Abstract
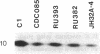
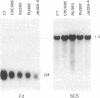
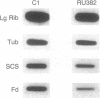
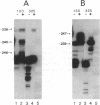
Metronidazole [1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole] is used to treat infections caused by Trichomonas vaginalis, a sexually transmitted human parasite. This drug is administered in an inactive form and is reduced to its cytotoxic form within the hydrogenosome, an unusual organelle found in trichomonads. Metronidazole reduction occurs via ferredoxin-mediated electron transport. We have investigated the role of ferredoxin in metronidazole resistance. Immunoblot analysis of drug-resistant and -sensitive T. vaginalis strains shows that intracellular levels of ferredoxin are invariably reduced in the resistant strains relative to a sensitive strain. Similarly, Northern blot analysis shows that ferredoxin mRNA levels are reduced 50-65% in resistant strains. Using nuclear run-on assays, we show that ferredoxin gene transcription is reduced 40-65% in resistant strains. Sequence comparison of the region 5' of the ferredoxin gene among drug-sensitive and -resistant strains reveals two point mutations, at -178 and -239 nucleotides relative to the start of transcription, in a resistant strain. Interestingly, a protein of approximately 23 kDa binds to a 28-base-pair region that encompasses the mutation at -239 nucleotides. The binding affinity of this protein appears to be reduced in the mutant. These data strongly correlate drug resistance with altered regulation of ferredoxin gene transcription. A reduction in gene transcription results in decreased intracellular levels of ferredoxin. This, in turn, may play a role in metronidazole resistance by decreasing the ability of the cell to activate the drug.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boreham P. F., Phillips R. E., Shepherd R. W. A comparison of the in-vitro activity of some 5-nitroimidazoles and other compounds against Giardia intestinalis. J Antimicrob Chemother. 1985 Nov;16(5):589–595. doi: 10.1093/jac/16.5.589. [DOI] [PubMed] [Google Scholar]
- Breuil J., Dublanchet A., Truffaut N., Sebald M. Transferable 5-nitroimidazole resistance in the Bacteroides fragilis group. Plasmid. 1989 Mar;21(2):151–154. doi: 10.1016/0147-619x(89)90060-7. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Chapman A., Cammack R., Linstead D. J., Lloyd D. Respiration of Trichomonas vaginalis. Components detected by electron paramagnetic resonance spectroscopy. Eur J Biochem. 1986 Apr 1;156(1):193–198. doi: 10.1111/j.1432-1033.1986.tb09567.x. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Sharp P. A. A single polypeptide possesses the binding and transcription activities of the adenovirus major late transcription factor. Mol Cell Biol. 1986 Dec;6(12):4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D. L., Rabin H. R., Laishley E. J. Role of hydrogenase 1 of Clostridium pasteurianum in the reduction of metronidazole. Biochem Pharmacol. 1988 Apr 15;37(8):1525–1534. doi: 10.1016/0006-2952(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Diamond L. S. Inhibition of clonal growth of Giardia lamblia and Entamoeba histolytica by metronidazole, quinacrine, and other antimicrobial agents. J Antimicrob Chemother. 1981 Oct;8(4):305–316. doi: 10.1093/jac/8.4.305. [DOI] [PubMed] [Google Scholar]
- Honigberg B. M., Livingston M. C., Frost J. K. Pathogenicity of fresh isolates of Trichomonas vaginalis: "the mouse assay" versus clinical and pathologic findings. Acta Cytol. 1966 Sep-Oct;10(5):353–361. [PubMed] [Google Scholar]
- Ings R. M., McFadzean J. A., Ormerod W. E. The mode of action of metronidazole in Trichomonas vaginalis and other micro-organisms. Biochem Pharmacol. 1974 May 1;23(9):1421–1429. doi: 10.1016/0006-2952(74)90362-1. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Kooter J. M., Borst P. Inactivation of transcription by UV irradiation of T. brucei provides evidence for a multicistronic transcription unit including a VSG gene. Cell. 1987 Oct 23;51(2):273–281. doi: 10.1016/0092-8674(87)90154-1. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., d'Oliveira C. E., Gorrell T. E., Müller M. Molecular analysis of the hydrogenosomal ferredoxin of the anaerobic protist Trichomonas vaginalis. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6097–6101. doi: 10.1073/pnas.87.16.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter J. M., Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 1984 Dec 21;12(24):9457–9472. doi: 10.1093/nar/12.24.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti C. J., Johnson P. J. Trichomonas vaginalis hydrogenosomal proteins are synthesized on free polyribosomes and may undergo processing upon maturation. Mol Biochem Parasitol. 1991 Jun;46(2):307–310. doi: 10.1016/0166-6851(91)90055-b. [DOI] [PubMed] [Google Scholar]
- Lathe R., Kieny M. P., Skory S., Lecocq J. P. Linker tailing: unphosphorylated linker oligonucleotides for joining DNA termini. DNA. 1984;3(2):173–182. doi: 10.1089/dna.1984.3.173. [DOI] [PubMed] [Google Scholar]
- Lloyd D., Yarlett N., Yarlett N. C. Inhibition of hydrogen production in drug-resistant and susceptible Trichomonas vaginalis strains by a range of nitroimidazole derivatives. Biochem Pharmacol. 1986 Jan 1;35(1):61–64. doi: 10.1016/0006-2952(86)90556-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mertens E., Müller M. Glucokinase and fructokinase of Trichomonas vaginalis and Tritrichomonas foetus. J Protozool. 1990 Sep-Oct;37(5):384–388. doi: 10.1111/j.1550-7408.1990.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Müller M. Energy metabolism of protozoa without mitochondria. Annu Rev Microbiol. 1988;42:465–488. doi: 10.1146/annurev.mi.42.100188.002341. [DOI] [PubMed] [Google Scholar]
- Müller M., Gorrell T. E. Metabolism and metronidazole uptake in Trichomonas vaginalis isolates with different metronidazole susceptibilities. Antimicrob Agents Chemother. 1983 Nov;24(5):667–673. doi: 10.1128/aac.24.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Lindmark D. G. Uptake of metronidazole and its effect on viability in trichomonads and Entamoeba invadens under anaerobic and aerobic conditions. Antimicrob Agents Chemother. 1976 Apr;9(4):696–700. doi: 10.1128/aac.9.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Lossick J. G., Gorrell T. E. In vitro susceptibility of Trichomonas vaginalis to metronidazole and treatment outcome in vaginal trichomoniasis. Sex Transm Dis. 1988 Jan-Mar;15(1):17–24. doi: 10.1097/00007435-198801000-00004. [DOI] [PubMed] [Google Scholar]
- Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983 Jan;93(1 Pt 2):165–171. [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem. 1985 Mar 25;260(6):3697–3702. [PubMed] [Google Scholar]
- Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984 Sep 6;311(5981):81–84. doi: 10.1038/311081a0. [DOI] [PubMed] [Google Scholar]
- Wu C. An exonuclease protection assay reveals heat-shock element and TATA box DNA-binding proteins in crude nuclear extracts. Nature. 1985 Sep 5;317(6032):84–87. doi: 10.1038/317084a0. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Yarlett N. C., Lloyd D. Ferredoxin-dependent reduction of nitroimidazole derivatives in drug-resistant and susceptible strains of Trichomonas vaginalis. Biochem Pharmacol. 1986 May 15;35(10):1703–1708. doi: 10.1016/0006-2952(86)90327-8. [DOI] [PubMed] [Google Scholar]