Tang L, Zhang XW, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011;43:309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- Yang L, Rau R, Goodell MA. DNMT3A in haematological malignancies. Nat Rev Cancer. 2015;15:152–165. doi: 10.1038/nrc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.