Abstract
Apolipoprotein E 4 (ApoE 4) has been linked to pathogenesis of Alzheimer’s disease and has been suggested to be maintained through evolutionary pressure via a protective role in malaria infection. We evaluated Plasmodium falciparum viability at the intraerythrocyte stage by exposure to plasma from human subjects with ApoE 4/4 or ApoE 3/3. Plasma samples from ApoE 4/4 but not ApoE 3/3 donors inhibited growth and disrupted morphology of P. falciparum. Evolutionary history is characterized by war between pathogenic microorganisms and defense mechanisms countering their pathogenicities. ApoE 4 frequency is highest in sub-Saharan Africa and other isolated populations (e.g., Papua New Guinea) that exhibit endemic malaria. High ApoE frequency may offer selective advantage protecting against some infectious diseases (e.g., Plasmodium falciparum). These results implicate evolutionary pressure by malaria selecting humans with ApoE 4/4, even considering lower survival in late life. These selective advantages may be relevant in the exploration of possible disparities between Black and Whites in the incidence of Alzheimer’s Disease.
Keywords: Aging, diet, evolution, health disparity, lipids, malaria
Apolipoprotein E (ApoE) has three major isoforms, ApoE 4, ApoE 3, and ApoE 2, that differ from one another only by single amino acid substitutions.1, 2 ApoE 4 increases the risk of cardiovascular disease, atherosclerosis, and also is a major risk factor associated with 40–65% of cases of sporadic and familial Alzheimer’s disease (AD).3 Deleterious effects of ApoE 4 are not manifested until late in life. In contrast, if ApoE 4 provides even a minor protection against malaria or other infections in young persons until they reach their reproductive years, then the presence of the ApoE 4 gene will confer a selective advantage that would lead to its fixation in the population, and thus could explain why the ApoE 4 gene is common among most populations.1 These selective advantages may be relevant in the exploration of possible disparities between Black and Whites in the incidence of Alzheimer’s Disease.4–7
The geographic and ethnic differences in the ApoE allele frequencies raise fascinating questions. In all populations, the ApoE 3 allele is the most frequent, with a range of 50%–90%, and the range of the ApoE 4 allele is about 5–35%.1 In North American populations derived from Europe, the genotype E 4/4 is less than 1%; however, in the African subcontinent (sub-Saharan Africa) and certain other isolated populations, such as in Papua New Guinea, the frequency of the ApoE 4 allele is extremely high.8–10 In these populations, the ApoE 4 allele might be increased due to a role in protection against infectious diseases.11–13
A primary metabolic role for ApoE is to transport and deliver lipids from one tissue or cell type to another.2, 14 During the erythrocyte cycle of Plasmodium falciparum parasite, there is a 500–700% increase in phospholipid levels in the infected erythrocyte and thus the parasite requires incorporation of intact phospholipids from the plasma.15–16 Compared with other ApoE isoforms, the ApoE 4 isoform is relatively ineffective in encouraging neurite outgrowth, possibly due to less efficient phospholipid transport.17
There is evidence that ApoE plays a role in resistance to bacterial infections. The enhanced susceptibility of ApoE-null mice against Listeria monocytogenes,18 and Klebsiella pneumoniae19 has been reported. Additionally, there is evidence that ApoE plays a role in protection against malaria. The malaria circumsporozoite protein (CSP) utilizes the heparan sulfate proteoglycan (HSPG)/low density lipoprotein receptor-related protein (LRP) pathway to invade hepatocytes. Remnant lipoprotein, which use ApoE as the ligand and which interacts with the HSPG/LRP pathway, can inhibit host cell invasion of the CSP.20
Malaria parasites have probably had a profound impact on recent human evolution.21 This “malaria hypothesis” posits that certain hemoglobinopathies have been selected to high frequencies in particular populations because they protect against the effects of malaria infections.22–26 Although these hemoglobinopathies are dangerous and often fatal, they sometimes confer an advantage in the form of protection against infectious diseases.24, 25 This line of thinking leads us to hypothesize that apolipoproteins can play a role in the critical intraerythrocyte development stage of malaria and that if any one isoform has different lipid carrying or transferring properties, it could interfere with disease progression.
In this study we directly test the effect of ApoE 4 on the growth of malaria parasites in red blood cells. Our findings demonstrate ApoE 4 inhibits malaria parasite growth.
Methods
ApoE 3/3 and ApoE 4/4 donors
Approved by the University Hospitals-Case Medical Center, Institutional Review Board. All subjects were adults. Each subject was assigned a unique ID number. To ensure confidentiality, tubes of blood were labeled only with the ID number. All other subject information recorded for the study was kept in locked file cabinets, and/or password-protected computer databases. Both research assistants and investigators were blind to individual results. Vulnerable donors who had been infected with malaria or had taken anti-malarial drugs were not utilized in this experiment. To eliminate donors carrying any hemoglobinopathy and other red blood cell disorders, HbA genotype was determined by cellulose acetate and citrate agar electrophoresis and confirmed by HPLC analysis. All donors carried normal HbAA and no red blood cell disorder. Seven ApoE 4/4 and six ApoE 3/3 donors were available in this project, and analyzed in various portions of the study and genotyped by standard molecular techniques. Related red blood cells were washed three times with medium and stored at 50% hematocrit in RPMI-1640 for up to four weeks at 4°C.
P. falciparum isolates
Nine different P. falciparum parasite lines from a variety of geographical locations were evaluated in this study: 3D7, 1905 (PNG), Dd2 (Indochina), 11B3 (Honduras), FCB (Columbia), FCR3 (Gambia), FAB6 (South Africa), G134 (Ghana), and ItG2 (Southeast Asia).
In vitro parasite culture in ApoE 4/4 or ApoE 3/3 and growth experiment
Nine established P. falciparum lines from different continents (see above) were maintained in HbAA human red cells collected from healthy blood bank donors. Parasites were cultured by the method of Trager and Jensen.27 Albumax II (Gibco-BRL) was used instead of human serum. Parasite lines cultured in HbAA cells were enriched to 95% mature forms by Percoll-sorbitol methods.28 The initial parasitemia were adjusted to 0.5% by using uninfected HbAA cells and cultured in complete medium containing 10% Apo E4/4 or E3/3 plasma from each donor. Culture media containing ApoE plasma were changed daily and parasite counts were determined by examination of Giemsa-stained blood smears. Parasitemia (mean ± SD) were calculated from triplicate counts of 2000 red cells.
Serum ApoE 4/4 level and parasite growth inhibition
To determine whether the serum ApoE 4/4 levels are correlated with the parasite growth inhibition rate, we measured the serum ApoE 4/4 levels in each donor. Serum ApoE levels were determined, on coded samples without the knowledge of the donors, using standard methods.29–30
Electron microscopy
Nine P. falciparum lines were used in this experiment. Synchronized parasites were cultured in the ApoE 4/4 plasma from each donor or reconstituted ApoE 4/4 and processed for electron microscopy at the different incubation time points (0, 3, 6, 12, and 24 hr), and morphology analyzed.31–37 Morphological changes of the parasites incubated with ApoE 4/4 were compared with those cultured in normal plasma.
Results
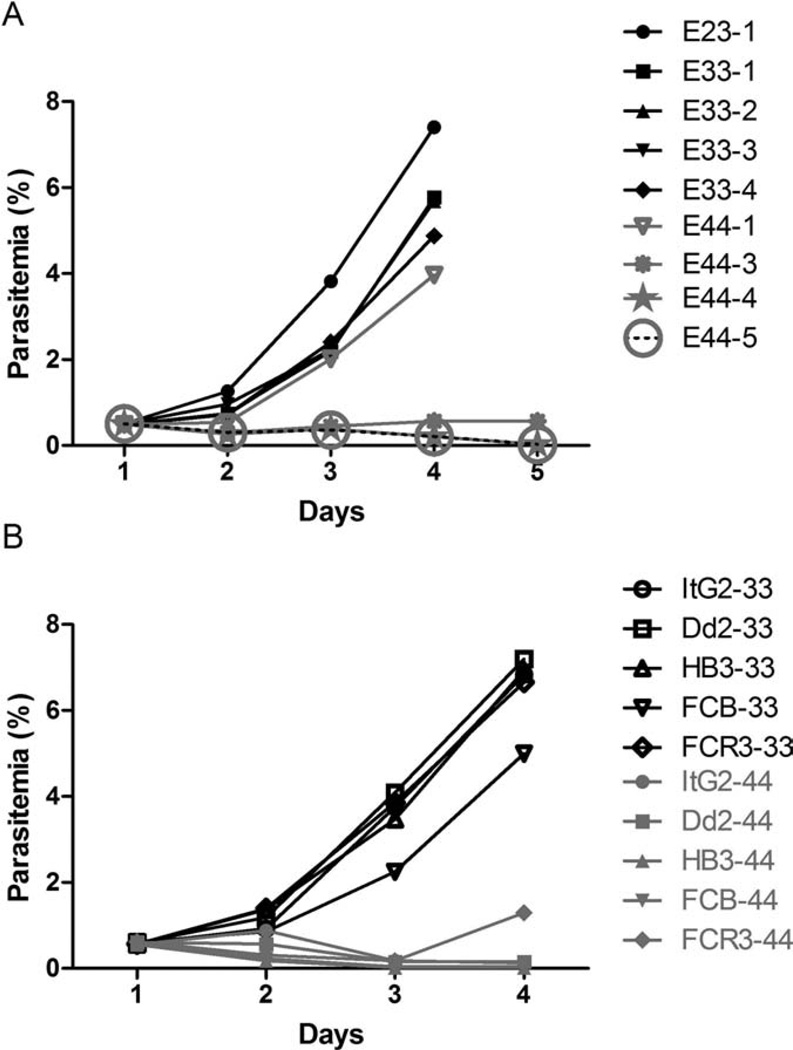
We investigated 13 plasma samples (7 ApoE 4/4, 6 ApoE 3/3) from donors (Figure 1A). Six out of seven plasma samples from ApoE 4/4 donors inhibited the growth of P. falciparum. The one exception could be explained by the lower level of ApoE 4/4 in plasma (data not shown).
Figure 1.
A) Growth of Plasmodium falciparum (ItG2) in eight of the thirteen Apo E plasma samples. Parasites were cultured for 4 days in HbAA cells. Day 1 is the starting time of the in vitro culture. E44 indicates apo E4/4 isoform. Parasites were enriched to 95% mature forms by the Percoll-sorbitol method (Schlichtherle et al, 2000). The initial parasitemia was adjusted at 0.5% by HbAA type RBC and cultured at 37°C in an atmosphere of 5% CO2, 5% O2, and 90% N2. Parasitemias were calculated from triplicate counts of 2000 RBC. B) Growth of P. falciparum lines (ItG2, Dd2, HB3, FCB, and FCR3) in ApoE 4/4–16 or ApoE 3/3–4. Parasites were cultured for 4 days in HbAA cells. Day 1 is the starting time of the in vitro culture. ItG2–44 means that the ItG2 line is cultured in apoE4/4 plasma.
We tested the responses to one ApoE 4/4 plasma using four additional P. falciparum lines originating from a variety of geographical locations: Dd2 (Indochina), HB3 (Honduras), FCB (Columbia), FCR3 (Gambia). The tested ApoE 4/4 plasma inhibited the parasite growth of all P. falciparum lines (Figure 1B).
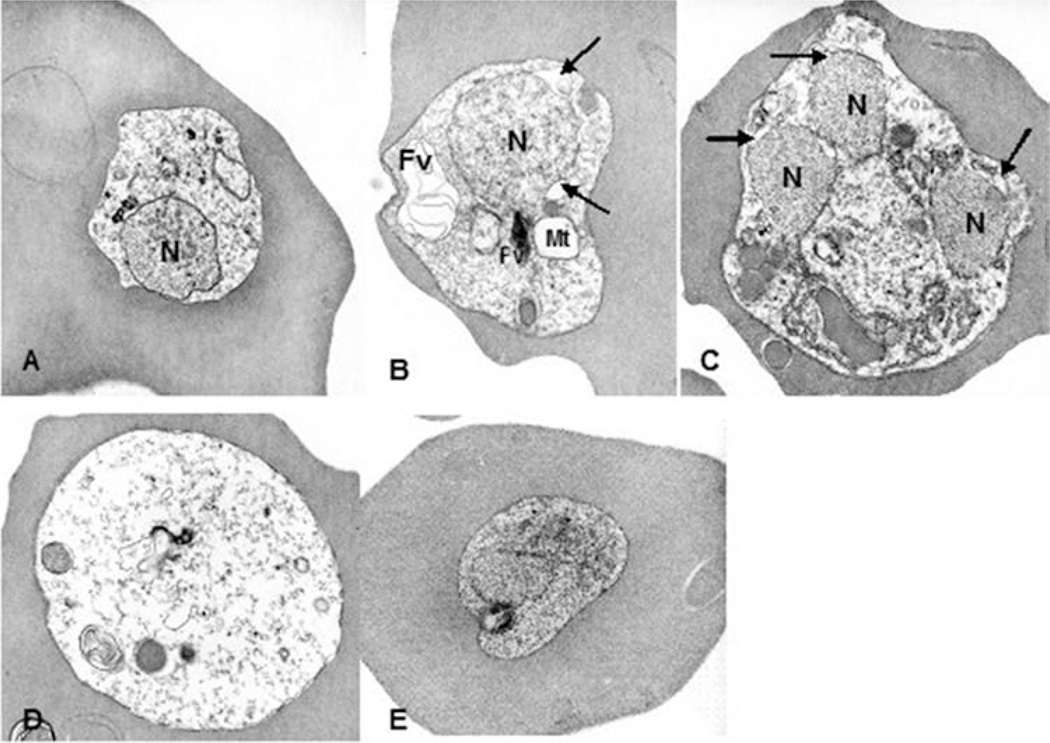
ApoE 4/4 treatment led to parasite disintegration within intact RBC; parasite death was found at all stages of maturation. This result indicated that human ApoE 4/4 plasma could be a factor mediating malaria death (Figure 2). The P. falciparum ItG2 line exhibited disorganization in vitro when grown in ApoE 4/4 plasmas. Condensation of cytoplasm, dilatation of plasma membrane, mitochondrial swelling, dilatation of cisternae of the rough endoplasmic reticulum, and the perinuclear space characterized parasite disintegration. The morphological changes suggested the target site of ApoE 4/4 in infected erythrocytes is different from those of known anti-malarial drugs. Similar morphological changes observed in ApoE 4/4 plasma were not seen for malaria grown in ApoE 3/3 plasma (not shown).
Figure 2.
Electron micrographs of P. falciparum (ItG2) parasites cultured in Apo E4/4 (A~D). Parasites infected with RBC exhibited mitochondrial swelling and dilatation of the perinuclear space. Cytoplasm became electron lucent, and membranous structures accumulated in the food vacuole. Micrograph E shows a control parasite cultured in Apo E 3/3. N; nucleus, FV; food vacuole, Mt; mitochondrion. Arrows indicate dilated perinuclear spaces.
Discussion
In this study, in vitro experiments showed that ApoE 4 but not ApoE 3 inhibits P. falciparum growth. These experiments provided the first direct evidence that ApoE 4 plays a role in inhibition of P. falciparum. An interesting possible outcome of these studies is the key role ApoE 3 plays in parasite growth. ApoE 3 preferentially binds to the smaller, more phospholipid enriched high density lipoprotein, while ApoE 4 preferentially binds to the larger, triglyceride-rich very low density lipoproteins. These differences in lipoprotein association between ApoE 3 and ApoE 438 might be responsible for parasite growth inhibition. Three-dimensional structures of regions of Apo E highlight isoform differences.39 For example, the critical rearrangement of the arginine 61 side chain alters the conformation of ApoE 4 and is probably responsible for several ApoE 4-specific roles. Identification of the critical locus for the effect of Apo E 4 in the infected erythrocytes may aid development of new drugs for the treatment of P. falciparum malaria.
It seems there is a need for a critical balance between taking in fatty acids (from the plasma high-density lipoprotein (HDL) and losing red blood cell membrane cholesterol (to the plasma HDL) that is required for successful development and exit of parasites from the red blood cell.40 The accumulation of fatty acids by malaria within the RBC is critical for development from the early invasion stage to the late invasion stage, peaking just before exit. This progression must be tightly regulated; otherwise too many fatty acids accumulate in the immediate vicinity of the infected RBC and parasite within. A prime example of why this progression and process of fatty acid uptake becomes critical is that more membrane will be needed to produce the multiple parasites from the one that originally invaded, just before RBC rupture and exit. That time, from early stage development to late stage development of parasites, seems to comprise the stages most affected by ApoE 4 in this study.
In summary, these findings support the view that ApoE 4 persists in human populations in part due to protection from infection diseases. The increased frequency of ApoE 4 in some populations, e.g. Africans, is a direct result of evolutionary balance between protection from malaria and increased risk of mortality from a variety of degenerative diseases. This study supports the need for careful ethnobiological studies to uncover the molecular basis of health disparities.
Acknowledgments
Partial support provided by National Institute of Allergy and Infection AI-058186-01. Support provided by the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health, and The Alzheimer’s Association, and the Semmes Foundation.
Notes
- 1.Seripa D, D’Onofrio G, Panza F, et al. The genetics of the human APOE polymorphism. Rejuvenation Res. 2011 Oct;14(5):491–500. doi: 10.1089/rej.2011.1169. Epub 2011 Sep 29. [DOI] [PubMed] [Google Scholar]
- 2.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009 Apr;50(Suppl):S183–S188. doi: 10.1194/jlr.R800069-JLR200. Epub 2008 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corder EH, Saunders AM, Strittmatter WI, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 4.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14(6):481–493. [PubMed] [Google Scholar]
- 5.Manly J, Mayeux R. Ethnic differences in dementia and Alzheimer’s disease. In: Anderson N, Bulatao R, Cohen B, editors. Critical perspectives on racial and ethnic differentials in health in late life. Washington, DC: National Academies Press; 2004. pp. 95–141. [PubMed] [Google Scholar]
- 6.Dilworth- Anderson P, Hendrie HC, Manly JJ, et al. Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimers Dement. 2008;4(4):305–309. doi: 10.1016/j.jalz.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Potter GG, Plassman BL, Burke JR, et al. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement. 2009;5(6):445–453. doi: 10.1016/j.jalz.2009.04.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerdes LU, Gerdes C, Hansen PS, et al. Are men carrying the apolipoprotein epsilon 4- or epsilon 2 allele less fertile than epsilon 3 epsilon 3 genotypes? Hum Genet. 1996 Aug;98(2):239–242. doi: 10.1007/s004390050200. [DOI] [PubMed] [Google Scholar]
- 9.Zekraoui L, Lagarde JP, Raisonnier A, et al. High frequency of the apolipoprotein E *4 allele in African pygmies and most of the African populations in sub- Saharan Africa. Hum Biol. 1997 Aug;69(4):575–581. [PubMed] [Google Scholar]
- 10.Sayi JG, Patel NB, Premkumar DR, et al. Apolipoprotein E polymorphism in elderly east Africans. East Afr Med J. 1997 Oct;74(10):668–670. [PubMed] [Google Scholar]
- 11.Martin GM. APOE alleles and lipophilic pathogens. Neurobiol Aging. 1999 Jul-Aug;20(4):411–413. doi: 10.1016/s0197-4580(99)00078-0. [DOI] [PubMed] [Google Scholar]
- 12.Aucan C, Walley AJ, Hill AV. Common apolipoprotein E polymorphisms and risk of clinical malaria in the Gambia. J Med Genet. 2004 Jan;41(1):21–24. doi: 10.1136/jmg.2003.011981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wozniak MA, Faragher EB, Todd JA, et al. Does apolipoprotein E polymorphism influence susceptibility to malaria? J Med Genet. 2003 May;40(5):348–351. doi: 10.1136/jmg.40.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson JA, Itkovitz- Elder J, Shapiro S. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 15.Vial HJ, Ancelin ML, Philippot JR, et al. Biosynthesis and dynamics of lipids in Plasmodium - infected mature mammalian erythrocytes. Blood Cells. 1990;16(2–3):531–555. [PubMed] [Google Scholar]
- 16.Vignali M, McKinlay A, LaCount DJ, et al. Interaction of an atypical Plasmodium falciparum ETRAMP with human apolipoproteins. Malar J. 2008 Oct 20;7:211. doi: 10.1186/1475-2875-7-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitas RE, Ji ZS, Weisgraber KH, et al. Role of apolipoprotein E in modulating neurite outgrowth: potential effect of intracellular apolipoprotein E. Biochem Soc Trans. 1998 May;26(2):257–262. doi: 10.1042/bst0260257. [DOI] [PubMed] [Google Scholar]
- 18.Roselaar SE, Daugherty A. Apolipoprotein E- deficient mice have impaired innate immune responses to Listeria monocytogenesis in vivo. J Lipid Res. 1998 Sep;39(9):1740–1743. [PubMed] [Google Scholar]
- 19.de Bont N, Netea MG, Demacker PN, et al. Apolipoprotein E knock- out mice are highly susceptible to endotoxemia and Klebsielle pneumoniae infection. J Lipid Res. 1999 Apr;40(4):680–685. [PubMed] [Google Scholar]
- 20.Sinnis P, Willnow TE, Briones MR, et al. Remnant lipoproteins inhibit malaria sporozoite invasion of hepatocytes. J Exp Med. 1996 Sep 1;184(3):945–954. doi: 10.1084/jem.184.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalli- Sforza LL, Menozzi P, Piazza A. The history and geography of human genes. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- 22.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002 Oct;15(4):564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flint J, Hill AV, Bowden DK, et al. High frequencies of alpha- thalassaemia are the result of natural selection by malaria. Nature. 1986 Jun 19–25;321(6072):744–750. doi: 10.1038/321744a0. [DOI] [PubMed] [Google Scholar]
- 24.Ruwende C, Khoo SC, Snow RW, et al. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature. 1995 Jul 20;376(6537):246–249. doi: 10.1038/376246a0. [DOI] [PubMed] [Google Scholar]
- 25.Modiano D, Luoni G, Sirima BS, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001 Nov 15;414(6861):305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 26.Fairhurst RM, Fujioka H, Hayton K, et al. Aberrant development of Plasmodium falciparum in hemoglobin CC red cells: implications for the malaria protective effect of the homozygous state. Blood. 2003 Apr 15;101(8):3309–3315. doi: 10.1182/blood-2002-10-3105. Epub 2002 Dec 12. [DOI] [PubMed] [Google Scholar]
- 27.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 28.Schlichtherle M, Wahigren M, Perlmann H, et al. Methods in Malaria Research. 3rd. Manassas, VA: MR4/ATCC; 2000. [Google Scholar]
- 29.Slooter AJ, de Knijff P, Hoff man A, et al. Serum apolipoprotein E level is not increased in Alzheimer’s disease: the Rotterdam study. Neurosci Lett. 1998 May 22;248(1):21–24. doi: 10.1016/s0304-3940(98)00339-5. [DOI] [PubMed] [Google Scholar]
- 30.Smit M, de Knijif P, Rosseneu M, et al. Apolipoprotein E polymorphism in The Netherlands and its effect on plasma lipid and apolipoprotein levels. Hum Genet. 1998 Nov;80(3):287–292. doi: 10.1007/BF01790099. [DOI] [PubMed] [Google Scholar]
- 31.Aikawa M, Atkinson CT. Immunoelectron microscopy of parasites. Adv Parasitol. 1990;29:151–214. doi: 10.1016/s0065-308x(08)60106-2. [DOI] [PubMed] [Google Scholar]
- 32.Su X-Z, Kirkman LA, Fujioka H, et al. Complex polymorphisms in an approximately 330 kDa protein are linked to chloroquine- resistant P. falciparum in Southeast Asia and Africa. Cell. 1997 Nov 28;91(5):593–603. doi: 10.1016/s0092-8674(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 33.Aikawa M. High-resolution autoradiography of malaria parasites treated with 3H- chloroquine. Am J Pathol. 1972 May;67(2):277–284. [PMC free article] [PubMed] [Google Scholar]
- 34.Aikawa M, Beaudoin RL. Effects of chloroquine on the morphology of the erythrocyte stages of Plasmodium gailinaceum. Am J Trop Med Hyg. 1969 Mar;18(2):166–181. [PubMed] [Google Scholar]
- 35.Atkinson CT, Bayne MT, Gordeuk VR, et al. Stage- specific ultrastructural effects of desferioxarnine on Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1991 Nov;45(5):593–601. doi: 10.4269/ajtmh.1991.45.593. [DOI] [PubMed] [Google Scholar]
- 36.Fujioka H, Emancipator SN, Aikawa M, et al. Immunocytochemical colocalization of specific immunoglobulin A with sendai virus protein in infected polarized epithelium. J Exp Med. 1998 Oct 5;188(7):1223–1229. doi: 10.1084/jem.188.7.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujioka H, Toni M, Barnwell JW, et al. Localization of F- actin on the caveola-vesicle complex of the erythrocytes infected with Plasmodium vivax and Plasmodium cynomolgi. Jpn J Parasitol. 1992;41:361–364. [Google Scholar]
- 38.Dong LM, Wilson C, Wardell MR, et al. Human apolipoprotein E. Role of arginine 61 in mediating the lipoprotein preferences of the E3 and E4 isoforms. J Biol Chem. 1994 Sep 2;269(35):22358–22365. [PubMed] [Google Scholar]
- 39.Mahley RW, Huang Y. Apolipoprotein E: from atherosclerosis to Alzheimer’s disease and beyond. Curr Opin Lipidol. 1999 Jun;10(3):207–217. doi: 10.1097/00041433-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Wernsdorfer WH, Trigg PI. Recent progress of malaria research: chemotherapy. In: Wernsdorfer WH, McGregor I, editors. Malaria: principles and practice of malariology (vol 2) Edinburgh: Churchill Livingstone; 1988. pp. 1569–1674. [Google Scholar]