Abstract
After a sharp drop of rotavirus (RV) infections at Children’s Hospital of Michigan, Detroit, USA in 2010 season, we noted an increase in the number of cases during the 2011 season including some RV vaccine (RVV) recipients. This study was conducted to determine the circulating genotypes during 2011 season and whether the increase in RV diarrhea was caused by replacement genotypes. G and P genotypes were determined by RT PCR and nucleotide sequencing of selected strains was performed. The vaccination rate among study patients was 24 %. RV strains from 68 stool samples were genotyped including 18 from vaccinated children and 50 from unvaccinated children. The predominant G genotype was G1 (58.8 %) followed by G9 (17.7 %) and G4 (15.5 %). P[8] was the predominant P genotype (68 %) followed by P[6] (17.6 %) and P[4] (3 %). All G9 strains were associated with P[6]. The most prevalent G–P combination was G1P[8] (56 %), followed by G9P[6] (17.6 %). Similar proportions of RV genotypes were found among vaccinated and unvaccinated children. Our local data suggest that 5 years after the introduction of RVV there has been no genotype replacement. Although a small increase in G9P[6] frequency was noted, G1P[8] remained the predominant strain of RV in our inner city community in the Midwestern USA.
Keywords: Children, Rotavirus genotype, Vaccination
Rotavirus (RV) gastroenteritis caused over 2.4 million hospital admissions and 450,000 deaths among children younger than 5 years worldwide in 2008 [21]. The outer layer or the virion is composed of two proteins: VP4 (P) and VP7 (G), which provide the basis for both virus classification and immunity. Prior to 1990, the most common RV types were G1P[8], G2P[4], G3P[8] and G4P[8], with G9P[8] recognized as the fifth most common [7, 8, 14]. The number of infections has decreased markedly since the introduction of two live rotavirus vaccines (RVV). The vaccines do not contain all of the common G and P types. RotaTeq (Merck) is a pentavalent human–bovine reassortant vaccine that contains G1, G2, G3 and G4 combined with P[8], the most common P type. Rotarix (GlaxoSmithKline) contains only G1P[8]. Replacement of RVV serotypes with others has been observed with G5 becoming more prevalent in South America, and P[6] being detected in more than half of symptomatic infections in Africa, and suggestions that G9 may be emerging as the most common G type world wide [6, 15, 19, 20]. In addition, G12 genotype has also been emerging in different countries [17, 22, 25].
In our urban 230-bed hospital, we conducted an epidemiologic study during the period 2007–2009. We found that the most prevalent genotype combinations in our community were G1P[8] (21.4 %), which is included in both vaccines, followed by G9P[8] (20.4 %), G4P[8] (13 %) which is included in one of the two vaccines and G9P[6] (8.8 %) [1]. Although G9 was the most prevalent G type at 39.5 %, P[8], which is in both vaccines, was the most prevalent P type [1] in the early time period. There was a sharp drop in the number of children who presented to our hospital with RV gastroenteritis during 2010 (49 RV-positive cases), however, the number increased to 238 cases in 2011. This is consistent with the new biennial activity of RV that has been noted in different parts of the USA in the post-vaccine era [3].
We therefore conducted a follow up study to determine the circulating genotypes during 2011. The aim of the study was to determine whether a change in RV genotypes accounted for the significant increase in RV infections in 2011 as compared to previous seasons. We postulated that the increase in RV diarrhea would be caused by replacement genotypes: that G9 would have replaced G1, and/or that the most prevalent P type would be other than P[8], perhaps P[6]. Of the 238 cases, 150 had available stool left over samples for analysis. Of these, 72 samples were selected for RV genotype analysis. Four specimens did not provide enough PCR product for analysis. Study subjects ranged in age from 8 days through 70 months (mean 24.4 months, median 16 months). They presented to our emergency department during the RV season of 2011 and were diagnosed as having RV diarrhea by the Sure-Vue RotaTest (Fisher HealthCare, Houston, TX, USA). The remaining 68 subjects included both vaccinated (18) and unvaccinated (50) children. Each vaccinated patient specimen was matched with specimens from unvaccinated patients presenting within 1 week. Vaccinated children were defined as those who could be shown to have received at least one dose of RVV at least 14 days prior to presentation, as determined from our hospital records or after querying the Michigan Care Improvement Registry of the Department of Community Health of the state of Michigan. The vaccination rate in this study group (24 %) exceeded that in our previous study (7 %), in our area where children tend to be under-vaccinated. The study was approved by the Institutional Review Board of Wayne State University School of Medicine.
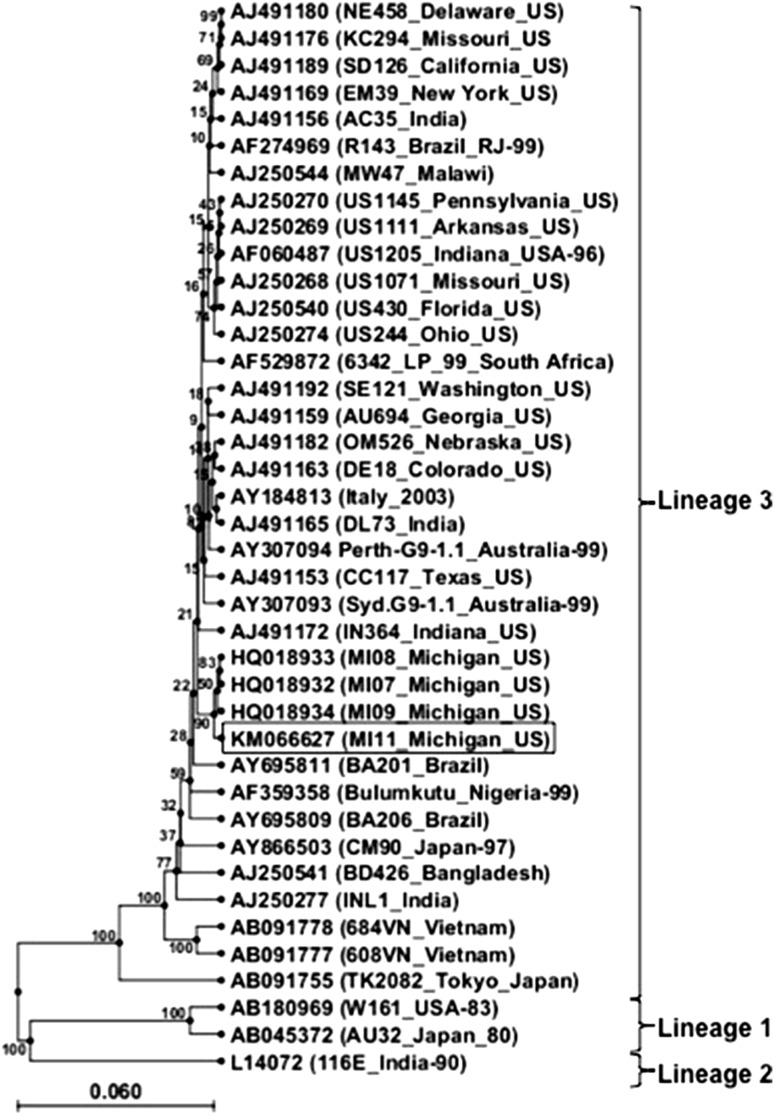
Stool samples were stored at −80 °C until tested. The molecular techniques used in the determination of G and P types were similar to those described previously [1, 7, 9, 12]. Specific primers to detect G1, G2, G3, G9 and G10 as well as P[4], P[6], P[8], P[9], P[10] and P[11] were used. PCR products of all G9 and P[6] genotypes were verified by nucleotide sequencing. The VP7 sequences of G9 strains isolated were compared with other strains isolated in the recent years and the reference strain AF060487 (UN 1204/Indiana/US). Phylogenetic analysis and sequence alignment (Fig. 1) indicated 99 % identitiy with strains isolated in 2007, 2008 and 2009 from Southeast Michigan. The representative G9 VP7 gene sequence (GenBank accession number KM066627) belonged to lineage 3.
Fig. 1.
Phylogenetic analysis of the VP7 gene nucleotide sequence of human rotavirus G9 strains isolated during the years 2007 (MI07_Michigan_US), 2008 (MI08_Michigan_US), 2009 (MI09_Michigan_US), and 2011 (MI11_Michigan_US). The dendogram was constructed by the UPGMA method. Confidence values based on 100 bootstrap replicates are shown on the branches of the tree. Consensus sequence reported in this study along with GenBank accession number is indicated in the highlighted box
The predominant strains during the 2011 study period were G1P[8] at 55.9 %, and G9P[6] at 17.6 %, including 13 patients who had more than one viral genotype (Table 1). G1 was found in 50/68 (73.5 %) of stool samples, while G9 was found in 20 (29.4 %). P[8] was found in 54 (79.4 %) stool samples. Similar proportions of RV genotypes were found among vaccinated and unvaccinated children.
Table 1.
Distribution of G and P genotypes and G/P combinations of RV strains detected in 68 children during the 2011 season
| Genotype combination | Number (%) | G Genotype | Number (%) | P Genotype | Number (%) |
|---|---|---|---|---|---|
| G1P[4] | 2 (2.9 %) | G1 | 40 (58.8 %) | P[4] | 2 (2.9 %) |
| G1P[8] | 38 (55.9 %) | G4 | 3 (4.4 %) | P[6] | 12 (17.6 %) |
| G4P[8] | 3 (4.4 %) | G9 | 12 (17.6 %) | P[8] | 46 (67.6 %) |
| G9P[6] | 12 (17.6 %) | G1 + G4 | 5 (7.3 %) | P[6] + P[8] | 8 (11.7 %) |
| G1 + 4P[8] | 5 (7.3 %) | G1 + G9 | 5 (7.3 %) | Total* | 68 |
| G1 + 9P[6 + 8] | 5 (7.3 %) | G4 + G9 | 3 (4.4 %) | ||
| G4 + 9P[6 + 8] | 3 (4.4 %) | Total* | 68 | ||
| Total* | 68 |
* Including 18 vaccinated and 50 non-vaccinated children
The most frequent circulating G–P combinations in the present study were G1P[8] and G9P[6]. All G9 strains in this study were associated with P[6] in contrast to the 2007–2009 seasons when G9 was most frequently associated with P[8] [1]. G9 and P[6] are not included in the current two vaccines; however, clinical studies have shown efficacy of these vaccines against G9 strains [18, 23], probably due to the presence of P[8] in both vaccines and predominance of G9P[8] in areas where vaccine trials were conducted. However, protection by either RotaTeq or Rotarix against G9P[6] strains, which share no neutralization antigens with the vaccine strains, could be a major challenge.
The introduction of mass RV vaccination programs in 2006 has resulted in a sharp drop of RV gastroenteritis cases requiring hospitalization worldwide [5, 24]. In some countries distinct genotypes were detected more frequently after introducing RV vaccines. In Australia and Brazil, G2P[4] prevailed in children given Rotarix [10, 13]. In Australia and USA infection due to G3P[8] occurred more frequently among children vaccinated with RotaTeq [4, 11, 16]. However, in another study the distribution of RV genotypes did not differ between vaccinated and unvaccinated children [2]. Thus, it is likely that in the setting of sporadic exposure to natural RV infections, there might not be a constant selection of a particular genotype among vaccinated and unvaccinated children [16].
Although the small number of patients analyzed precludes valid statistical comparisons between the vaccinated and non-vaccinated groups, the G1 and P[8] proteins present in both vaccines predominated in both the vaccinated and the unvaccinated groups. Thus although vaccination overall has greatly reduced the number of deaths and hospitalizations due to RV, our local data suggest that 5 years after the introduction of RVV there has been no genotype replacement, a small increase in G9P[6] genotype frequency was noted and G1P[8] remained the predominant strain of RV in our urban community.
Acknowledgments
We thank Dr. Ronald Thomas and Dr Marwan Zidan for assistance with statistical analysis and Tanaz Salimnia, B.Sc. for technical assistance. The study was supported by a Grant from Children’s Research Center of Michigan, Wayne State University.
References
- 1.Abdel-Haq N, Amjad M, McGrath E, Chearskul P, Amer A, Salimnia H, et al. Emergence of human rotavirus genotype G9 in metropolitan Detroit between 2007 and 2009. J Med Microbiol. 2011;60:761–767. doi: 10.1099/jmm.0.026807-0. [DOI] [PubMed] [Google Scholar]
- 2.Adlhoch C, Hoehne M, Littmann M, Marques AM, Lerche A, Dehnert M, et al. Rotavirus vaccine effectiveness and case-control study on risk factors for breakthrough infections in Germany, 2010–2011. Pediatr Infect Dis J. 2013;32:e82–e89. doi: 10.1097/INF.0b013e3182720b71. [DOI] [PubMed] [Google Scholar]
- 3.Aliabadi N, Tate JE, Haynes AK, Parashar UD. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination: United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2015;64:337–342. [PMC free article] [PubMed] [Google Scholar]
- 4.Clark HF, Lawley D, DiStefano D, Matthijnssens J, Dinubile MJ. Distribution of rotavirus genotypes causing nosocomial and community-acquired acute gastroenteritis at the Children’s Hospital of Philadelphia in the new rotavirus vaccine era. Hum Vaccines. 2011;7:1118–1123. doi: 10.4161/hv.7.11.17820. [DOI] [PubMed] [Google Scholar]
- 5.Cortes JE, Curns AT, Tate JE, Cortese MM, Patel MM, Zhou F, et al. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med. 2011;365:1108–1117. doi: 10.1056/NEJMoa1000446. [DOI] [PubMed] [Google Scholar]
- 6.de Rougemont A, Kaplon J, Lebon P, Huet F, Denis F, Alain S, et al. Unexpected substitution of dominant rotavirus G genotypes in French hospitalized children over five consecutive seasons. Eur J Clin Microbiol Infect Dis. 2009;28:403–407. doi: 10.1007/s10096-008-0640-1. [DOI] [PubMed] [Google Scholar]
- 7.Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentsch JR, Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, et al. G and P types of circulating rotavirus strains in the United States during 1996–2005: nine years of prevaccine data. J Infect Dis. 2009;200(Suppl 1):S99–S105. doi: 10.1086/605038. [DOI] [PubMed] [Google Scholar]
- 9.Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurgel RQ, Cuevas LE, Vieira SC, Barros VC, Fontes PB, Salustino EF, et al. Predominance of rotavirus P[4]G2 in a vaccinated population, Brazil. Emerg Infect Dis. 2007;13:1571–1573. doi: 10.3201/eid1310.070412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Gentsch JR, et al. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J. 2011;30:S42–S47. doi: 10.1097/INF.0b013e3181fefd78. [DOI] [PubMed] [Google Scholar]
- 12.Iturriza-Gomara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31:259–265. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood CD, Boniface K, Bishop RF, Barnes GL. Australian rotavirus surveillance program annual report, 2008/2009. Commun Dis Intell Q Rep. 2009;33:382–388. doi: 10.33321/cdi.2009.33.41. [DOI] [PubMed] [Google Scholar]
- 14.Koshimura Y, Nakagomi T, Nakagomi O. The relative frequencies of G serotypes of rotaviruses recovered from hospitalized children with diarrhea: a 10-year survey (1987–1996) in Japan with a review of globally collected data. Microbiol Immunol. 2000;44:499–510. doi: 10.1111/j.1348-0421.2000.tb02525.x. [DOI] [PubMed] [Google Scholar]
- 15.Leite JP, Alfieri AA, Woods PA, Glass RI, Gentsch JR. Rotavirus G and P types circulating in Brazil: characterization by RT-PCR, probe hybridization, and sequence analysis. Arch Virol. 1996;141:2365–2374. doi: 10.1007/BF01718637. [DOI] [PubMed] [Google Scholar]
- 16.Matthijnssens J, Nakagomi O, Kirkwood CD, Ciarlet M, Desselberger U, Van Ranst M. Group A rotavirus universal mass vaccination: how and to what extent will selective pressure influence prevalence of rotavirus genotypes? Expert Rev Vaccines. 2012;11:1347–1354. doi: 10.1586/erv.12.105. [DOI] [PubMed] [Google Scholar]
- 17.Payne DC, Szilagyi PG, Staat MA, Edwards KM, Gentsch JR, Weinberg GA, et al. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr Infect Dis J. 2009;28:948–953. doi: 10.1097/INF.0b013e3181a6ad6e. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Fauquier A, Montero V, Moreno S, Sole M, Colomina J, Iturriza-Gomara M, et al. Human rotavirus G9 and G3 as major cause of diarrhea in hospitalized children, Spain. Emerg Infect Dis. 2006;12:1536–1541. doi: 10.3201/eid1210.060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steele AD, Ivanoff B. Rotavirus strains circulating in Africa during 1996–1999: emergence of G9 strains and P[6] strains. Vaccine. 2003;21:361–367. doi: 10.1016/S0264-410X(02)00616-3. [DOI] [PubMed] [Google Scholar]
- 21.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 22.Tra My PV, Rabaa MA, Vinh H, Holmes EC, Hoang NV, Vinh NT, et al. The emergence of rotavirus G12 and the prevalence of enteric viruses in hospitalized pediatric diarrheal patients in southern Vietnam. Am J Trop Med Hyg. 2011;85:768–775. doi: 10.4269/ajtmh.2011.11-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human–bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 24.Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010;125:e208–e213. doi: 10.1542/peds.2009-1246. [DOI] [PubMed] [Google Scholar]
- 25.Wulan WN, Listiyaningsih E, Samsi KM, Agtini MD, Kasper MR, Putnam SD. Identification of a rotavirus G12 strain, Indonesia. Emerg Infect Dis. 2010;16:159–161. doi: 10.3201/eid1601.091010. [DOI] [PMC free article] [PubMed] [Google Scholar]