Abstract
The aim of this study was to evaluate the diagnostic performance of osteocalcin (OC), as measured by automated electrochemiluminescence immunoassay (ECLIA), in identifying Cushing's syndrome (CS) in two separate populations: among obese and overweight subjects and among women of postmenopausal age with osteoporosis. Among the 106 referral patients with obesity, CS was confirmed in 42 cases. The patients of the referred population provided late-night salivary cortisol (LNSC), underwent low-dose dexamethasone suppression testing (DST) and were further evaluated until CS was pathologically confirmed. A threshold of OC—8.3 ng ml−1 differentiated CS among obese and overweight subjects with a sensitivity of 73.8% (95% confidence interval (CI) 58.9–84.7) and a specificity of 96.9% (95% CI 89.3–99.1). The total area under the receiver operating characteristic curve (AUC) was 0.859 (95% CI 0.773–0.945), which was lower than LNSC or DST (P=0.01). In the retrospective portion of the study, the OC levels were evaluated in 67 subjects with newly diagnosed postmenopausal osteoporosis and in 23 patients (older than 45) with newly diagnosed CS and osteoporosis (presence of low traumatic fractures or T-score P–2.5). The diagnostic performance of OC for osteoporosis due to CS was within an AUC of 0.959 (95% CI 0.887–1.00). A threshold for OC of 8.3 ng ml-1 yielded a sensitivity of 95.4% (95% CI 78.2–99.2%) and a specificity of 98.5% (95% CI 92.0–99.7%). Thus, osteocalcin could be used in the diagnostic testing for endogenous hypercortisolism in patients referred to exclude CS and to identify CS among patients of postmenopausal age with osteoporosis.
Introduction
Osteocalcin (OC) is a major non-collagen bone Gla protein and is a well-established bone formation marker produced by osteoblasts during bone formation; OC is bound to hydroxyapatite,1 reflecting a late stage of osteoblast activity, and is released into the circulation during formation and resorption. According to the IOF position statement, bone turnover markers (BTMs) predict fracture risk, and treatment-induced changes in specific markers account for a substantial proportion of fracture risk reduction.2 High levels of BTMs (mainly resorption) are an independent risk factor for fracture in postmenopausal women.3,4 Prospective studies have shown a direct relationship between the level of BTMs and the loss of bone mineral density (BMD) measured by dual-energy X-ray absorptiometry (DXA) in postmenopausal women.5,6 However, BTMs are not recommended as a diagnostic test for patients with osteoporosis and are therefore not included in the fracture risk assessment tool.2 BTMs do not control skeletal metabolism and are not disease specific; these markers simply reflect the entire skeleton remodeling regardless of the underlining cause.7 In clinical practice on individual patients, BTMs could be used to evaluate the effectiveness of treatment with antiresorptive or anabolic compounds and patient adherence to therapy.8,9,10,11 Nevertheless, many researchers have demonstrated a statistically significant reduction in OC levels in patients receiving glucocorticoid therapy12,13 and in patients with endogenous hypercortisolism.14,15,16 The resulting suppression of bone formation is so profound that OC levels were tested and shown to be effective in differentiating endogenous Cushing's syndrome (CS) from healthy control subjects with an area under the curve (AUC) of 0.922, which is similar to the late-night salivary cortisol (LNSC) of 0.994 obtained from a receiver operating characteristic (ROC) analysis in the same cohort of people.17 However, the most challenging cases involve differentiating patients with endogenous CS from obese subjects due to similar clinical features and complications such as the presence of functional hypercortisolism with relatively higher cortisol levels in obese subjects compared with healthy controls.18,19,20 Furthermore, elderly patients with CS usually do not show typical changes in their appearance, and many of these patients remain undiagnosed, receiving treatment for hypertension, diabetes and osteoporosis. An active screening for CS among 219 consecutive patients (200 women and 19 men) referred for treatment of osteoporosis revealed the prevalence of subclinical CS in 4.8% of subjects.21
Taking advantage of our position as the leading referral clinic for patients with suspected CS in Russia, we enrolled a sizeable population with a high percentage of CS to estimate the diagnostic performance of OC as measured by automated electrochemiluminescence immunoassay (ECLIA) in two separate populations; we differentiated endogenous CS among obese and overweight subjects and among women of postmenopausal age with osteoporosis.
Results
Diagnostic performance of OC measurements to identify CS among obese and overweight patients
One hundred and six obese and overweight patients referred by physicians to exclude CS were enrolled in the study. The median age (Q25–Q75) was 39 (26–49) years, and the median body mass index (BMI) was 35 (31–41) kg m−2). Endogenous hypercortisolism was confirmed in 42 of these patients. Adrenal CS was diagnosed in two cases, and Cushing's disease was established in 39 subjects, all of whom underwent transsphenoidal adenomectomy; a bronchial carcinoid tumor was successfully removed in one case. The artificially high prevalence of patients with CS among obese and overweight subjects could be explained by the invitation of a prescreened referral population of highly suspected, and in some cases confirmed, CS by the local laboratory of referring physicians.
The general characteristics of obese and overweight patients with CS and constitutional obesity are summarized in Table 1.
Table 1. General characteristics of enrolled patients with Cushing's syndrome and constitutional obesity.
| Cushing's syndrome Me (Q25–Q75) | Constitutional obesity Me (Q25–Q75) | P-value | |
|---|---|---|---|
| N, (number) | 42 | 64 | |
| Sex, female:male (%) | 31:11 (26.2%) | 44:20 (31.2%) | 0.4 |
| Age (years) | 37.5 (24.0–51.0) | 39.5 (29.0–48.5) | 0.6 |
| Body mass index (kg m−2) | 31.5 (28.0–37.0) | 37.5 (34.0–42.0) | <0.001 |
| Late-night salivary cortisol (nmol l−1) | 23.3 (16.1–43.8) | 3.2 (2.1–4.4) | <0.001 |
| Osteocalcin (ng ml−1) | 6.5 (4.1–10.2) | 16.5 (13.2–23.3) | <0.001 |
Abbreviations: Me, median, Q25–Q75, interquartile range.
Patients with CS and constitutional obesity did not differ in age or sex. However, patients with CS had lower BMI, statistically significantly lower levels of serum OC and higher levels of LNSC.
A threshold for OC of 8.3 ng ml−1, generated based on the maximum sum of sensitivity and specificity, yielded a sensitivity of 73.8% (95% confidence interval (CI) 58.9–84.7%) and a specificity of 96.9% (95% CI 89.3–99.1%) to identify CS among obese and overweight subjects. A predefined threshold of 12.9 ng ml−1 17 in our cohort of patients demonstrated only a slightly better sensitivity (81.1%), but a considerable decline in specificity (78.1%). The individual values of OC in subjects with obesity and CS are presented in Figure 1.
Figure 1.
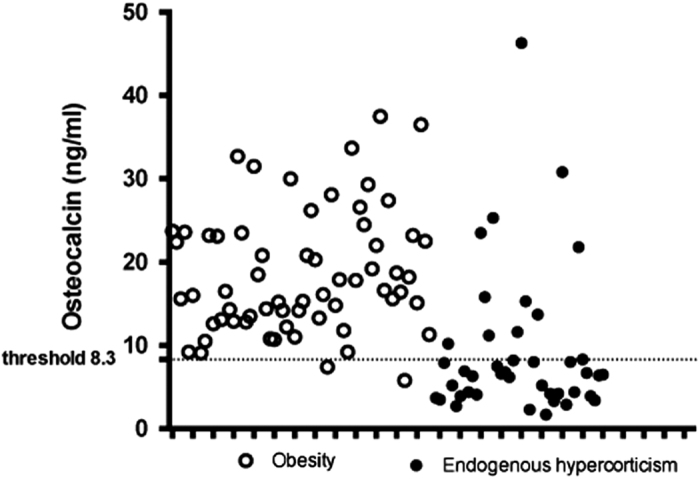
The individual serum osteocalcin values in obese and overweight patients diagnosed with Cushing's syndrome and in subjects with constitutional obesity. The threshold of 8.3 ng ml−1 with two false positive results and eleven false negative values for CS yielded a sensitivity of 73.8% (95% CI 58.9–84.7%) and a specificity of 96.9% (95% CI 89.3–99.1%) to differentiate CS among obese and overweight subjects.
ROC curves were generated for the OC levels and LNSC to characterize and compare their diagnostic performance in identifying CS in obese subjects (Figure 2). The diagnostic performances of the OC measurement AUC of 0.859 (95% CI 0.773–0.945) proved to be significantly lower compared with the LNSC AUC of 0.961 (95% CI 0.928–0.994; P=0.015) or the low-dose dexamethasone suppression test (DST) AUC of 0.977 (95% CI 0.954–1.0; P=0.003). There were no differences between the AUCs of LNSC and DST (P=0.316).
Figure 2.
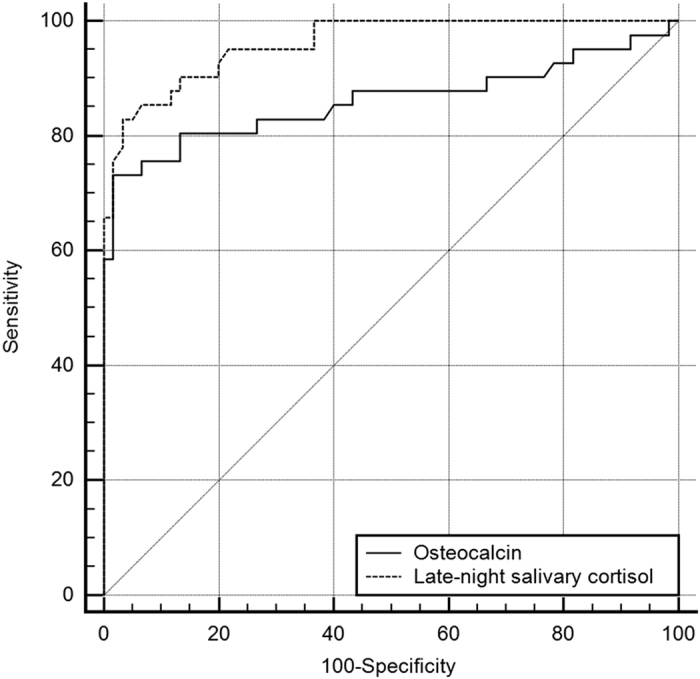
Direct comparison of areas under the curve (AUC) of osteocalcin (OC) and late-night salivary cortisol (LNSC) to evaluate Cushing's syndrome (CS) in referred population of obese and overweight patients. The diagnostic performance of the OC measurement AUC of 0.859 (95% CI 0.773–0.945) was statistically significantly lower compared with the LNSC AUC of 0.961 (95% CI 0.928–0.994); P=0.015) among the referral population of obese and overweight subjects with suspected CS.
It appears that combining OC measurements with LNSC does not add to LNSC evaluation in obese subjects. There were 4 discordant results of OC and LNSC in obese subjects and 12 discordant results of OC and LNSC in CS subjects. In three cases, the OC was true negative, and the LNSC was false positive for CS; therefore, the OC could help to identify these three obese subjects. However, in one case, the OC was below 8.3 ng ml−1, and the LNSC and DST were true negatives. In one case, both the OC and LNSC were false negative, but the subject was confirmed of having CS with a DST and 24-h urinary free cortisol (24hUFC). Among subjects with CS confirmed based on further histological evaluation after surgery, there were 11 false-negative cases of OC measurements, and all of these cases were correctly identified with LNSC. The OC was true positive in one case, whereas the LNSC was false negative. The overall AUC of the combined results for OC and LNSC was 0.853 (95% CI 0.768–0.968), which was not superior to OC or LNSC separately analyzed.
All patients with elevated LNSC underwent DST. All subjects with CS had results above the currently recommended threshold of 50 nmol l−1, proving that these subjects had CS. Four subjects with obesity had elevated LNSC (19.6 nmol l−1; 9.8 nmol l−1; 10.1 nmol l−1; 15.5 nmol l−1), and three of them had positive DST results (16.4; 10.10; 50.3 nmol l−1). Patients provided 24hUFC, which were within the reference range in these three subjects. In one case (LNSC 15.5 nmol l−1), the DST result was 616 nmol l−1. This subject was hospitalized and underwent further testing (24hUFC, late-night serum cortisol), and CS was ruled out. His OC level was 14.4 nmol l−1.
Among the subjects with CS confirmed by the histological evaluation after neurosurgery, the results of LNSC were false negative in six cases (3.95; 5.8; 5.6; 6.9; 3.9; 7.7 nmol l−1); the DST was true positive for CS in all of these cases (200; 573; 73; 292; 642; 446 nmol l−1), and the 24hUFC levels were elevated. The consequent individual values for the OC levels were as follows: 10.2; 6.3; 23.5; 11.6; 46.3; 6.4 ng ml−1. We analyzed the two unusually high values of OC. An OC level of 23.5 ng ml−1 was registered in a woman 64 years old with a BMI of 38 kg m−2 who was suffering from mild Cushing's disease. Contrary to the cohort of subjects referred due to osteoporosis, this woman did not have any fractures or BMD decline. An OC level of 46.3 ng ml−1 was found in a woman 19 years old with a BMI of 37 kg m−2 who was suffering from mild Cushing's disease. It is likely that in these cases of mild CS, the elevation of bone markers that is typical for age remained undisturbed.
Because there were inconsistent results in two excluded cases, their data were not analyzed and are not provided.
Diagnostic performance of serum OC levels to differentiate patients older than 45 with osteoporosis and CS from postmenopausal osteoporosis
The serum OC levels were investigated in 23 subjects older than 45 years with osteoporosis and established active CS (adrenal CS in 3 cases, ACTH-ectopic CS in 3 and Cushing's disease in 17), and in 67 women with newly diagnosed postmenopausal osteoporosis (PMO).
The general characteristics of the participants with osteoporosis are presented in Table 2.
Table 2. General characteristic of enrolled patients with postmenopausal osteoporosis and Cushing's syndrome older than 45 suffering from osteoporosis (low traumatic fracture or/and BMD T-score ⩽−2.5).
| Cushing's syndrome Me (Q25–Q75) | Postmenopausal osteoporosis Me (Q25–Q75) | P-value | |
|---|---|---|---|
| N (number) | 23 | 67 | |
| Age (years) | 52 (51–56) | 69 (61–73) | <0.001 |
| BMI (kg m−2) | 28 (26–33) | 24 (20–27) | <0.001 |
| Number of patients with low traumatic fractures (%) | 20 (87%) | 36 (54%) | <0.001 |
| Vertebral | 17 | 14 | |
| Non-vertebral | 7 (1 hip fracture) | 20 (4 hip fracture) | |
| L1–L4 T-score | −1.4 (−2.1 to −0.4) | −2.9 (−3.7 to −2.5) | <0.001 |
| Neck T-score | −1.5 (−1.7 to −0.9) | −2.4 (−2.8 to −1.8) | <0.001 |
| Osteocalcin (ng ml−1) | 4.0 (2.6–7.2) | 23.2 (15.8–23.4) | <0.001 |
Abbreviations: BMD, bone mineral density; Me, median, Q25–Q75, interquartile range.
Patients with CS differed from consecutive newly diagnosed subjects with PMO in regard to many parameters. The patients with CS were younger and the BMD loss was mild compared with subjects with PMO; however, the prevalence of fracture was very high (87%) compared with women with ordinary PMO (54%). All subjects with CS had amenorrhea, the cause of which was not identified and could be due to CS or menopause. The most specific difference between these groups of patients concerned the OC levels, and this difference was recognizable even in the individual patient data (Figure 3). The optimal threshold for the OC levels of 8.3 ng ml−1 showed a sensitivity of 95.6% (95% CI 79.0–99.2%) and a specificity of 98.5% (95% CI 92.0–99.7%). The ROC curve analysis for the OC levels showed an AUC=0.960 (95% CI 0.892–1.00; Figure 4).
Figure 3.
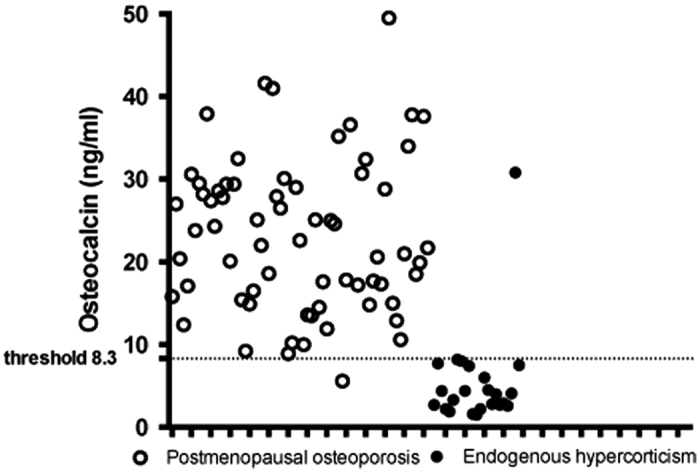
Individual serum osteocalcin (OC) values in patients with Cushing's syndrome (CS) older than 45 suffering from osteoporosis and in women with newly diagnosed postmenopausal osteoporosis (PMO). The optimal threshold for OC levels of 8.3 ng ml−1 with 1 false-positive and 1 false-negative result showed a sensitivity of 95.6% (95% CI 79.0–99.2%) and a specificity of 98.5% (95% CI 92.0–99.7%) to differentiate women older than 45 with CS and osteoporosis from subjects with postmenopausal osteoporosis.
Figure 4.
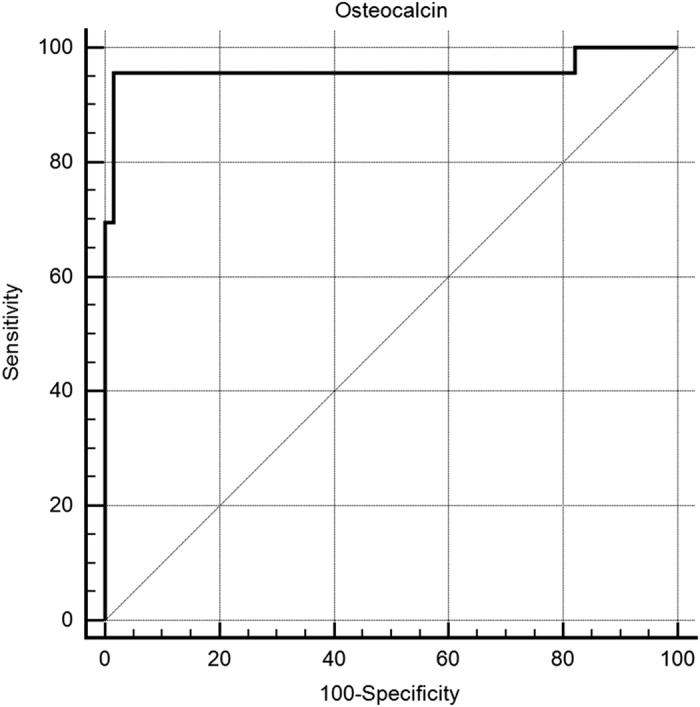
Receiver-operator characteristic curves of osteocalcin measurements to differentiate Cushing's syndrome among subjects with osteoporosis who were older than 45. The receiver-operator characteristic curve (ROC) analysis for OC levels showed an area under the curve (AUC)=0.960 (95% CI 0.892–1.00) among subjects with osteoporosis who were older than 45.
The diagnostic indices for the OC thresholds in both cohorts of patients with obesity and osteoporosis are summarized in Table 3.
Table 3. Diagnostic performance of serum osteocalcin measurements by ECLIA (Roche Cobas e601).
| Prospective evaluation of Cushing's syndrome patients among obese and overweight subjects | Retrospective evaluation of patients with CS older than 45 with osteoporosis versus newly diagnosed postmenopausal osteoporosis | |
|---|---|---|
| Threshold for serum osteocalcin value (ng ml−1) | 8.3 | 8.3 |
| Sensitivity (95% CI) | 87.2% (73.3–94.4) | 95.6% (79.0–99.2) |
| Specificity (95% CI) | 98.5% (92.1–99.7) | 98.5% (92.0–99.7) |
| Positive predictive value (95% CI) | 59.2 (8.4–416.3) | 64.1 (9.1–449.2) |
| Negative predictive value (95% CI) | 0.13 (0.057–0.295) | 0.044 (0.006–0.3) |
| Likelihood ratio for positive result (95% CI) | 0.13 (0.057–0.295) | 1452 (87.1–24201.9) |
| Area under the curve AUC (95% CI) | 0.859 (0.773–0.945) | 0.960 (0.892–1.00) |
Abbreviations: AUC, area under the curve; CI, confident interval; ECLIA, electrochemiluminescence immunoassay.
Discussion
This is the first study evaluating the diagnostic performance of OC in the most challenging conditions by identifying CS in consecutive obese and overweight patients and elderly patients with osteoporosis. OC was slightly less effective in differentiating CS among patients with obesity compared with usual testing methods (LNSC, DST); nevertheless, OC appears promising in differentiating CS among patients with osteoporosis who were older than 45. This study cohort comprised patients whose BTMs might be assessed by various specialists before treating osteoporosis. In a previous study, Sereg et al.17 proved that serum OC is effective in differentiating overt CS among healthy subjects and inactive adrenal adenomas with an AUC of 0.922 (95% CI 0.860–0.984), which was statistically significantly better compared with morning cortisol in serum or in saliva and did not differ from LNSC or late-night serum cortisol or DST and 24hUFC. Contrary to the result of Sereg et al.,17 the diagnostic performance of OC measurement was lower compared with LNSC or DST in our study. These findings might be explained by the functional hypercortisolism reported in patients with obesity, which makes differential diagnosis from CS more challenging compared with healthy control subjects or subjects suffering inactive benign adrenal adenomas. The difference in the studied populations also explains the variation in suggested threshold values; the values were 8.3 ng ml−1 in our study compared with 12.9 ng ml−1 determined in the study of Sereg et al.17 The lower threshold value yielded slightly worse sensitivity but better specificity compared with that previously reported. Numerous studies evaluating bone complications in patients with CS reported low bone formation markers compared with healthy control subjects. Although the clinical presentation of overt CS is usually typical in a younger population, the presentation could be rather masked in elderly individuals manifesting with severe osteoporosis or other complications of CS. BTMs are elevated in postmenopausal women compared with women of premenopausal age. However, in the present study, the OC remained low in subjects with CS of postmenopausal age, indicating that the difference in OC levels was even more prominent in the elderly population. Consequently, retrospective analyses of OC measurements in patients of postmenopausal age suffering from osteoporosis demonstrated an excellent diagnostic performance for this marker to allow for the identification of patients with endogenous CS and osteoporosis.
OC measurements have certain limitations. Given that OC is rapidly degraded in vivo and in vitro, it is present as an intact molecule (1–49) or N-mid 1–43 fragment or can be undercarcarboxylated;22 consequently, the developed threshold is valid for the used assay only. The thresholds suggested for automated ECLIA by Sereg et al.17 and in our study were very close, supporting the reliability of the automated assay. In addition, the concentration of OC in the serum depends on the time of blood sample collection, reaching a maximum at 0400 hours, and is not dependent on food consumption during the previous day.23 The serum OC concentration decreases if samples are frozen or in the event of blood hemolysis,24,25 giving advantages to automated assays that do not require sampling storage for optimal kit usage. Moreover, OC most likely loses its diagnostic accuracy in patients who have already received anabolic or antiresorptive treatment for osteoporosis.
Genetic and pharmacological experiments in mice have shown OC to be a hormone that increases insulin production and sensitivity, enhancing glucose utilization and energy expenditure,26 making OC different from other bone formation markers such as procollagen type I N-propeptide (PINP). However, PINP is usually also suppressed in patients with CS, and its diagnostic accuracy in this rare disorder might be the subject of future research.27
The decrease in OC levels is highly specific for patients with endogenous hypercortisolism. Although the diagnostic performance of OC measurement proves less accurate compared with LNSC and DST, this method could be used as a diagnostic test in patients with CS among obese individuals referred to exclude CS and should be explored as even more promising in differentiating CS among patients with osteoporosis of postmenopausal age.
Materials and methods
The Institutional Review Board of the National Research Centre for Endocrinology (NRCE) approved the study protocol.
Patients with clinical findings suggestive of CS were unrestrictedly referred to the NRCE by clinicians from Moscow and other regions of Russia between January 2010 and January 2012.
The study consisted of two parts, and two separate populations of patients were enrolled.
In the prospective portion of our research, consecutive patients who were suspected by their physician of having CS and who complained of obesity (BMI ⩾30 kg m−2) or sudden weight gain to a BMI of 26–29 kg m−2 were invited to participate. Patients who gave informed consent were enrolled in the study (n=106).
In the retrospective portion of the study, OC levels were analyzed in 23 consecutive patients older than 45 years with biochemically proven CS and osteoporosis defined by either low traumatic fracture or BMD T-score ⩽–2.5 by DXA and in 64 newly diagnosed women with PMO (low traumatic fracture or BMD T-score ⩽–2.5 DXA) who were under observation and receiving treatment in the NRCE.
There were completely different cohorts of patients. None of the subjects with obesity and highly suspected CS were included in the evaluation of subjects with osteoporosis either of PMO or women older than 45 having CS.
The exclusion criteria for both parts of the study were as follows: pregnancy, glucocorticoid use, alcohol abuse, gingival bleeding, acute infection, exacerbation of chronic disease, severe conditions (that is, renal and liver insufficiency, heart attack, stroke, terminal conditions), mental insanity, prolonged immobilization (>1 week), any other cause of secondary osteoporosis28,29 presently or within a 2-year medical history or any prolonged treatment with drugs documented to influence bone metabolism in humans during the previous 12 months,28,29 including treatment for osteoporosis with antiresorptive or anabolic compounds, or treatment to resolve hypercortisolism.
Diagnostic evaluations followed recent clinical practice guidelines.19 The initial use of LNSC measured by ECLIA (reference range 0.5–9.4 nmol l−1)20 was independently followed by DST (cutoff value forsuppression: 50 nmol l−1).19 Any patients with discordant results from the initial evaluations underwent an additional third or fourth test: 24hUFC (reference range 60–413 nmol per 24 h) and/or awake serum cortisol at 2300 hours (reference range 46–270 nmol l−1).
All subjects provided fasting (0800–0900 hours) blood samples to establish OC levels. The serum OC was measured by ECLIA on an automatic analyzer Roche Cobas e 601 (Hoffmann-La Roche diagnostic kit N-Mid OC catalog number 12212149133).
Serum and saliva samples were measured for cortisol levels using the Cobas e601 ECLIA kit.
The final diagnosis of CS was confirmed by histological evaluation, with the histological material obtained after surgery being sufficient to confirm CS in all patients.
Patients in whom CS was excluded were under observation for an average of 6 months (minimum: 3 months; maximum: 12 months) in our center or in the practice of the referring physicians and received appropriate treatment for constitutional obesity or osteoporosis; during this time, we could observe the absence of, or progression towards, overt CS.
In two cases, we could not positively verify the diagnosis, and the data were excluded and are not presented.
BMD and fracture assessment
At the time of enrollment, all participants were questioned regarding any recent low traumatic fractures, back pain and height changes. The height was measured by stadiometre, and the BMI was calculated as kilograms per meter squared.
Patients with CS and PMO underwent standard spinal radiographs in anterior–posterior and lateral positions of the vertebrae Th4–L4 (Axiom Icons R200 ‘Siemens'). A deformity was considered a fracture if the visual inspection perceived a reduction in vertebral height (anterior, posterior or middle) of 20% or more.30
The BMD was measured by DXA Prodigy Lunar in patients with CS and PMO at the anteroposterior lumbar spine (L1–L4) and femoral neck positions according to the standard protocol. Quality control procedures were carried out in accordance with the manufacturers' recommendations.
Statistical analysis
Descriptive statistics: quantitative parameters are presented as median values and ranges (Q25–Q75), and qualitative parameters are presented as percentages and binomial 95% CIs. Because normality tests (skewness and kurtosis) rejected normality for the majority of quantitative parameters, non-parametric tests were utilized. The Mann–Whitney U-test was utilized to compare quantitative parameters in two independent samples. A two-tailed approach for the calculation of P-value was utilized. A P-value <0.05 was considered statistically significant. Spearman's rank test was used for correlations. The threshold for OC was selected to achieve maximum diagnostic accuracy (maximum sum of sensitivity (proportion of true positives correctly identified by testing) and specificity (proportion of true negatives correctly identified by testing) values)) obtained from the ROC analysis.31 The positive predictive value (the chance of disease given a positive result; number of cases true positive by testing/(number of cases true positive by testing+number of cases false positive by testing)), the negative predictive value (the chance of no disease given a negative result; number of cases true negative by testing/(number of cases true negative by testing+number of cases false negative by testing)), the likelihood ratio for a positive result (the likelihood of having the disease, as opposed to not having the disease, having tested positive for it; sensitivity/(1−specificity)) and sensitivity and specificity for earlier predefined threshold17 were calculated as generally recommended.32 The total areas under the ROC curve (AUC) were measured to represent the probability of the tests correctly identifying true positives and negatives. The AUC values of the different tests (OC, LNSC (ECLIA) and DST) were directly compared with the ROC curve.33
SPSS 16.0 (IBM, Armonk, NY, USA) and Med Calc MedCalc(C) Version 10.4.6.0 (MedCalc Software bvba, Ostend, Belgium) software were used for the analyses.
Acknowledgments
Russian Scientific Foundation No 15-15-30032.
Footnotes
The authors declare no conflict of interest.
References
- Gossiel F, Finigan J, Jacques R, Reid D, Felsenberg D, Roux C et al. Establishing reference intervals for bone turnover markers in healthy postmenopausal women in a nonfasting state. BoneKey Rep 2014; 3: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. J Osteoporos Int 2011; 22: 391–420. [DOI] [PubMed] [Google Scholar]
- Johnell O, Oden A, De Laet C, Garnero P, Delmas PD, Kanis JA. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int 2002; 13: 523–526. [DOI] [PubMed] [Google Scholar]
- Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: The OFELY Study. J Bone Miner Res 2005; 20: 1813–1819. [DOI] [PubMed] [Google Scholar]
- Vasikaran SD. Utility of biochemical markers of bone turnover and bone mineral density in management of osteoporosis. Crit Rev Clin Lab Sci 2008; 45: 221–258. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the international Osteoporosis Foundation. Osteoporos Int 2000; 11: (suppl 6): s2–s17. [DOI] [PubMed] [Google Scholar]
- Wheater G, Elshaly M, Tuck SP, Datta HK, Laar JM. The clinical utility of bone marker measurements in osteoporosis. J Translat Med 2013; 11: 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Reginster JY, Crans GG, Diez-Perez A, Pinette KV, Delmas PD. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res 2004; 19: 394–401. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Sipos A, Jiang Y, Fahrleitner-Pammer A, Ste-Marie LG, Gallagher JC et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab 2005; 90: 3970–3977. [DOI] [PubMed] [Google Scholar]
- Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 2004; 89: 1117–1123. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Vrijens B, Eastell R, Roux C, Pols HA, Ringe JD et al. Improving Measurements of Persistence on Actonel Treatment (IMPACT) Investigators. Effect of monitoring bone turnover markers on persistence with risendronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 2007; 92: 1296–1304. [DOI] [PubMed] [Google Scholar]
- Lukert BP, Higgins JC, Stoskopf MM. Serum osteocalcin is increased in patients with hyperthyroidism and decreased in patients receiving glucocorticoids. J Clin Endocrinol Metab 1986; 62: 1056–1058. [DOI] [PubMed] [Google Scholar]
- Barahona MJ, Sucunza N, Resmini E, Fermandez-Real JM, Ricart W, Moreno-Navarrete JM et al. Deletorious effects of glucocorticoid replacement on bone in women after long-term remission of Cushing's syndrome. J Bone Miner Res 2009; 24: 1841–1846. [DOI] [PubMed] [Google Scholar]
- Sartorio A, Conti A, Ferrario S, Passini E, Re T, Ambrosi B. Serum bone Gla protein and carboxyterminal cross-linked telopeptide of type I collagen in patients with Cushing's syndrome. Postgrad Med J 1996; 72: 419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szappanos A, Toke J, Lippai D, Patocs A, Igaz P, Szucs N et al. Bone turnover in patients with endogenous Cushing's syndrome before and after successful treatment. Osteoporos Int 2010; 21: 637–645. [DOI] [PubMed] [Google Scholar]
- Kristo C, Jemtland R, Ueland T, Godang K, Bollerslev J. Restoration of the coupling process and normalization of bone mass following successful treatment of endogenous Cushing's syndrome: a prospective, long-term study. Eur J Endocrinol 2008; 154: 109–118. [DOI] [PubMed] [Google Scholar]
- Sereg M, Toke J, Patocs A, Varga I, Igaz P, Szucs N et al. Diagnostic performance of salivary cortisol and serum osteocalcin measurements in patients with overt and subclinical Cushing's syndrome. Steroids 2011; 76: 38–42. [DOI] [PubMed] [Google Scholar]
- Baid SM, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for Cushing's syndrome in an overweight and obese population. J Clin Endocrinol Metab 2009; 94: 3857–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman LK, Biller BMK, Finding JW, Newell-Price J, Savage MO, Stewart PM et al. The diagnosis of Cushing's syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008; 93: 1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaya ZE, Iljin AV, Melnichenko GA, Rozhinskaya LY, Dragunova NV, Dzeranova LK et al. Diagnostic performance of late-night salivary cortisol measured by automated electrochemiluminescence immunoassay in obese and overweight patients referred to exclude Cushing's syndrome. Endocrine 2012; 41: 494–500. [DOI] [PubMed] [Google Scholar]
- Chiodini I, Mascia ML, Muscarella S, Battista C, Minisola S, Arosio M et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med 2007; 147: 541–548. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Christiancen C, Mann KG, Price PA. Bone Gla protein (osteocalcin) assay standardization report. J Bone Miner Res 1990; 5: 5–11. [DOI] [PubMed] [Google Scholar]
- Schlemmer A, Hassager C. Acute fasting diminishes the circadian rhythm of biochemical markers of bone resorption. Eur J Endocrinol 1990; 140: 332–337. [DOI] [PubMed] [Google Scholar]
- Gundberg CM, Wilson PS, Gallop PM, Parfitt AM. Determination of osteocalcin in human serum: results with two kits compared with those by a well-characterized assay. Clin Chem 1985; 31: 1720–1730. [PubMed] [Google Scholar]
- Diaz Diego EM, Guerrero R, de la Piedra C. Six osteocalcin assays compared. Clin Chem 1994; 40: 2071–2077. [PubMed] [Google Scholar]
- Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab 2009; 20: 230–236. [DOI] [PubMed] [Google Scholar]
- Gifre L, Ruiz-Gaspà SA, Nomdedeu MB, Filella X, Guañabens N, Peris P. Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone 2013; 57: 272–276. [DOI] [PubMed] [Google Scholar]
- Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF et al. AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010; 16(Suppl 3): 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizolli R, Reginster R. on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2013; 24: 23–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genant HK, Wu CY, van Kujik C, Nevitt MC. Vertebral fracture assessment using a semiquantative technique. J Bone Miner Res 1993; 8: 1137–1148. [DOI] [PubMed] [Google Scholar]
- Zou KH, O'Maley J, Mauri L. Receiver-Operating Characteristic Analysis for evaluating diagnostic tests and predictive models. Circulation 2007; 115: 654–657. [DOI] [PubMed] [Google Scholar]
- Ajetunmobi O. Making sense of critical appraisal Hodder Arnold part of Hachette UK2002; 69–84.
- Hanley JA, McNeil BJ. 1983 A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983; 148: 839–843. [DOI] [PubMed] [Google Scholar]


