Abstract
Introduction: The penetration depth of irrigating solutions in dentinal tubules is limited; consequently, bacteria can remain inside dentinal tubules after the cleaning and shaping of the root canal system. Therefore, new irrigation systems are required to increase the penetration depth of irrigating solutions in dentinal tubules.
Methods: A comparative study regarding the penetration depth of sodium hypochlorite (NaOCl) solution in dentinal tubules using four methods, (1) conventional irrigation (CI), (2) smear layer removal plus conventional irrigation (gold standard), (3) passive ultrasonic agitation (PUA) and (4) Nd:YAG laser activated irrigation (LAI), took place on 144 extracted mandibular teeth with a single root canal. After decoronation with a diamond disc and working length determination, the apical foramen was sealed with wax. The canals were prepared up to #35 Mtwo rotary file and 5.25% NaOCl was used for irrigation during preparation. To study the penetration depth of NaOCl, smear layer was eliminated in all samples. Dentinal tubules were stained with crystal violet and after longitudinal sectioning of teeth, the two halves were reassembled and root canal preparation was performed up to #40 Mtwo rotary file. Then the samples were distributed into four experimental groups. Depth of the bleached zone was evaluated by stereomicroscope (20X). Data were analyzed by Kruskal-Wallis test.
Results: The highest and lowest average for NaOCl penetration depth in all three coronal, middle and apical sections belonged to CI + smear layer removal and CI. A statistically significant difference was seen when comparing the penetration depth of CI + smear layer removal group to CI and PUA groups in coronal and middle third, in which the average NaOCl penetration depth of the gold standard group was higher (P < 0.05). A statistically significant difference was seen between CI + smear layer removal group and the other three groups including CI, PUA and LAI in apical third, in which the average NaOCl penetration depth in the gold standard group was higher (P < 0.001).
Conclusion: The standard protocol for smear layer removal led to more effective smear layer elimination and deeper penetration depth of irrigation solutions. PUA and LAI groups exhibited less smear layer elimination and penetration depth of irrigation solutions. Therefore, CI+smear layer removal should still be considered as the gold standard.
Keywords: Agitation, Irrigation, Lasers, Nd:YAG, Ultrasonic
Introduction
Complete cleaning and shaping of root canal system, is an important part in endodontic treatments, which is performed by mechanical and chemical methods. Elimination of the pulp tissue, mineral and organic debris, micro-organisms and their by-products with the use of inter canal irrigation instruments is one of the main aims of this treatment phase.1 While treating infected root canals, special attention should be guided towards eliminating bacteria and their byproducts from the root canal system. The practicality of irrigating solutions depends on different factors such as the irrigation mechanism, its contact potential with substances, materials and root canal structures, their penetration depth in the main canal and lateral spaces.2
When evaluating the practicality of debris elimination in 3 irrigating solutions, Baker et al proposed that the flushing effect was more important than the power of tissue resolution.3 Therefore, sodium hypochlorite’s efficiency might be affected by the method of use.
For instance, it has been shown that when sodium hypochlorite is activated with ultrasonic, its efficiency increases.4 Better penetration of chemical substances, irrigation solutions and intra canal medications into dentinal tubules caused by smear layer removal allows a better disinfection and seal in the root canal system.5
Root canal irrigation systems can be divided into two main groups, including hand agitation techniques and agitation techniques with devices. Hand agitation consists of irrigation with positive forces, commonly by the use of syringes and side-vented needles.
Agitation techniques with devices include sonic, ultra sonic, also newer systems like Endo VAC (apical negative pressure)6and plastic rotary files.7,8
Paragliola et al concluded that ultrasonic agitation can increase the effect of the final irrigant on root canal walls in the apical third.9
The penetration depth of irrigation solutions and therefore their disinfecting effect on dentinal tubules are limited. These limitations lead to incomplete bacterial removal from dentinal tubules after cleaning and shaping of the root canal system.10
Irrigation systems which increase the penetration depth of irrigants in dentinal tubules and the root canal while encountering minimal apical extrusion of irrigants, eliminating cytotoxic effects on periapical tissues, exhibit superior effects.11,12
Dental lasers have probable photo biologic effects including photoacoustic, photochemical and photothermal effects.13
Laser activated irrigation (LAI) was introduced with the aim of increasing irrigant activation. This technique causes acoustic cavitation, defined by the formation of large oval vapor bubbles which will expand and implode subsequent to the use of ablative lasers in an aqueous environment. It has been shown that these vapor bubbles may increase the volume up to 1600 times. Expansion creates high pressure and the pressure causes the expulsion of fluid. When the bubbles implode after 100-200 ms, the pressure declines. By means of subsequent re-entry of fluid into the root canal, the secondary effects of cavitation are created. Thus, the laser acts as a fluid pump.14
In the steps of cleaning and shaping the root canal system, application of laser has some advantages such as debris removal, smear layer removal and bactericidal effects.15 Few studies have reported adverse effects from this type of laser. It also has been confirmed that this laser can strengthen disinfection and sterilization due to thermal effects.16 This laser can reduce the post-operative pain owing to the effective removal of bacteria in the apical region of the root canal.17 Other benefits include melting of dentin and sealing the dentinal tubules,18removal of debris and smear layer from root canal walls and tissue vaporization.19
Little information exists about the effects of Neodymium-Doped Yttrium Aluminum Garnet )Nd:YAG( LAI on the penetration depth of sodium hypochlorite. And no study has compared this laser with other methods. The aim of this Ex vivo study was to evaluate the extent of penetration of sodium hypochlorite into dentinal tubules by passive ultrasonic agitation (PUA), Nd:YAG LAI and compare them with conventional clinical methods and determine the more effective and practical irrigation method for endodontic treatments.
Methods
Seventy-two extracted human anterior mandibular teeth with one root canal were selected. The roots of teeth were mature and straight, without caries, resorption and observable cracks under 3X magnification. The teeth were extracted due to periodontal problems. The teeth were stored in normal saline and disinfected by chloramines T with 1:7 proportion. The crowns of teeth were cut off by diamond discs with a thickness of 1 mm (D & Z, Cologne, Germany) to provide samples with 19 mm length from the apical foramen. The position of apical foramen was determined by a stainless steel K-type hand file #15 (Dentsply, Maillefer, Ballaigues, Switzerland). After seeing the tip of file in the apical foramen, 1 mm was reduced from the length of file and that file length was considered as working length.
Then, the samples were randomly divided into 4 groups:
Group 1: Conventional irrigation (CI): First, the root canals were irrigated with 2 ml 5.25% NaOCl by an endodontic irrigating syringe and 30 gauge needle (Max-i-Probe; Dentsply, Rinn, Elgin, IL) for 20 seconds. The tip of the needle was placed 1 mm to the apical foramen and was moved up and down in the apical third with latitude of 4 mm. Then NaOCl was left in the root canal for 20 seconds without any movement, and the canals were irrigated again for 20 seconds as described previously with 30 gauge Max-i-Probe needle. Finalirrigation was accomplished by normal saline for 60 seconds.
Group 2: CI plus smear layer removal: The canals were irrigated by 2 ml 5.25% NaOCl with a syringe and 30 gauge Max-i-Probe needle for 20 seconds as described previously in group 1. Then for smear layer removal, 2 ml of 17% ethylenediaminetetraacetic acid (EDTA) (Apadana Tak Co, Tehran, Iran) was used for one minute followed by 2 ml 5.25% NaOCl for another minute. Final irrigation was accomplished by normal saline for 60 seconds. This group was considered as the gold standard group.
Group 3: PUA group: The root canals were irrigated by 2 ml 5.25% NaOCl with a syringe and 30 gauge Max-i-Probe needle for 20 seconds. Then, a stainless steel NSK U file 33 mm #20 mounted at E11 ultrasonic tip (Varios, NSK Nakanishi Inc., Kanuma, Japan) was activated for 20 seconds with an ultrasonic device (Varios 350, NSK Nakanishi Inc., Kanuma, Japan) with the power set on E4 to agitate the solution. The tip of file was placed 1 mm above apical foramen according to the method explained by De Moor et al.20 Then, the canals were irrigated by 2 ml 5.25% NaOCl with a syringe for 20 seconds. Final irrigation was accomplished by normal saline for 60 seconds.
Group 4: LAI group: The teeth were filled by 2 ml 5.25% NaOCl with syringe and 30 gauge Max-i-Probe needle for 20 seconds. Then, the Nd:YAG laser (Fidelis plus, Fotona; Ljubljana, Slovenia) with 300 µm endodontic fiber and 100 mJ energy pulse and power of 2 W, frequency of 20 Hz and pulse width of 100 µs (VSP: Very Short Pulse) was activated for 20 seconds. The fiber was placed in the apical part of the root canal for 5 seconds and moved in spiral motion out of the root canal. Then the fiber was reinserted in the root canal and this procedure was repeated for 4 more times (total time = 20 seconds). The laser was only fired during fiber removal from the root canal according to Blanken et al.21 Then, the root canal was irrigated with 2 ml 5.25% NaOCl for 20 seconds with syringe and 30 gauge Max-i-Probe needle. Final irrigation was accomplished by normal saline for 60 seconds.
After irrigation, the samples were separated in half. One half was randomly selected and then the surface layer was grounded with a thickness of approximately 100 microns by a soft abrasive paper and grinding machine (Malek Teb, Tehran, Iran). Then the created surfaces were evaluated with stereomicroscope Model LA-SZM45-B1 (Nanjing Sunny Optical Instrument Co., Ltd, Nanjing, China) with 20X magnification. Images were saved in JPEG format by Dinocapture 2.0 software using AM423X Dino-Eye digital eyepiece and RL-L64 LED light source LED model. The depth of the bleached zone (depth of penetration of sodium hypochlorite) was calculated in microns.
The Method of Calculating the Bleached Zone
The captured images from stereomicroscopic with 20X magnifications, were opened with Photoshop software. The limits of bleached zone were determined by polygonal lasso tool, number of selected pixels was read in Photoshop histogram menu and likewise, height of canal interior wall was read based on pixel in Info menu. The penetration depth of irrigation solution was calculated according to the following formula based on pixel:
(b=Average penetration depth of irrigation solution, a=Height of canal interior wall) (Figure 1).
Figure 1 .
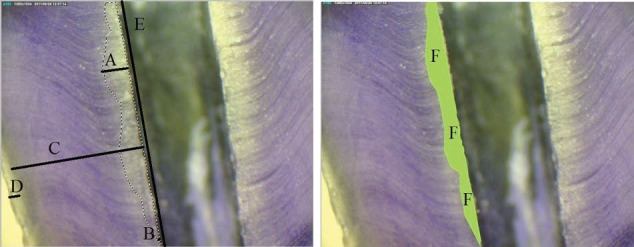
Determining Bleached Level by Photoshop Software. A) Maximum penetration depth of sodium hypochlorite, B) Minimum penetration depth of sodium hypochlorite, C) Dentin, D) Cementum, E) Height of canal interior wall, and F) Limits of bleached zone.
For images with 72 dpi resolution, every 100 pixels were equivalent to 35.28 mm and by dividing that to the magnification, the real size which is convertible to micron was obtained and in that way, the average, minimum and maximum penetration in the total penetrated surface was obtained. The results of penetration depth were announced by two trained blind observers in such a way that they came to the same conclusion after some disagreement. Afterwards, statistical analysis was performed.
For data analysis, quantitative variables were described as average and standard deviation and qualitative variables as raw abundance and relative abundance.
To compare the average penetration depth of sodium hypochlorite among four study groups, analysis of variance was used and for pair wise comparison, Dunnett T3 test was applied due to non-homogenous variance of data. A statistical significant limit of 0.05 was considered for this purpose.
Results
Average penetration depth of sodium hypochlorite in the coronal third was different among the four groups (P<0.001). The average penetration depth of sodium hypochlorite was significantly different when comparing the CI+smear layer removal group (309.81 microns) with CI groups (249.93 microns ) and PUA (260.56 microns) groups (P<0.001 and P=0.001 respectively). Also, the average penetration depth of sodium hypochlorite was significantly different when comparing the LAI group (293.03 microns) with CI (249.93 microns) and PUA (260.56 microns) groups (P<0.001 and P=0.004 respectively).
Average penetration depth of sodium hypochlorite in the middle third was different among the four groups under study (P<0.001). A statistically significant difference was seen when comparing the average penetration depth of sodium hypochlorite between the CI+smear layer removal group (212.7 microns) with CI groups (163.9 microns) and PUA (188.12 microns) (P<0.001 and P=0.04 respectively). A statistically significant difference was also seen when comparing the average penetration depth of sodium hypochlorite between the CI groups (163.9 microns) with LAI groups (207.79 microns) and PUA (188.12 microns) (P<0.009 and P=0.001 respectively).
The average penetration depth of sodium hypochlorite in apical one third was also different in the four experimental groups (P<0.001). A statistically significant difference was seen when comparing the average penetration depth of sodium hypochlorite between CI+smear layer removal group (175.41 microns) with CI groups (42.07 microns), PUA (71.61 microns) and LAI (90.2 microns) (P<0.001). The average penetration depth of sodium hypochlorite in group CI (42.07 microns) had a significant difference with that of the LAI (90.2 microns) and PUA (71.61 microns) groups (P<0.001). Furthermore, a significant difference was seen in the average penetration depth of sodium hypochlorite between LAI (90.2 microns) and PUA (71.61 microns) groups (P=0.05). The results are shown in Table 1.
Table 1 . Statistical Indicators for the Penetration Depth of Sodium Hypochlorite in the Coronal, Middle and Apical Third of Four Experimental Groups (Based on Microns) .
| Groups | LAI | PUA | CI + Smear Layer Removal | CI |
| Coronal | ||||
| Mean | 293.03 a,b | 260.56 a,c | 309.81 c,d | 249.93 b,d |
| SD | 17.56 | 31.15 | 39.01 | 24.57 |
| Min | 245.46 | 202.14 | 215.96 | 188.62 |
| Max | 316.34 | 301.09 | 389.43 | 283.34 |
| Middle | ||||
| Mean | 207.79 e | 188.12 f,g | 212.7 f,h | 163.9 e,g,h |
| SD | 33.49 | 23.22 | 27.28 | 18.17 |
| Min | 140.65 | 140.25 | 175.76 | 129.76 |
| Max | 269.12 | 245.45 | 279.13 | 190 |
| Apical | ||||
| Mean | 90.2 i | 71.61 i | 175.41 i | 42.07 i |
| SD | 18.64 | 38.75 | 38.75 | 17.71 |
| Min | 52.32 | 119.32 | 110.32 | 10.54 |
| Max | 121.52 | 234.57 | 234.57 | 70.09 |
Abbreviations: LAI, laser activated irrigation; PUA, passive ultrasonic agitation; CI, conventional irrigation
Similar alphabetical superscripts indicate significant difference amongst the groups.
Discussion
Studying the extent and measuring the penetration depth of sodium hypochlorite in dentin is not possible in vivo due to practical and moral limitations. Therefore, to standardize the study, human extracted teeth were used (ex vivo).
William et al22 used the motile microorganisms model to study penetration depth of smear layer in an in vitro study. They reported that in severely infected root canals, bacteria might be found in depth of dentinal tubules and the smear layer covering the root canal walls following endodontic instrumentation contained bacteria and remains of necrotic pulp tissues.
Also, the presence of smear layer can prevent antiseptics and root canal fillers from entering dentinal tubules.23
Nair et al24 showed that 88% of root canals of mesial roots of one-visit treated mandibular molars revealed residual infection after instrumentation, irrigation with sodium hypochlorite, and obturation. Due to these limitations, searching for the enhancement of the results of sodium hypochlorite irrigation by devices or a better root canal irrigant must be continued.24
Likewise, a study by Hata et al showed that instrumentation and irrigation with 5% sodium hypochlorite followed by 15% EDTA using a syringe was the most effective irrigation technique for smear layer removal.25Therefore, in the current study, conventional irrigation and smear layer removal by the aforementioned protocol was considered as the gold standard for comparison.
Since mechanical instrumentation only eliminates 50% of bacteria from root canal, irrigating solutions are required to eliminate microbiota from the zones out of reach of instruments.26 Different activation techniques are recommended for promoting the efficiency of irrigating solutions. Some of them include agitation by hand file, Gutta Percha, mechanical agitation by plastic instruments, also sonic and ultrasonic agitation.27
Minamisako et al have shown the efficiency of Nd:YAG laser in eliminating debris, smear layer and pulp tissues.19 Therefore, in the current study, Nd:YAG was used with 2 W power, 20 Hz frequency and 100 microsecond pulse width (VSP: very short pulse) for 20 seconds. Longer irradiation time with less output power decreases the risk of tissue damage due to significantly less temperature increase. On the other hand, to diffuse the heat generated from laser radiation and in tensify the effects of laser radiation, canals may be filled with sodium hypochlorite or EDTA.19
Mello et al28 concluded that constant irrigation with 5 ml of EDTA for 3 minutes can effectively remove smear layer.
In vivo conditions such as moisture, temperature and dentinal tubule content may impress the penetration depth to dentinal tubules.29 Likewise, the penetration depth of irrigating solutions into dentinal tubules, and as a result, their bactericidal effects are clearly impressed by the presence or lack of smear layer. It has been indicated that smear layer formation, decreases penetration into root dentin about 25%-49%.30
In some studies, dye penetration in sodium hypochlorite solution has been used as an index for evaluating penetration, but, there is doubt whether there is a conformity between the penetration depth of sodium hypochlorite and dye penetration depth or not. It has been reported that the surface tension of sodium hypochlorite limits its capability to distribute inside the root canal.31Adding some acid fuchsin to sodium hypochlorite may have some effect on its surface tension which should come into consideration in future studies. Due to the small molecular size of sodium hypochlorite (molecular weight of NaOCl solved in distilled water is 74.45 g/mole), its penetration depth may be higher than that of the dye itself (acid fuchsin molecular weight is 585.54 g/mole). Therefore, advancement of the solution in the root canal is assessed indirectly and the results may not bedefinite.32
Previous studies indicate that there are some certain dyes that may easily penetrate into the whole depth of dentin even without removing root surface cementum.33 Crystal violet and safranin red showed the same results in dye and sodium hypochlorite penetration studies.34
The reason for selecting crystal violet in the current study was better observation under stereomicroscope. Because sodium hypochlorite is a strong oxidant, it whitens the purple color of crystal violet and reveals the clear natural color of dentin. The width of the discolored zone was easily recorded and considered as the penetration depth of sodium hypochlorite. After exposing floated samples in crystal violet to sodium hypochlorite, 100 micron was removed from surface of dentin samples by abrasive paper to let the dentin zones that were only under the impression of sodium hypochlorite via dentinal tubules come up. Of course, it is assumed that coloring the walls of dentinal tubules by dye may change liquid penetration patterns by changing dentine surface features.34
Penetration depth and presence of bacteria in various surfaces of dentin and inside dentinal tubules have been studied by some researchers. For instance, Peters et al in an in vivo study showed that in 62% of cases, bacteria grew up to the cemental surface of the root and in 24% of cases, over 50000 CFU/g were seen on root cementum surface.35 Further studies, reported the maximum penetration depth of bacteria in the pulpal dentin wall to be up to 0.25 mm.36 On the other hand, Siqueira et al observed bacterial cells up to an approximate depth of 300 microns and believed that low penetration depth was more conventional.13
Also, resistant infections resulting from reproduction of the remaining bacteria inside dentinal tubules are one of the probable reasons for treatment failure.37
Based on the aforementioned studies, increasing the penetration depth of irrigation solutions inside dentinal tubules may be effective in promoting the success rate of endodontic treatments and decreasing failure in the treatment of resistant infections. According to the findings of the present study, the average penetration depth of sodium hypochlorite in the coronal third was different among the four studied groups. The highest penetration depth was seen in the CI+smear layer removal group (309.81 microns) and the lowest was seen in the CI group (249.93 micron). Differences were statistically significant (P<0.001). The differences seen in the coronal third do not seem to be of clinical significance.
In the middle third, the highest penetration depth was seen in the CI + smear layer removal group (212.70 micron) and the lowest was seen in the CI group (163.90 micron). The difference was statistically significant (P<0.001).
In terms of clinical significance, it seems that by approaching the apical zone, such differences(about 60 microns) clearly become more important and effective. Similar to the middle third zone, the average penetration depth of sodium hypochlorite in LAI group was 207.79 microns and that amount did not show a significant difference with the gold standard group (212.70 microns). On the contrary, the difference seen between the PUA and gold standard group was significant. It can be concluded that in middle and coronal third, only LAI was as effective as the gold standard protocol in increasing the penetration depth of irrigation solution.
In apical third, similar to that of the middle and coronal third, the highest penetration depth belonged to the CI + smear layer removal group, and the CI group showed the lowest penetration depth (175.41 and 40.07 microns respectively). In the apical third, the average penetration depth of sodium hypochlorite in the PUA and LAI groups were 71.61 and 90.20 micron respectively, which had a significant difference with the gold standard group (175.41 micron). An 85-105 microns difference has a clear clinical impact in the apical third zone.
What comes into spotlight in all the aforementioned comparisons is that EDTA was never used in CI, PUL and LAI techniques and probably its significant role might be the reason for such significant difference. In this study, despite of implementing activation techniques, NaOCl did not show complete penetration in any of the samples and this can justify failure in ideal root canal treatments.
In this study, the penetration depth of sodium hypochlorite was reported to be in a range of 40 to 309 microns. Ando and Hoshino showed presence of bacteria in dentinal tubules up to the half way between root canal and cementodentinal junction (CDJ).38 Haapasalo et al reported that Enterococcus faecalis quickly attacked tubules and in some blocks, they could penetrate up to a depth of 1000 microns after three weeks incubation.37 Therefore, access to all bacteria in all cases by irrigation with sodium hypochlorite would be difficult or almost impossible and various factors such as viscosity, time, temperature, surface tension, needle gauge and needle head depth position may affect the penetration depth of irrigation solutions such as NaOCl.34
In the study of Zou et al,34 the highest amount of penetration depth of 6% sodium hypochlorite was 300 microns which was seen after application of this solution for 20 minutes in 45°C. It was shown that temperature, time and viscosity were variables affecting the penetration depth of sodium hypochlorite into dentinal tubules. The deepest penetration took place when all the three variables existed simultaneously contributing to intensification.34
De Gregorio et al39 evaluated the penetration of irrigation solution into synthetic accessory canals. Dissimilar to the results of the current study, they concluded that adding EDTA did not lead to better penetration of irrigation solution into synthetic accessory canals. The use of accessory canals instead of dentinal tubules may be the reason for such differences.
Minamisako et al reported that Nd:YAG laser was capable of removing smear layer, debris and pulpal tissues even without black initiator ink.19 The results of present study indicated that the penetration depth of sodium hypochlorite in the coronal and middle sections did not show significant differences when comparing the use of laser with the gold standard; whereas, in apical third, the LAI group, showed a significant difference when compared to the gold standard group in such a way that the average penetration depth of sodium hypochlorite was 90.2 microns in the v group and 175.41 micron in the gold standard group. The lower penetration depth can be attributed to higher amounts of the remaining smear layer, the melting effect of Nd:YAG laser on dentin and occlusion of dentinal tubule orifices.
According to the results of the current study it can be concluded that the standard protocol for smear layer removal leads to a more effective elimination of smear layer and deeper penetration of irrigation solution. It should be noted that penetration of irrigating solution into dentinal tubules does not necessarily indicate its sufficient concentration for eliminating microorganisms,40 which is of more importance in infected root canals.
Lloyd et al41 showed that laser-activated photon-induced photo-acoustic streaming irrigation can eliminate debris from complex canal spaces of mandibular molars at a significantly greater level in comparison with standard needle irrigation.
Further studies regarding effective elimination of biofilm and microorganism inside dentinal tubules using other types of lasers with various settings and environments are highly recommended.
Conclusion
The use of PUA and LA resulted in less smear layer elimination and consequently less penetration of irrigating solutions. CI + smear layer removal can be considered as the gold standard technique for deeper penetration of NaOCl in dentinal tubules and techniques that do not remove smear layer cannot be considered as a good alternate for it.
Ethical Considerations
The teeth used in this study were extracted due to periodontal problems.
Conflict of Interest
The authors has no conflict of interest to declare.
Please cite this article as follows: Ghorbanzadeh A, Aminsobhani M, Sohrabi K, et al. Penetration depth of sodium hypochlorite in dentinal tubules after conventional irrigation, passive ultrasonic agitation and Nd:YAG laser activated irrigation. J Lasers Med Sci. 2016;7(2):105-111. doi:10.15171/jlms.2016.18.
References
- 1.Clegg MS, Vertucci FJ, Walker C, Zand V. The effect of exposure to irrigant solutions on apical dentin biofilms in vitro. J Endod. 2006;32(5):434–437. doi: 10.1016/j.joen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Chow TW. Mechanical effectiveness of root canal irrigation. J Endod. 1983;9(11):475–479. doi: 10.1016/s0099-2399(83)80162-9. [DOI] [PubMed] [Google Scholar]
- 3.Baker NA, Eleazer PD, Averbach RE. Scanning electron microscopic study of the efficacy of various irrigation solutions. J Endod. 1975;1(4):127–135. doi: 10.1016/S0099-2399(75)80097-5. [DOI] [PubMed] [Google Scholar]
- 4.Cheung GS, Stock CJR. In vitro cleaning ability of root canal irrigants with and without Endosonics. Int Endod J. 1993;26(6):334–343. doi: 10.1111/j.1365-2591.1993.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 5.Foster KH, Kulild JC, Weller RN. Effect of smear layer removal on the diffusion of calcium hydroxide through radicular dentin. J Endod. 1993;19(9):136–140. doi: 10.1016/S0099-2399(06)80508-X. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen BA, Baumgartner JC. Comparision of the Endovac system to needle irrigation of the root canals. J Endod. 2007;33(5):611–615. doi: 10.1016/j.joen.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Chopra S, Murray PE, Namerow KN. A scanning electron microscopic evaluation of the effectiveness of the F-file versus ultrasonic activation of a K-file to remove smear layer. J Endod. 2008;34(12):1234–1235. doi: 10.1016/j.joen.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Bahcall J, Oslen FK. Clinical introduction of a plastic rotary endodontic finishing file. Endo Prac. 2007;10(5):17–20. [Google Scholar]
- 9.Paragliola R, Franco V, Fabiani C, Mazzoni A, Nato F, Tay FR. et al. Final Rinse Optimization: Influence of Different Agitation protocols. J Endod. 2010;36(2):282–285. doi: 10.1016/j.joen.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Wu Mk, Dummer PM, Wesselink PR. Consequences of and strategies to deal with residual post-treatment root canal infection. Int Endod J. 2006;39(5):343–356. doi: 10.1111/j.1365-2591.2006.01092.x. [DOI] [PubMed] [Google Scholar]
- 11.Serper A, Ozbek M, Calt S. Accidental sodium hypochlorite-induced skin injury during endodontic treatment, J Endod. 2004;30(3):180–181. doi: 10.1097/00004770-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Bradford CE, Eleazer PD, Downs KE, Scheetz JP. Apical pressures developed by needles for canal irrigation. J Endod. 2002;28(9):333–335. doi: 10.1097/00004770-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Siqueira JF, Rocas IN, Lopez HP. Patterns of microbial clonization in primary root canal infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(12):174–178. doi: 10.1067/moe.2002.119910. [DOI] [PubMed] [Google Scholar]
- 14.Van der Sluis LM, Wu MK, Versluis M, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40(6):415–426. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 15.Stabholz A, Sahar-Helft S, Moshonov J. lasers in endodontics. Dent clin north Am. 2004;48(4):809–832. doi: 10.1016/j.cden.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Zhang C, Yin X. Evaluation of bactericidal effect of Er;Cr:YSGG and Nd:YAG lasers in experimentally infected root canals. J Endod. 2007;33(7):830–832. doi: 10.1016/j.joen.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Koba K, Kimura Y, Matsumoto K, Takeuchi T, Ikarugi T, Shimizu T. A histopathologial study of the morphological changes at the apical seat in the periapical region after irradiation with a pulsed Nd:YAG laser. Int Endod J. 1998;31(6):415–420. doi: 10.1046/j.1365-2591.1998.00177.x. [DOI] [PubMed] [Google Scholar]
- 18.Miserendino LJ, Levy GC, Rizoiu IM. Effects of the Nd:YAG laser on the permeability of root canal wall dentin. J Endod. 1995;21(2):83–87. doi: 10.1016/S0099-2399(06)81101-5. [DOI] [PubMed] [Google Scholar]
- 19.Minamisako MC, Kinoshita JL, Matsumoto K, Stolf DP, Marques JL. A study on root canal cleaning by Nd:YAG laser with black dye solution. J Oral Laser Applications. 2009;9(2):101–109. [Google Scholar]
- 20.De Moor RJ, Torbeyns D, Meire M. Lasers in endodontics Part 2: root canal wall cleanliness and modification. Endod Pract Today. 2009;3:19–33. [Google Scholar]
- 21.Blanken J, De Moor RJ, Meire M, Verdaasdonk R. Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal Part 1: a visualization study. Lasers Surg Med. 2009;41(7):514–519. doi: 10.1002/lsm.20798. [DOI] [PubMed] [Google Scholar]
- 22.William S, Goldman M. Penetrrability of the smeared layer by a strain of Proteus vulgaris. J Endod. 1985;11(5):385–388. doi: 10.1016/s0099-2399(85)80026-1. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg F, Abramovich A. Analysis of the effect of EDTAC on the dentinal walls of the root canal. J Endod. 1977;3(5):101–105. doi: 10.1016/s0099-2399(77)80203-3. [DOI] [PubMed] [Google Scholar]
- 24.Nair BG, Reddy KA. Advances in root canal disinfection. J Pharm Biomed Sci. 2011;5(24):1–3. [Google Scholar]
- 25.Hata G, Hayami S, Weine FS, Toda T. Effectiveness of oxidative potential water as a root canal irrigant. Int Endod J. 2001;34(12):308–317. doi: 10.1046/j.1365-2591.2001.00395.x. [DOI] [PubMed] [Google Scholar]
- 26.Ciucchi B, Khettabi M, Holz J. The effectiveness of different endodontic irrigation procedures on the removal of smear layer: A scanning electron microscopic study. Int Endod J. 1989;22(9):21–28. doi: 10.1111/j.1365-2591.1989.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 27.Gu LS, Kim JR, Ling j, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35(6):791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Mello I, Kammerer BA, Yoshimoto D, Macedo MC, Antoniazzi JH. Influence of final rinse technique on ability of Ethylenediaminetetraacetic acid of removing smear layer. J Endod. 2010;36(3):512–514. doi: 10.1016/j.joen.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Kokkas AB, BoutsioukisACh BoutsioukisACh, Vassiliadis LP, Stavrianos CK. The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers : an in vitro study. J Endod. 2004;30(2):100–102. doi: 10.1097/00004770-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Fogel HM, Pashley DH. Dentin permeability : effects of endodontic procedures on root slabs. J Endod. 1990;16(9):442–445. doi: 10.1016/s0099-2399(06)81888-1. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham WT, Cole JS 3rd, Balekjian AY. Effect of alcohol on the spreading ability sodium hypochlorite endodontic irrigant. Oral surgery, Oral medicine and oral Pathology. 1982;54(12):333–335. doi: 10.1016/0030-4220(82)90105-0. [DOI] [PubMed] [Google Scholar]
- 32.Hauser V, Braun A, Frentzen M. Penetration depth of a dye marker into dentin using a novel hydrodynamic system (RinsEndo) Int Endod J. 2007;40(8):644–652. doi: 10.1111/j.1365-2591.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 33.Paque F, Luder HU, Sener B, Zehnder M. Tubular sclerosis rather than smear layer impedes dye penetration into the dentine of endodontically instrumented root canals. Int Endod J. 2006;39(1):18–25. doi: 10.1111/j.1365-2591.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- 34.Zou L, Shen Y, Li W, Haapasalo M. Penetration of Sodium Hypochlorite into dentin. J Endod. 2010;36(5):793–796. doi: 10.1016/j.joen.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Peters LB, Wesselink PR, Buijs JF, Winkelholf AJ. Viable bacteria in root dentinal tubules of teeth with apical periodontitis. J Endod. 2001;27(5):76–81. doi: 10.1097/00004770-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Schafer E, Bossmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Entrococcus fecalis. J Endod. 2005;31(9):53–56. doi: 10.1097/01.don.0000134209.28874.1c. [DOI] [PubMed] [Google Scholar]
- 37.Haapasalo M, Qrstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66(5):1375–1379. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 38.Ando N, Hoshino E. Predominant obligate anaerobes invading the deep layers of root canal dentin. Int Endod J. 1990;23(12):20–27. doi: 10.1111/j.1365-2591.1990.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 39.De Gregorio C, Steves R, Cisneros R, Heilborn C, Cohenca N. Effect of EDTA, sonic and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: An in vitro study. J Endod. 2009;35(6):891–895. doi: 10.1016/j.joen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Buck R, Eleazer PD, Staat RH. In vitro disinfection of dentinal tubules by various endodontic irrigants. J Endod. 1999;25(9):786–788. doi: 10.1016/s0099-2399(99)80297-0. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd A, Uhles JP, Clement DJ, Garcia-Godoy F. Elimination of intracanal tissue and debris through a novel laser-activated system assessed using high-resolution micro-computed tomography: a pilot study. J Endod. 2014;40(4):584–587. doi: 10.1016/j.joen.2013.10.040. [DOI] [PubMed] [Google Scholar]


