Abstract
How are emotional processes associated with the increased rates of substance use and psychological disorders commonly observed during adolescence? An index of emotion-related physiological arousal—cortisol reactivity—and subjective emotion regulation have both been independently linked to substance use and psychological difficulties among youth. The current study (N = 134 adolescents) sought to elucidate the interactive effects of cortisol reactivity following a stressful parent–child interaction task and self-reported emotion regulation ability on adolescents’ substance use and externalizing and internalizing behavior problems. Results revealed that adolescents with low levels of cortisol reactivity and high emotion regulation difficulties were more likely to use substances, and also had the highest parent-reported symptoms of oppositional defiant disorder. With respect to internalizing symptoms, high emotion-related physiological reactivity coupled with high emotion regulation difficulties were associated with higher self-reported major depression symptoms among youth. Findings reveal that different profiles of HPA axis arousal and emotion regulation are associated with substance use and symptoms of psychopathology among adolescents.
Keywords: Adolescence, Emotion regulation, Emotional arousal, Emotional reactivity, Cortisol, Substance use, Externalizing, Internalizing
Introduction
How are emotional processes associated with the increased rates of substance use and psychological disorders commonly observed during adolescence? Which emotion-related components (e.g., arousal, regulation)—or interaction of components—are most strongly implicated in the development of maladaptive behaviors (e.g., substance use) and symptoms of psychopathology among youth? These are critical questions that have perplexed clinical and developmental researchers for decades. Adolescence is a developmental period characterized by a number of cognitive, neurobiological, social, and emotional changes. At the same time, it is also a period of initiation and increases in drug and alcohol use and increases in several types of externalizing and internalizing disorders, such as conduct disorder and major depressive disorder (Chambers et al. 2003; Costello et al. 2003). Substance use among youth is a significant public health concern (Paglia and Room 1999), with 1-month rates of alcohol, cigarette, and illicit drug use among adolescents increasing from 7–14 % in eighth grade to 24–41 % by 12th grade (Johnston et al. 2011). Adolescent substance use has significant consequences—it is associated with academic problems, impaired driving, violent behaviors, and risky sexual behavior in adolescence (Windle et al. 2008) and is also related to substance use disorders and other forms of psychopathology in adulthood (Chassin et al. 2001; Lewinsohn et al. 1996). Many adolescents who use substances also have other comorbid psychological problems, including externalizing problems such as oppositional defiant disorder (ODD) and conduct disorder (CD) and internalizing problems such as major depressive disorder and anxiety disorders (Chan et al. 2008; Kandel et al. 1999).
The NIMH has recently launched an initiative aimed at transcending traditionally-delineated diagnostic categories and, instead, investigating dimensions of functioning implicated across many clinical phenomena at multiple units of analysis (RDoC; Insel et al. 2010). Emotion in particular has been conceptualized as a transdiagnostic factor; it is involved in multiple forms of psychopathology (Aldao et al. 2010; Harvey et al. 2004) and is manifested across several domains, including physiological, neurobiological, and behavioral systems (Mauss et al. 2005). In addition, emotional processes in different systems may interact with one another (e.g., Eisenberg et al. 1996). A transdiagnostic perspective may also better account for the high levels of comorbidity observed among disorders, especially given that dysfunctional emotion-related processes have been associated with substance use and also most psychological disorders (Kring 2008). Adolescents in particular experience rapid changes in emotion regulatory systems and also show high rates of comorbidity (Chan et al. 2008; Kandel et al. 1999; Steinberg 2007), making this a critical developmental period for links between emotion and the development of multiple forms of psychological problems. The current study addresses this by examining the interaction between one aspect of adolescents’ emotion-related physiological arousal (HPA axis reactivity) and adolescent’s self-reported emotion regulation behaviors as these relate to their substance use and psychological symptoms.
Emotion-Related Physiological Arousal and Emotion Regulation
Emotions have been defined as short-lived, affectively-valenced reactions to meaningful stimuli, with accompanying changes in subjective experience, behavioral tendencies, and peripheral physiology, including changes in skin conductance, heart rate, blood pressure, and HPA axis activation (Mauss et al. 2005; Mauss and Robinson 2009). HPA axis activation and the release of cortisol is one form of physiological arousal that has been associated with several forms of negative emotional arousal in childhood and adolescence including fear, sadness, crying, and anger arousal (Adam 2006; Baucom et al. 2012; Fortunato et al. 2008). HPA axis activation appears to be especially linked to responses to threatening situations (Dickerson et al. 2008; Dickerson and Kemeny 2004; Gruenewald et al. 2004; Rohleder et al. 2007; Gunnar and Quevedo 2007). In addition, daily cortisol levels and cortisol reactivity to stress have been linked in several studies to both substance use and to several forms of externalizing and internalizing psychological problems in adolescence (e.g., Chaplin et al. 2012, 2014; Flinn and England 1995; Klimes-Dougan et al. 2001). Thus, given that the HPA axis responds to emotional and stressful events and given that cortisol reactivity is strongly linked to both substance use and psychopathology, it is important to examine cortisol reactivity in association with substance use and externalizing and internalizing problems in adolescence.
In addition to direct links between cortisol reactivity and psychological problems in adolescence, adolescents’ emotion regulation behaviors may moderate links between HPA axis arousal and the development of substance use and psychopathology. For example, an adolescent with high levels of cortisol reactivity to a stressor may be more likely to then develop psychological problems if they use dysfunctional emotion regulation strategies to regulate their high reactivity. A youth who experiences high cortisol reactivity and responds to that reactivity by ruminating on negative emotion may, over time, develop depression in response to emotion-eliciting stressful events (Nolan-Hoeksema 1991). However, no study to our knowledge has examined interactions between cortisol reactivity and emotion regulation behaviors in predicting negative outcomes for adolescents.
The Role of Cortisol Reactivity and Emotion Regulation in Substance Use
Although no research to date has examined the interactive effects of cortisol reactivity and emotion regulation on substance use behaviors among youth, a small but growing body of research has documented direct links between cortisol stress reactivity and substance use in adolescents. These initial studies have found that cortisol reactivity is linked to substance use, with some of the studies finding higher cortisol reactivity (Chaplin et al. 2012, 2014) and others finding lower cortisol reactivity (van Leeuwen et al. 2011) related to increased past and current substance use behaviors.
In addition to altered cortisol stress responses, emotion regulation problems are also independently linked to adolescent substance use. For example, research with both adults and adolescents finds that substance users report that their most common reasons for choosing to use substances is to decrease negative affect, increase positive affect, or otherwise modulate the quality of their emotional experiences (e.g., Cahalan et al. 1969; Cooper et al. 1995). In addition, studies have found links between self-reported emotion regulation difficulties and increased alcohol use, tobacco, use, drug use, and drug use disorders in adolescents (Weinberg and Klonsky 2009; Wilens et al. 2013). Based on these findings, it is possible that adolescents with both altered cortisol stress responses and impoverished emotion regulation abilities will be at greatest risk for substance use.
The Role of Cortisol Reactivity and Emotion Regulation in Externalizing Symptoms
Altered cortisol reactivity has also been linked to externalizing problems among youth. Several studies have found that children and adolescents who experience aggressive, delinquent, or antisocial behaviors evidence blunted cortisol reactivity to stressors (e.g., Flinn and England 1995; Moss et al. 1995; Tennes et al. 1986) and blunted basal cortisol levels (Tennes and Kreye 1985; Vanyukov et al. 1993). Low cortisol reactivity may reflect under-arousal of the HPA axis system, which may signify a lack of appropriate arousal in response to negative events (Gorenstein and Newman 1980; Moffitt 1993). Low cortisol reactivity among antisocial and aggressive children and adolescents may reflect attenuated noradrenergic functioning and a fearless, stimulation-seeking temperament (Raine 2002). However, other studies have not found evidence of HPA axis hypo-reactivity among youth with externalizing symptoms (e.g., Kruesi et al. 1989; McBurnett et al. 1991; Scerbo and Kolko 1994; Targum et al. 1990). Interestingly, one study found that adolescents with ODD and anxiety experienced increases in salivary cortisol following a stressful task (van Goozen et al. 1998), which could have been due to the comorbid anxiety symptoms.
Prior research has also documented relations between self-reported emotion regulation difficulties and externalizing symptoms in adolescence. Studies have found that children with attention-deficit/hyperactivity disorder (ADHD) evidence impaired emotional inhibition (Walcott and Landau 2004). In addition, problems with controlling impulsive angry behaviors (e.g., anger, irritability, fear) have been linked to aggression and conduct problems cross-sectionally (Eisenberg et al. 2001; Hubbard et al. 2002; Olson et al. 2005; Shields and Cicchetti. 1998; Silk et al. 2003) as well as longitudinally (Caspi 2000; Eisenberg et al. 1997).
The Role of Cortisol Reactivity and Emotion Regulation in Internalizing Symptoms
HPA axis reactivity to stress has also been linked to internalizing symptoms (such as depression and anxiety) among adolescents, with internalizing symptoms correlated with heightened cortisol reactivity to stressors (Birmaher et al. 1996; Hankin et al. 2010; Rao et al. 2008), including stressful conflict discussions with a parent (Granger et al. 1996; Klimes-Dougan et al. 2001). Furthermore, behaviorally inhibited young children (who are at risk for anxiety disorders) show high levels of basal cortisol, in addition to heightened cortisol responses to stress (Davis and Buss 2012; Kagan et al. 1987; Schmidt et al. 1997).
In addition to altered cortisol reactivity, emotion regulation difficulties have also been linked to internalizing symptoms in youth. For example, Eisenberg et al. (1996) found that children with internalizing symptoms experience sadness more frequently and more intensely, have lower emotional self-awareness, demonstrate poor attention regulation abilities, and are more likely to inhibit their displays of anger compared to controls and those with externalizing symptoms. Similarly, children with internalizing symptoms have been found to evidence deficits in emotional awareness, emotional expression, and emotion coping, all of which are important aspects of effective emotion regulation efforts (Zeman et al. 2002). Additionally, early adolescents with depressive symptoms experience greater emotional intensity and lability with regard to anger, sadness, and worry, which may reflect difficulties in emotion regulation (Silk et al. 2003). Thus, difficulties in regulating emotions may be linked to internalizing problems in childhood and adolescence (Zahn-Waxler et al. 2000).
Current Study
Substance use and externalizing and internalizing disorders are commonly comorbid, particularly in adolescence, but few studies have examined how cortisol reactivity and emotion regulation are related to all three outcomes and no study has examined whether emotion regulation moderates effects of cortisol reactivity on adolescent outcomes. Consistent with the stated goals of the RDoc, the primary goal of the current study was to examine whether adolescents’ self-reported emotion regulation behaviors moderate links between cortisol reactivity and symptoms of substance use and externalizing and internalizing psychopathology. We predicted that adolescents’ emotion regulation abilities would moderate the association between cortisol reactivity and substance use, externalizing, and internalizing symptoms. Specific hypotheses by outcome were as follows:
For substance use, we predict that adolescents who have altered cortisol reactivity and high emotion regulation difficulties will show increased substance use. Given the mixed prior findings on cortisol reactivity and substance use, the direction of association between cortisol reactivity and substance use was exploratory.
For externalizing symptoms, we predict that adolescents who evidence low cortisol reactivity and high emotion regulation difficulties will show greater parent-reported symptoms of ADHD, ODD, and CD.
For internalizing symptoms, we predict that adolescents who evidence high cortisol reactivity and high emotion regulation difficulties will show higher self-reported symptoms of depression and anxiety.
Methods
Participants
Participants included 134 adolescents ages 12–14 (M = 12.61, SD = 0.50; 45 % female) and their primary caregiver (98.3 % female). Most adolescents were Caucasian (71.4, 10.5 % African American, 11.2 % Hispanic, 3.0 % Asian American, 3.8 % mixed/other) and annual family incomes were 66.7 % over $100,000/year, 13.6 % between 75,000 and 99,999, 3.8 % between $60,000 and 74,999, 12.9 % below $60,000, and 3.1 % “don’t know/other.” Families with adolescents in a metropolitan area in the Northeastern region and a suburban area in a Mid-Atlantic region of the United States were recruited through newspaper advertisements, flyers, and mailings. Families were representative of their respective communities in terms of ethnicity and income level.
Procedure
Participants attended two sessions, spaced approximately 1 week apart. In the first session, adolescents and their parent (note: for the rest of the paper we will refer to primary caregivers as “parents”) completed questionnaires and interviews assessing adolescent psychopathology and other aspects of youth and parent life stress and social-emotional functioning. In the second session, adolescents and their parents participated in a parent–adolescent interaction session.
Parent–Adolescent Interaction Laboratory Session
The central part of the laboratory sessions was the parent–adolescent discussion task, which was based on conflict tasks performed in prior research (e.g., Sheeber et al. 1997). Laboratory sessions were scheduled at 4:00 pm to control for diurnal variation in cortisol. Youth and their parents refrained from eating during the session, were asked to refrain from alcohol or drug use in the day before the session, and menstruating girls were scheduled during days 5–10 of their cycle to control for effects on cortisol levels. In order to account for the effect of food intake on cortisol levels on the day of the interaction task, all participants ate immediately prior to coming into the lab. If they did not have a snack before the lab, they were given a snack when they arrived at the lab (about an hour prior to the baseline cortisol measurement). Thus, all participants ate 1–2 h before the parent–adolescent interaction task.
Upon arriving at the lab, adolescents and parents went to separate rooms, were seated in comfortable chairs, and met with separate research assistants who introduced the session, stating, “We are studying how parents and adolescents talk about everyday topics and about drug use.” At 4 pm, there was a 25-min adaptation period, during which time participants listened to a guided relaxation tape. At 4:25 pm, baseline salivary cortisol measurements were collected. At 4:30 pm, the parent was brought into the adolescent’s room and seated next to him/her. The parent and adolescent had the drug use discussion for 10 min and discussed a conflict topic for 10 min, with discussion order randomly assigned. For the conflict discussion portion of the discussion task, the parent and adolescent were asked to discuss their mutual highest-rated conflict issue from the Issues Checklist (IC; Prinz et al. 1979). The entire discussion task was videotaped. After the discussion task, parents returned to their rooms and measures of salivary cortisol were taken immediately post-task and every 15 min thereafter through a 60-min recovery period.
Measures
Cortisol Reactivity
For the current study, change in salivary cortisol levels following the parent–adolescent interaction was measured as a marker of adolescents’ physiological arousal. Consistent with past studies (Chaplin et al. 2012, 2014; Rudolph et al. 2010), a change score was computed such that the average of the first two timepoints (i.e., baseline) was subtracted from the score at the timepoint after the discussion when cortisol reactivity was at peak elevation (i.e., before recovery). Saliva was collected using a cotton swab which participants placed between their tongue and cheek for 2 min, or until adequately saturated. Saliva samples were assayed in duplicate using standard radioimmunoassay kits with no modifications (intra-assay coefficients of variation from 3.0 to 5.1 %). In concordance with prior studies (Buss et al. 2005), all cortisol scores were Winsorized prior to analysis. For purposes of the current study, an overall cortisol reactivity score was calculated by subtracting baseline cortisol (the average of the first two timepoints occurring before the discussion task) from each individual’s peak reactivity score (highest salivary cortisol value at any timepoint after the discussion task), which has been used as a valid measure of reactivity to an acute stressor (Buss et al. 2005; Chaplin et al. 2012, 2014; Rudolph et al. 2010).
Emotion Regulation
In the first questionnaire/interview session, adolescents reported on their own emotion regulation using the 36-item Difficulties in Emotion Regulation Scale (DERS; Gratz and Roemer 2004), which yields six subscales: nonacceptance of emotional responses (Non-acceptance), difficulties engaging in goal-directed behavior (Goals), impulse control difficulties (Impulse), lack of emotional awareness (Awareness), limited access to emotion regulation strategies (Strategies), and lack of emotional clarity (Clarity). Participants responded to items on a 5-point Likert scale (1 = almost never, 5 = almost always). The DERS total scale has shown high reliability and consistency (α = 0.93; Gratz and Roemer 2004) and demonstrated excellent reliability in the current study (α = 0.90). Higher scores reflected poorer emotion regulation.
Lifetime Substance Use
Lifetime substance use in adolescence was assessed with a combination of measures including, (1) self-report of any substance use (including alcohol, tobacco, marijuana, cocaine, and other drugs) on the Youth Risk Behavior Survey questionnaire (YRBS; Brener et al. 2002), (2) self-report in an interview using the Teen Addiction Severity Index interview (T-ASI; Kaminer et al. 1991), (3) urine screens using the Redicup (Redwood Toxicology) urine screen for opiates, cocaine, Tetrahydro-cannabinol, amphetamines, benzodiazapines, and the Nicotine Urine Test Kit test for cotinine, (4) alcohol breath tests using alcohol breathalyzers (BACTrack Select model) and (5) carbon monoxide breath test for inhaled tobacco use. Youth that endorsed or tested positively on any measure scored a 1 for lifetime substance use (20.3 %); those who did not received a score of 0 (79.7 %).
Internalizing Symptoms
Because adolescents are regarded as the most reliable reporters of their internalizing symptoms (Achenbach et al. 1987; Durbin 2010), adolescents rated their depressive symptoms on the widely used 27-item Children’s Depression Inventory (CDI; Kovacs 2004). Adolescents were asked to choose one of three statements that best described how they felt over the past 2 weeks, with each corresponding to an absence of symptoms, a mild or probable symptom, or a definite symptom. The CDI yields five subscales in addition to a total score: Negative Mood, Interpersonal Problems, Ineffectiveness, Anhedonia, and Negative Self-Esteem. Internal consistency for the current study was excellent (α = 0.91).
Adolescents also self-reported on their symptoms of anxiety on the 37-item Revised Children’s Manifest Anxiety Scale (RCMAS; Reynolds and Richmond 1985). The RCMAS yields three subscales: Physiological anxiety, Worry/oversensitivity, and Social concerns/concentration. The RCMAS has shown high reliability and validity among adolescent samples (Lee et al. 1988; Muris et al. 2002) and internal consistency for the current study was good (α = 0.83).
Externalizing Symptoms
Parents rated adolescent externalizing symptoms using the Child Symptom Inventory (CSI, Gadow and Sprafkin 1997). The CSI produces symptom count scores and dimensional symptom severity scores for most psychological disorders based on the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV, American Psychiatric Association 2000). Symptoms are rated on a scale from 0 “never” to 3 “very often.” Symptom severity scores for attention deficit hyperactivity disorder-combined (ADHD-C, α = 0.94), oppositional defiant disorder (ODD, α = 0.91), and conduct disorder (CD, α = 0.75) scales were examined. The CSI has been used with adolescents (Chaplin et al. 2012) and has demonstrated satisfactory psychometric properties (Sprafkin et al. 2002).
Data Analysis Plan
To address primary hypotheses, six analyses, each with one predictor (cortisol reactivity), one moderator (emotion regulation difficulties) and one of six outcomes (lifetime substance use, ADHD, ODD, CD, depression, anxiety) were conducted. First, means and standard deviations were calculated, and variables were inspected for normality. The total score for conduct disorder was positively skewed so an inverse transformation was applied. We examined as covariates any demographic variables that had been linked to our independent or dependent variables in the literature. Adolescent gender and race were chosen as covariates in subsequent analyses due to associations with emotional functioning in the literature (e.g., Desantis et al. 2007; Rudolph 2002; Zeman et al. 2006). Age has also been linked to emotional functioning and symptoms in the literature, but age differences are found when comparing youth across different developmental levels. Since this study examined a narrow age range only within one developmental period (early adolescence), we did not include age as a covariate. For the purpose of analyses, gender was dummy coded with 0 representing girls and 1 representing boys. Race was dummy coded with 0 representing White and 1 representing Black and other ethnic minorities. Linear regressions testing the main effects of cortisol reactivity and emotion regulation difficulties and the cortisol × emotion regulation interaction on each outcome were performed. For the binary outcome of lifetime substance use, logistic regression was utilized (Hayes and Matthes 2009). Following recommendations of Aiken and West (1991), we entered predictor variables hierarchically in order to assess the size of incremental effects, with covariates entered first, then main effects, followed by interaction terms. For linear regressions, we examined the change in the percentage of variance explained (ΔR2) as a measure of effect size, where 0.01 is small, 0.09 is medium, and 0.25 is large (Cohen 1998). For the logistic regression, we calculated odds ratios as a measure of effect size where 1.5 is small, 3.5 is medium, and 9 is large (Cohen 1998). Chi squared tests were also conducted to assess model fit for the logistic regression. Prior to all analyses, the predictor and moderator were mean-centered (Aiken and West 1991). Significant interactions were probed by examining simple slopes at −1 standard deviation and +1 standard deviation of the moderator using the PROCESS macro developed by Hayes (2013) in SPSS 18.0. In addition, regions of significance were examined for significant interactions.
Results
Descriptive Statistics and Preliminary Analyses
Table 1 presents the means, standard deviations, and bivariate correlations among primary study variables. Of the sample, 20.3 % were positive for lifetime substance use, which was overwhelmingly either alcohol (14.3 %) or tobacco (6.8 %). One of our participants had used marijuana and inhalants (0.8 %), one had used marijuana only (0.8 %), one had used inhalants only (0.8 %), and one had used opiates only (0.8 %). Relations with demographic variables showed that girls had higher levels than boys of depression (t [127] = 3.85, p <0.001) and anxiety (t [127] = 4.13, p <0.001). White participants had lower levels than non-white participants of substance use (x2 [1] = 12.52, p <.001), depression (t [127] = −2.83, p <0.01), and anxiety (t [127] = −2.30, p <0.05).
Table 1.
Mean, standard deviations, and bivariate correlations among study variables
| Mean (SD) | Correlation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1. Cortisol baseline | 0.153 (0.09) | 1 | |||||||||
| 2. Cortisol peak | 0.133 (0.07) | 0.587** | 1 | ||||||||
| 3. Cortisol reactivity | −0.022 (0.07) | −0.693** | 0.177* | 1 | |||||||
| 4. DERS | 75.15 (20.16) | −0.215* | −0.063 | 0.186* | 1 | ||||||
| 5. SU | 0.37 (0.49) | −0.077 | −0.053 | 0.059 | 0.169† | 1 | |||||
| 6. ADHDC | 31.66 (10.41) | 0.013 | −0.087 | −0.084 | 0.189* | 0.093 | 1 | ||||
| 7. ODD | 13.08 (4.44) | −0.054 | −0.093 | −0.019 | 0.258** | 0.257** | 0.478** | 1 | |||
| 8. CD | −0.06 (0.01) | −0.127 | −0.110 | 0.053 | 0.104 | 0.290** | 0.308** | 0.568* | 1 | ||
| 9. Dep | 33.98 (7.27) | −0.099 | 0.098 | 0.211* | 0.630** | 0.187* | 0.154† | 0.218* | 0.179* | 1 | |
| 10. Anx | 6.88 (5.68) | −0.186* | 0.037 | 0.257** | 0.577** | 0.122 | 0.193* | 0.197* | 0.220* | 0.786** | |
SD standard deviation, DERS difficulties in emotion regulation, SU substance use, ODD oppositional defiant disorder, ADHD-C attention-deficit hyperactivity disorder-combined, Dep depression, Anx anxiety
p <0.10;
p <0.05;
p <0.01
Eight participants were missing data on at least one primary study variable. Participants with missing data were not significantly different with respect to age and gender (p’s <0.10). Cases with missing data were excluded from regression analyses including that variable.
Regression Analyses
Analyses shown in Table 2 provide the main and interactive effects of cortisol reactivity and emotion regulation difficulties on each outcome controlling for gender and race.
Table 2.
Unstandardized regression coefficients controlling for gender and race
| SU | ODD | ADHD-C | CD | Depression | Anxiety | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| B (SE) | X2 | B (SE) | ΔR2 | B (SE) | ΔR2 | B (SE) | R2 | B (SE) | ΔR2 | B (SE) | ΔR2 | |
| Step 1 | 1.80 | 0.01 | 0.02 | 0.00 | 0.14** | 0.14** | ||||||
| Gender | −0.30 (0.47) | 0.15 (0.78) | 3.05 (1.88) | 0.01 (0.37) | −2.77 (1.00)** | −2.75 (0.83)** | ||||||
| Race | 0.05 (0.51) | 1.35 (0.89) | 2.43 (2.13) | 0.13 (0.42) | 1.44 (1.13) | 0.15 (0.94) | ||||||
| Step 2 | 2.68 | 0.06* | 0.06* | 0.01 | 0.31** | 0.28** | ||||||
| DERS | 0.03 (0.01)* | 0.08 (0.02)** | 0.16 (0.05)** | 0.01 (0.01) | 0.18 (0.03)** | 0.13 (0.02) ** | ||||||
| Cortisol | 0.02 (3.51) | −11.68 (5.65)* | −29.12 (13.58)* | −0.14 (2.66) | 14.36 (7.24)† | 14.54 (6.01) * | ||||||
| Step 3 | 4.89* | 0.06** | 0.03† | 0.00 | 0.03** | 0.01 | ||||||
| DERS × cortisol | −0.36 (0.18)* | −0.74 (0.26)** | −1.15 (0.62)† | −0.12 (0.12) | 0.89 (0.33)** | 0.27 (0.28) | ||||||
Gender and race were both dummy coded (0 = girl; 1 = boy; 0 = White, 1 = Black and other minorities)
SE standard error, DERS difficulties in emotion regulation, SU substance use, ODD oppositional defiant disorder, ADHD-C attention-deficit hyperactivity disorder-combined, CD conduct disorder
< p 0.10;
p <0.05;
p <0.01
Relations with Substance Use
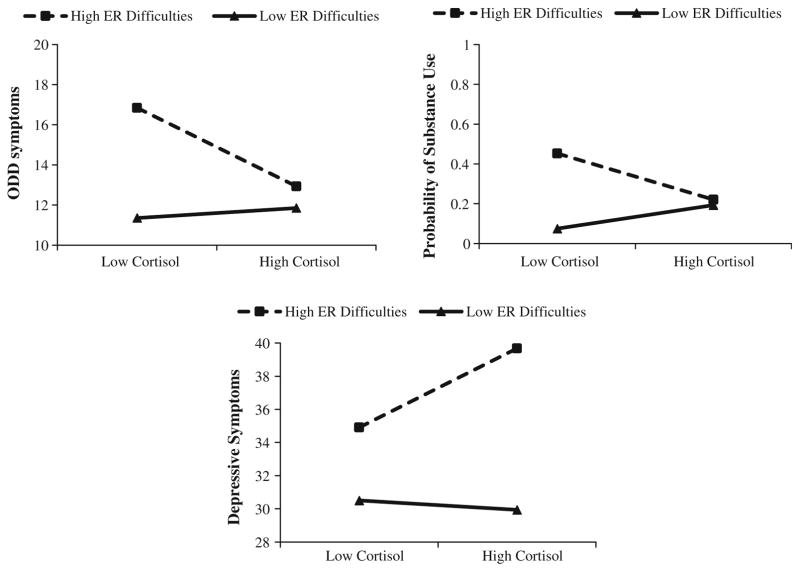
It was hypothesized that altered cortisol reactivity and high emotion regulation difficulties would predict substance use behaviors. Given the mixed prior findings on cortisol reactivity and substance use, the direction of association between cortisol reactivity and substance use was exploratory. As shown in Table 2, logistic regression analyses revealed a significant cortisol × emotion regulation interaction for substance use, (b = −0.36, SE = 0.18, p <0.05, OR = 0.70), which represented a small effect size. A significant Chi square value indicated the interaction model fit the data better than the main effects model (x2 (1) = 4.89, p <0.05. Follow-up simple slope analyses at +1 SD and −1 SD were nonsignificant (b = −7.28, SE = 5.40, p = 0.18; b = 7.38, SE = 4.74, p = 0.12, respectively), and no regions of significance were identified.1 However, Fig. 1 shows that adolescents with low levels of cortisol reactivity and high levels of emotion regulation difficulties were most likely to have used substances. In addition, the emotion regulation difficulties main effect was significant, with higher emotion regulation difficulties related to substance use.
Fig. 1.
Cortisol reactivity × emotion regulation difficulties interaction predicting adolescent oppositional defiant symptoms, substance use, and depressive symptoms. ER emotion regulation. Note High = +1 SD above the mean; Low = −1 SD below the mean
Relations with Externalizing Symptoms
It was hypothesized that lower levels of cortisol reactivity paired with high emotion regulation difficulties would predict externalizing symptoms. For ODD, linear regressions showed a significant cortisol reactivity × emotion regulation interaction, b = −0.74, SE = 0.26, p <0.01. The interaction accounted for 6 % of the variance explained beyond that accounted for by the main effects (ΔR2 = 0.06), representing a small to medium effect size. The simple slope for adolescents with high levels of emotion regulation difficulties (+1 SD: b = −26.63, SE = 8.90, p <0.01) was significantly different than zero (Fig. 1). The simple slope for adolescents with low levels of emotion regulation difficulties (−1 SD: b = 3.38, SE = 6.22, p = 0.54) was non-significant. As hypothesized, similar to patterns with substance use, the simple slope indicated that for adolescents with high levels of emotion regulation difficulties, lower cortisol reactivity was associated with ODD symptoms. The region of significance for this conditional effect was emotion regulation difficulties of −0.87 units (0.04 SD) and higher. In addition, cortisol reactivity and emotion regulation difficulties main effects were significant, with lower cortisol and higher emotion regulation difficulties related to ODD symptoms.2
With respect to ADHD, linear regressions revealed a marginally significant cortisol × emotion regulation interaction for ADHD, b = −1.15, SE = 0.62, p = 0.07; however, this effect did not account for significant variance beyond that accounted for by the main effects. Main effects of cortisol reactivity and emotion regulation difficulties were significantly related to ADHD with lower cortisol levels and higher emotion regulation difficulties related to ADHD symptoms. Finally, main and interactive effects for CD were nonsignificant.
Relations with Internalizing Symptoms
It was hypothesized that higher levels of cortisol reactivity paired with high emotion regulation difficulties would predict internalizing symptoms. Linear regressions revealed a significant cortisol × emotion regulation interaction for depressive symptoms, b = 0.89, SE = 0.33, p <0.01. The interaction accounted for 3 % of the variance explained beyond that accounted for by the main effects (ΔR2 = 0.03), representing a small to medium effect size. The simple slope for adolescents high in emotion regulation difficulties (+1 SD: b = 32.40, SE = 11.41, p <0.01) was significantly different than zero (Fig. 1). The simple slope for adolescents low in emotion regulation difficulties (−1 SD: b = −3.85, SE = 7.98, p = 0.63) was nonsignificant. As hypothesized, for adolescents with high levels of emotion regulation difficulties, higher cortisol reactivity was associated with depressive symptoms. The region of significance for this conditional effect was emotion regulation difficulties of −0.32 units (0.02 SD) and higher. Further, gender and emotion regulation difficulties main effects were significant, with female gender and higher emotion regulation difficulties related to depressive symptoms.
For adolescents’ anxiety symptoms, the cortisol × emotion regulation interaction was nonsignificant. Linear regressions showed significant main effects for gender, cortisol, and emotion regulation difficulties. Female gender, higher cortisol, and greater emotion regulation difficulties were associated with higher levels of anxiety.
Discussion
The goal of the current study was to examine how self-reported emotion regulation behaviors moderate the association between HPA axis arousal on symptoms of substance use and psychopathology during adolescence. This is particularly important given that adolescence is a time of increased emotional arousal, in addition to still-developing regulatory abilities (Dahl 2004; Steinberg 2007). Findings suggest that altered cortisol reactivity and difficulties in emotion regulation predicted increased probability of symptoms, with low cortisol reactivity and emotion regulation difficulties related to adolescent substance use and externalizing symptoms and high cortisol reactivity and emotion regulation difficulties related to depressive symptoms. This is the first study of its kind that uniquely extends upon previous research by supplementing physiological measures of reactivity with self-reported measures of emotion regulation abilities in order to understand the interactive effects of two biobehavioral units of analysis as they relate to multiple indices of functioning—specifically, adolescent substance use and psychopathology. The following key findings emerged and highlight the importance of understanding adolescent substance use and mental health through multiple measures of analysis.
Cortisol Reactivity and Emotion Regulation in Substance Use
Findings indicated a significant interaction between emotion regulation difficulties and cortisol reactivity predicting substance use. Although the follow-up simple slope analyses were not significant, the pattern of findings (shown in Fig. 1) and our additional post hoc follow-up analysis (see Footnote) both suggested that adolescents with low levels of cortisol reactivity and high emotion regulation difficulties were most likely to use substances. This finding is similar to prior separate studies that found adolescents with low levels of cortisol reactivity (van Leeuwen et al. 2011) and high levels of emotion regulation difficulties (e.g., Weinberg and Klonsky 2009) were at an increased risk for substance use, respectively. Extending upon previous research, our findings may suggest that adolescents may be most likely to use substances when they experience both low cortisol reactivity and poor emotion regulation abilities.
Stimulation-seeking theory postulates that low levels of HPA reactivity may lead to inclinations toward sensation-seeking behaviors, such as substance use, as a means of up-regulating low arousal, particularly during adolescence (Arnett 1992; Steinberg 2004). Therefore, adolescents with low levels of HPA reactivity may be at risk for substance use. Those who have low HPA reactivity and who also don’t have the ability to effectively regulate their low arousal through other means (such as by engaging in appropriate goal-directed behavior) may be more inclined to seek out maladaptive behaviors, such as substance use, to compensate for under-arousal. Indeed, previous studies in adolescent substance users found that youth report using substances in order to modulate emotional states (Cahalan et al. 1969; Cooper et al. 1995), which could include up-regulating low arousal. Thus, if low HPA axis arousal predisposes adolescents to seek out substances, those that also possess poor emotion regulation abilities may be at a unique risk for substance use.
Cortisol Reactivity and Emotion Regulation in Externalizing Symptoms
As predicted, adolescents with low levels of cortisol reactivity and high emotion regulation difficulties had the highest parent-reported symptoms of ODD. Our results are consistent with several previous studies that found that blunted physiological reactivity (e.g., Flinn and England 1995; Moss et al. 1995) was related to externalizing symptoms. Our data also mirror separate prior studies documenting that emotion regulation difficulties are related to externalizing symptoms (e.g., Eisenberg et al. 2001; Walcott and Landau 2004). Our findings add to the literature by suggesting that lower cortisol reactivity is particularly likely to lead to externalizing symptoms when coupled with emotion regulation difficulties. Low HPA axis arousal can lead to difficulties in age-appropriate inhibition, which can create risk for externalizing problems such as ODD (Kariyawasam et al. 2002). Our research suggests that adolescents who are both low in cortisol reactivity and endorse ineffectual emotion regulation abilities are most vulnerable to engaging in deviant behavior.
Interestingly, some studies find that high reactivity is associated with externalizing symptoms (for a review, see Alink et al. 2008), however, we did not find this in the current study. This may due to inconsistency in methodologies employed across studies; for instance, many researchers employ tasks related to social-evaluative threat or outcome uncontrollability as laboratory stressors, instead of parent–child discussions. With respect to ADHD, although we did not find a significant interaction, we found main effects for both cortisol reactivity and emotion regulation difficulties. This is consistent with the literature showing that youth who suffer from externalizing problems are more likely to evidence a blunted cortisol stress response (e.g., Flinn and England 1995) and experience difficulties modulating their emotional arousal (Walcott and Landau 2004). It is noteworthy that we did not find significant moderation with respect to conduct disorder, perhaps due to the limited variability of parent-reported symptoms of CD in the community-based sample of adolescents employed in the current study. However, for this sample’s higher-frequency Oppositional Defiant Disorder symptoms and behaviors, findings suggest that blunted cortisol reactivity, coupled with emotion regulation difficulties, contribute to adolescent externalizing problems.
Cortisol Reactivity and Emotion Regulation in Internalizing Symptoms
As predicted, high emotion-related cortisol reactivity coupled with high emotion regulation difficulties were associated with higher self-reported symptoms of depression among youth. This is consistent with past research showing that high cortisol reactivity (e.g., Granger et al. 1996; Klimes-Dougan et al. 2001) and ineffective emotion management strategies (e.g., Silk et al. 2003) are independently associated with depressive symptoms among adolescents. However, this is the first study to date that reveals the interactive relationship among youths’ emotion-related cortisol reactivity, emotion regulation abilities, and symptoms of depression. Our data suggest that young adolescents who experience an increase in HPA axis arousal following an interpersonal stressor, and who do not have the requisite emotion regulation abilities to cope with negative affect, are more likely to report depressive symptoms.
It is interesting that no significant moderation was found with respect to adolescents’ anxiety symptoms, although a significant main effect emerged with respect to emotion regulation difficulties. This is consistent with past research linking anxiety and emotion regulation deficits among youth (Zeman et al. 2002). It may be possible that basal cortisol levels (as opposed to cortisol reactivity) may be associated with anxiety. For instance, anxious youth may have entered the lab with elevated cortisol levels that may have been maintained across the entire laboratory session. These youth may have shown a restricted range of cortisol responses and, thus, their levels of HPA axis arousal would not have been accurately captured by our methodology.
Limitations and Future Directions
These findings may contribute to targeted interventions for adolescents at risk for substance use behaviors or symptoms of psychopathology. Specifically, the results may help in the identification of children at risk for internalizing disorders (i.e., those who show high cortisol reactivity) and teach them constructive ways of modulating their negative emotions, perhaps through biofeedback or mindfulness-based interventions (e.g., Burke 2010). Similarly, our findings suggest that adolescents with blunted cortisol reactivity may at an increased risk for substance use and/or externalizing problems. Contrary to most emotion-focused interventions, which almost exclusively focus on the down-regulation of negative affect, these children may particularly benefit from learning emotion regulation strategies that help them up-regulate their emotional arousal.
The principal limitations of the present study can be addressed in future research. Primarily, the present study assessed cortisol reactivity to a single laboratory interpersonal stressor. Future work examining responses across varying contexts, both intra- and interpersonal, may shed light on the continuity of cortisol reactivity and emotion regulation and its relation to maladaptive functioning. In this vein, the cross-sectional nature of the present study limits inferences concerning the development of substance use behaviors, externalizing, and internalizing symptoms and their temporal relation to emotional processes. Longitudinal studies are important for future work to assess the onset of these disorders and the extent to which adolescent emotional functioning may maintain substance use and psychopathology symptoms. Future research could also explore more nuanced interactions with regard to specific emotions (e.g., anger, sadness) and emotion regulation problems or strategies (e.g., up-regulation, down-regulation), which may differentially interact with blunted or high cortisol reactivity to predict psychological outcomes.
Despite these limitations, the present study offers novel insight concerning the interactive nature of youth’s emotion-related physiological reactivity and regulation as measured across multiple domains in the relationship with substance use and multiple associated mental health symptoms. In particular, we found differential patterns of cortisol reactivity and emotion regulation difficulties across substance use, externalizing, and internalizing psychopathology. Importantly, results indicated youth with low levels of cortisol reactivity and greater emotion regulation difficulties were most likely to have engaged in substance use and had higher levels of some externalizing symptoms; whereas, youth with high levels of cortisol reactivity and greater emotion regulation difficulties had higher levels of internalizing symptoms (i.e., depression). Given these findings, and consistent with the RDoc, the present study highlights the importance of investigating the interactions among multiple units of adolescents’ emotional functioning in order to achieve a more nuanced understanding of adolescent adjustment.
Acknowledgments
This work was carried out by a National Institute on Drug Abuse grant awarded to the fifth author (R01-DA033431). Please note that the content presented does not necessarily represent the official views of the National Institute on Drug Abuse, and that the funding sources had no other role other than financial support.
Footnotes
An additional post hoc analysis was performed to assess whether cortisol moderated the association between emotion regulation difficulties and substance use. This resulted in a similar pattern of results; the simple slope for adolescents with low levels of cortisol reactivity (−1 SD: b = 0.14, SE = 0.03, p <0.001) was significantly different than zero, indicating that higher emotion regulation difficulties were more associated with substance use for youth with lower cortisol reactivity.
Given that there are two emotion-regulation specific symptoms of ODD on the Child Symptom Inventory, we also reran analyses without these items, and the cortisol reactivity × DERS interaction results remained significant, t = −3.13, p = 0.002.
Conflict of Interest Jennifer A. Poon, Caitlin C. Turpyn, Amysue Hansen, Juliana Jacangelo and Tara M. Chaplin declare that they have no conflict of interest.
Compliance with Ethical Standards
Informed Consent All procedures followed were in accordance with the ethical standards of the Institutional Review Boards at Yale University and George Mason University. Informed consent was obtained from all individual participants in the study.
Animal Rights No animal studies were carried out by the authors for this paper.
References
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychological Bulletin. 1987;101(2):213. [PubMed] [Google Scholar]
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Alink LR, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology. 2008;50(5):427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. [Google Scholar]
- Arnett J. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12(4):339–373. [Google Scholar]
- Baucom BR, Saxbe DE, Ramos MC, Spies LA, Iturralde E, Duman S, Margolin G. Correlates and characteristics of adolescents’ encoded emotional arousal during family conflict. Emotion. 2012;12(6):1281. doi: 10.1037/a0028872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Dahl RE, Perel J, Williamson DE, Nelson B, Stull S, Ryan ND. Corticotropin-releasing hormone challenge in prepubertal major depression. Biological Psychiatry. 1996;39(4):267–277. doi: 10.1016/0006-3223(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Brener ND, Kann L, McManus T, Kinchen SA, Sundberg EC, Ross JG. Reliability of the 1999 youth risk behavior survey questionnaire. Journal of Adolescent Health. 2002;31(4):336–342. doi: 10.1016/s1054-139x(02)00339-7. [DOI] [PubMed] [Google Scholar]
- Burke CA. Mindfulness-based approaches with children and adolescents: A preliminary review of current research in an emergent field. Journal of Child and Family Studies. 2010;19(2):133–144. [Google Scholar]
- Buss KA, Hill Goldsmith H, Davidson RJ. Cardiac reactivity is associated with changes in negative emotion in 24-month-olds. Developmental Psychobiology. 2005;46(2):118–132. doi: 10.1002/dev.20048. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes. Monographs of the Rutgers Center of Alcohol Studies. 1969;6:260. [Google Scholar]
- Caspi A. The child is father of the man: Personality continuities from childhood to adulthood. Journal of Personality and Social Psychology. 2000;78(1):158–172. doi: 10.1037//0022-3514.78.1.158. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YF, Dennis ML, Funk RR. Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. Journal of Substance Abuse Treatment. 2008;34(1):14–24. doi: 10.1016/j.jsat.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Hansen A, Simmons J, Mayes LC, Hommer RE, Crowley MJ. Parental–adolescent drug use discussions: Physiological responses and associated outcomes. Journal of Adolescent Health. 2014;55(6):730–735. doi: 10.1016/j.jadohealth.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Sinha R, Simmons JA, Healy SM, Mayes LC, Hommer RE, Crowley MJ. Parent–adolescent conflict interactions and adolescent alcohol use. Addictive Behaviors. 2012;37(5):605–612. doi: 10.1016/j.addbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Collins RL, Ritter J, Shirley MC, Zvolensky MJ, Kashdan TB. Vulnerability to substance use disorders across the lifespan. In: Ingram, Prince, editors. Vulnerability to Psychopathology: Risk Across the Lifespan. New York: The Guilford Press; 2001. pp. 165–172. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1998. [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69(5):990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent development and the regulation of behavior and emotion: Introduction to part VIII. Annals of the New York Academy of Sciences. 2004;102(1):294–295. doi: 10.1196/annals.1308.034. [DOI] [PubMed] [Google Scholar]
- Davis EL, Buss KA. Moderators of the relation between shyness and behavior with peers: Cortisol dysregulation and maternal emotion socialization. Social Development. 2012;21(4):801–820. doi: 10.1111/j.1467-9507.2011.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychology. 2008;27(1):116–121. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- Durbin CE. Modeling temperamental risk for depression using developmentally sensitive laboratory paradigms. Child Development Perspectives. 2010;4(3):168–173. [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, Guthrie IK. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Development. 2001;72(4):1112–1134. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Murphy BC, Maszk P, Holmgren R, Suh K. The relations of regulation and emotionality to problem behavior in elementary school children. Development and Psychopathology. 1996;8(1):141–162. [Google Scholar]
- Eisenberg N, Fabes RA, Shepard SA, Murphy BC, Guthrie IK, Jones S, Maszk P. Contemporaneous and longitudinal prediction of children’s social functioning from regulation and emotionality. Child Development. 1997;68(4):642–664. [PubMed] [Google Scholar]
- Flinn MV, England BG. Childhood stress and family environment. Current Anthropology. 1995;36(5):854–866. [Google Scholar]
- Fortunato CK, Dribin AE, Granger DA, Buss KA. Salivary alpha-amylase and cortisol in toddlers: Differential relations to affective behavior. Developmental Psychobiology. 2008;50(8):807–818. doi: 10.1002/dev.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Child symptom inventory-4 norms manual. Stony Brook, NY: Checkmate Plus; 1997. [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: A new perspective and a model for research. Psychological Review. 1980;87(3):301–315. [PubMed] [Google Scholar]
- Granger DA, Weisz JR, McCracken JT, Ikeda SC, Douglas P. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Development. 1996;67(6):3250–3262. [PubMed] [Google Scholar]
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment. 2004;26(1):41–54. [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: Shame, social self-esteem, and cortisol activity. Psychosomatic Medicine. 2004;66(6):915–924. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic–pituitary–adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A, Watkins E, Mansell W, Shafran R. Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Hayes N, editor. Doing qualitative analysis in psychology. Hove: Psychology Press; 2013. [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41(3):924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hubbard JA, Smithmyer CM, Ramsden SR, Parker EH, Flanagan KD, Dearing KF, Simons RF. Observational, physiological, and self-report measures of children’s anger: Relations to reactive versus proactive aggression. Child Development. 2002;73(4):1101–1118. doi: 10.1111/1467-8624.00460. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975–2010. Volume I, secondary school students. Institute for Social Research; 2011. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58(6):1459–1473. [PubMed] [Google Scholar]
- Kaminer Y, Bukstein O, Tarter RE. The teen-addiction severity index: Rationale and reliability. Substance Use and Misuse. 1991;26(2):219–226. doi: 10.3109/10826089109053184. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Johnson JG, Bird HR, Weissman MM, Goodman SH, Lahey BB, Schwab-Stone ME. Psychiatric comorbidity among adolescents with substance use disorders: Findings from the MECA study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(6):693–699. doi: 10.1097/00004583-199906000-00016. [DOI] [PubMed] [Google Scholar]
- Kariyawasam SH, Zaw F, Handley SL. Reduced salivary cortisol in children with comorbid attention deficit hyperactivity disorder and oppositional defiant disorder. Neuroendocrinology Letter. 2002;23(1):45–48. [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13(3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s depression inventory (CDI) Toronto: Multi-Health Systems Inc; 2004. [Google Scholar]
- Kring AM. Emotion disturbances as transdiagnostic processes in psychopathology. Handbook of emotion. 2008;3:691–705. [Google Scholar]
- Kruesi MJ, Schmidt ME, Donnelly M, Hibbs ED, Hamburger SD. Urinary free cortisol output and disruptive behavior in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28(3):441–443. doi: 10.1097/00004583-198905000-00024. [DOI] [PubMed] [Google Scholar]
- Lee SW, Piersel WC, Friedlander R, Collamer W. Concurrent validity of the Revised Children’s Manifest Anxiety Scale (RCMAS) for adolescents. Educational and Psychological Measurement. 1988;48(2):429–433. [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR. Alcohol consumption in high school adolescents: Frequency of use and dimensional structure of associated problems. Addiction. 1996;91(3):375–390. doi: 10.1046/j.1360-0443.1996.9133757.x. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Robinson MD. Measures of emotion: A review. Cognition and Emotion. 2009;23(2):209–237. doi: 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, Hanson KS. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(2):192–196. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100(4):674. [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological Psychiatry. 1995;38(8):547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Ollendick T, King N, Bogie N. Three traditional and three new childhood anxiety questionnaires: Their reliability and validity in a normal adolescent sample. Behaviour Research and Rherapy. 2002;40(7):753–772. doi: 10.1016/s0005-7967(01)00056-0. [DOI] [PubMed] [Google Scholar]
- Nolan-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100(4):569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Olson SL, Sameroff AJ, Kerr DC, Lopez NL, Wellman HM. Developmental foundations of externalizing problems in young children: The role of effortful control. Development and Psychopathology. 2005;17(01):25–45. doi: 10.1017/s0954579405050029. [DOI] [PubMed] [Google Scholar]
- Paglia A, Room R. Preventing substance use problems among youth: A literature review and recommendations. Journal of Primary Prevention. 1999;20(1):3–50. [Google Scholar]
- Prinz RJ, Foster SL, Kent RN, O’Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. Journal of Applied Behavior Analysis. 1979;12(4):691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Annotation: The role of prefrontal deficits, low autonomic arousal, and early health factors in the development of antisocial and aggressive behavior in children. Journal of Child Psychology and Psychiatry. 2002;43(4):417–434. doi: 10.1111/1469-7610.00034. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64(6):521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO. Revised Children’s Manifest Anxiety Scale. RCMAS Manual. Los Angeles: Western Psychological Services; 1985. [Google Scholar]
- Rohleder N, Beulen SE, Chen E, Wolf JM, Kirschbaum C. Stress on the dance floor: The cortisol stress response to social-evaluative threat in competitive ballroom dancers. Personality and Social Psychology Bulletin. 2007;33(1):69–84. doi: 10.1177/0146167206293986. [DOI] [PubMed] [Google Scholar]
- Rudolph KD. Gender differences in emotional responses to interpersonal stress during adolescence. Journal of Adolescent Health. 2002;30(4):3–13. doi: 10.1016/s1054-139x(01)00383-4. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Peer victimization and aggression: Moderation by individual differences in salivary cortisol and alpha-amylase. Journal of Abnormal Child Psychology. 2010;38(6):843–856. doi: 10.1007/s10802-010-9412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33(8):1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30(2):127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sheeber L, Hops H, Alpert A, Davis B, Andrews J. Family support and conflict: Prospective relations to adolescent depression. Journal of Abnormal Child Psychology. 1997;25(4):333–344. doi: 10.1023/a:1025768504415. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Reactive aggression among maltreated children: The contributions of attention and emotion dysregulation. Journal of Clinical Child Psychology. 1998;27(4):381–395. doi: 10.1207/s15374424jccp2704_2. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Sprafkin J, Gadow KD, Salisbury H, Schneider J, Loney J. Further evidence of reliability and validity of the Child Symptom Inventory-4: Parent checklist in clinically referred boys. Journal of Clinical Child and Adolescent Psychology. 2002;31(4):513–524. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: What changes, and why? Annals of the New York Academy of Sciences. 2004;1021(1):51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence new perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16(2):55–59. [Google Scholar]
- Targum SD, Clarkson LL, Magac-Harris K, Marshall LE, Skwerer RG. Measurement of cortisol and lymphocyte subpopulations in depressed and conduct-disordered adolescents. Journal of Affective Disorders. 1990;18(2):91–96. doi: 10.1016/0165-0327(90)90064-f. [DOI] [PubMed] [Google Scholar]
- Tennes K, Kreye M. Children’s adrenocortical responses to classroom activities and tests in elementary school. Psychosomatic Medicine. 1985;47(5):451–460. doi: 10.1097/00006842-198509000-00005. [DOI] [PubMed] [Google Scholar]
- Tennes K, Kreye M, Avitable N, Wells R. Behavioral correlates of excreted catecholamines and cortisol in second-grade children. Journal of the American Academy of Child Psychiatry. 1986;25(6):764–770. doi: 10.1016/s0002-7138(09)60193-x. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43(7):531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- van Leeuwen AP, Creemers HE, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC. Hypothalamic–pituitary–adrenal axis reactivity to social stress and adolescent cannabis use: The TRAILS study. Addiction. 2011;106(8):1484–1492. doi: 10.1111/j.1360-0443.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Plail JA, Blackson T, Mezzich AC, Tarter RE. Antisocial symptoms in preadolescent boys and in their parents: Associations with cortisol. Psychiatry Research. 1993;46(1):9–17. doi: 10.1016/0165-1781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Walcott CM, Landau S. The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2004;33(4):772–782. doi: 10.1207/s15374424jccp3304_12. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klonsky ED. Measurement of emotion dysregulation in adolescents. Psychological Assessment. 2009;21(4):616. doi: 10.1037/a0016669. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Anderson JP, Shelley-Abrahamson R, Biederman J. Difficulties in emotional regulation and substance use disorders: A controlled family study of bipolar adolescents. Drug and Alcohol Dependence. 2013;132(1):114–121. doi: 10.1016/j.drugalcdep.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Dahl RE. Transitions into underage and problem drinking: Developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121(Supplement 4):S273–S289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Klimes-Dougan B, Slattery MJ. Internalizing problems of childhood and adolescence: Prospects, pitfalls, and progress in understanding the development of anxiety and depression. Development and Psychopathology. 2000;12(3):443–466. [PubMed] [Google Scholar]
- Zeman J, Cassano M, Perry-Parish C, Stegall S. Emotion regulation in children and adolescents. Journal of Developmental and Behavioral Pediatrics. 2006;27(2):155–168. doi: 10.1097/00004703-200604000-00014. [DOI] [PubMed] [Google Scholar]
- Zeman J, Shipman K, Suveg C. Anger and sadness regulation: Predictions to internalizing and externalizing symptoms in children. Journal of Clinical Child and Adolescent Psychology. 2002;31(3):393–398. doi: 10.1207/S15374424JCCP3103_11. [DOI] [PubMed] [Google Scholar]