Abstract
Matrix metalloproteinases (MMPs) are extracellular proteolytic enzymes that contribute to pericellular remodeling in a variety of tissues, including brain, where they function in adult hippocampal synaptic structural and functional plasticity. Synaptic plasticity and remodeling are also important for development of connectivity, but it is unclear whether MMPs—particularly MMP-2 and -9, the major MMPs operative in brain—contribute at these stages. Here, we use a combination of biochemical and anatomical methods to characterize expression and localization of MMP-2 and MMP-9 in early postnatal and adult rat hippocampus. Gene and protein expression of these MMPs are evident throughout hippocampus at all ages examined, but expression levels were highest during the first postnatal week. MMP-2 and MMP-9 immunolocalized to punctate structures within the neuropil that codistributed with foci of proteolytic activity, as well as with markers of growing axons and synapses. Taken together, discrete foci of MMP proteolysis are likely important for actively shaping and remodeling cellular and connectional architecture as hippocampal circuitry is becoming established during early postnatal life.
Indexing terms: extracellular proteolysis, synaptic plasticity, development, hippocampus, in situ zymography
INTRODUCTION
The establishment of neural circuitry during brain development requires considerable remodeling of the pericellular environment. In hippocampus and elsewhere, dynamic processes of cellular migration, axon and dendrite extension, elaboration of dendritic filopodia and spines, synaptogenesis, extension of fine astrocytic processes and myelination all require flexible and sometimes transient membrane appositions during early postnatal life as synaptic circuits are formed, become functional, refine and begin to mature (Bayer, 1980a; b; Amaral and Dent, 1981; Harris et al., 1992; Fiala et al., 1998; Haber et al., 2006). Such dynamic cellular remodeling is actively shaped by a composition of extracellular matrix proteins and other cell-surface molecular cues that serve to both inhibit and promote process contact, growth and movement (Gundelfinger et al., 2010). Together, these observations suggest that molecular mechanisms must exist to modulate the pericellular microenvironment during development to ensure that such dynamic processes proceed normally.
Matrix metalloproteinases (MMPs) are part of the Metzincin clan of zinc-binding metalloproteinases, which also includes the ADAM (A Disintegrin And Metalloproteinase) family among others (Huntley, 2012). MMPs are mostly secreted proteolytic enzymes that in many tissues, including brain, are important contributers to pericellular remodeling associated with both beneficial (e.g. wound repair) as well as maladaptive (e.g. cancer metastasis) events. These potent proteases are secreted into the extracellular environment as inactive zymogens, and undergo a number of processing steps in response to specific stimuli that are required to remove the autoinhibitory pro-domain to reveal the full catalytic activity of the enzyme (Ethell and Ethell, 2007). Such activity can be terminated by binding to one of four small, endogenous inhibitory proteins called TIMPs (Tissue Inhibitors of Metalloproteinases) (Okulski et al., 2007). Of the ~23 MMPs that are expressed in the body, at least 10 of these have been shown to be present in brain (Jaworski, 2000; Ayoub et al., 2005; Ulrich et al., 2005).
In adult rat hippocampus, MMP- 2 and 9, which are among the most extensively examined and abundant of the group, are localized perisynaptically and rapidly become proteolytically active upon tetanic stimulation protocols sufficient for inducing late-phase long-term potentiation (L-LTP) of CA1 synapses both in acute hippocampal slices (Nagy et al., 2006) as well as in urethane-anesthetized, adult rats (Bozdagi et al., 2007). Once proteolytically active, perisynaptic MMP-9 drives persistent dendritic spine enlargement and synaptic potentiation coordinately at CA1 synapses (Wang et al., 2008). When MMP-9 or other MMPs are blocked genetically or pharmacologically, successful performance in hippocampal-mediated learning and memory tasks that are thought to depend on such plasticity is abolished (Nagy et al., 2006; Brown et al., 2007; Nagy et al., 2007; Olson et al., 2008). While these data collectively have established important roles for MMP-mediated pericellular remodeling associated with synaptic plasticity in mature hippocampus (Huntley, 2012), it is less clear if MMP-2 and -9 are present and proteolytically active during the early postnatal period of hippocampal development when considerable remodeling occurs as circuits are becoming established.
The goal of this study, therefore, is to characterize expression and localization of MMP-2 and -9 and their presumptive proteolytic activities in developing rat hippocampus.
MATERIALS AND METHODS
Animals
This study was conducted on the postnatal brains of 125 Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) aged postnatal (P) day 0 (P0; the first 24 hours after birth) through P23, and adulthood (> P60). Both male and female animals were used. The treatment of all animals was in strict accordance with procedures approved by Mount Sinai’s Institutional Animal Care and Use Committee and guidelines established by the National Institutes of Health.
Total RNA isolation, preparation of cDNA, and RT-PCR
Whole hippocampus was dissected and snap-frozen (n = 6 rats per age). Total RNA was isolated using Trizol Reagent (Invitrogen, Carlsbad, CA) and treated with 1 U of DNAase I (Amplification Grade, Invitrogen) for 10 min to prevent genomic DNA contamination. The integrity of the RNA was analyzed on 10% agarose gels stained with ethidium bromide. cDNA was synthesized by incubating 5 µg of total RNA in a 25 µl reaction containing 50 U of SuperScrit II reverse transcriptase (Invitrogen), 0.5 mM dNTPs, 0.5 µg random primers and 1× buffer containing 1.5 mM MgCl2 according to the manufacturer’s instructions. Using 2 µl of cDNA, PCR reactions were performed for 40 cycles at optimized annealing temperatures (52°C, MMP-2; 54°C, MMP-9) with the following primers (Papp et al., 2007): MMP-2, 5’-CTATTCTGTCAGCACTTTGG-3’ (forward); 5’-CAGACTTTGGTTCTCCAACTT-3’ (reverse); MMP-9, 5’-AAATGTGGGTGTACACAGGC-3’ (forward); 5’-TTCACCCGGTTGTGGAAACT-3’ (reverse). The amplification products (300 bp for MMP-2; 309 bp for MMP-9) were electrophoresed on a 0.5% ethidium bromide-containing 1.5% agarose gel in Tris-borate-EDTA buffer to verify that the primers amplified the correct MMP isoform. Bands were visualized using a UV-transilluminator. At all ages, only a single amplification product of the expected size was visualized.
Quantitative RT-PCR
Changes in hippocampal RNA expression levels were determined from samples taken at P0, P4, P8, P12, P16 and adulthood by quantitative real-time RT-PCR (qRT-PCR) using fluorogenic Taqman probes to MMP-2, MMP-9 and glyeraldehyde-3-phosphate dehydrogenase (GAPDH; Applied Biosystems, Foster City, CA), used as a reference gene. PCR amplification was performed on a thermocycler (ABI Prism 7900HT; Applied Biosystems) by Mount Sinai’s Quantitative PCR Shared Resource Facility. qRT-PCR for MMP-2, MMP-9 and GAPDH was performed in three replicates (from n = 6 rats per age) using a total reaction volume of 20 µl, containing 1 µl of 20× Assays-on-Demand Gene Expression Assay (Applied Biosystems), 10 µl of 2× Taqman Universal PCR Master Mix (Applied Biosystems) and 50 ng of cDNA template. Fluorescent data from the PCR amplification were converted into cycle threshold (CT) values. No significant changes in GAPDH CT values were detected over the developmental ages studied. For each age per rat, the ΔCT value was determined by subtracting the three-replicate average GAPDH CT value from the three-replicate average MMP-2 or MMP-9 CT value. The ΔΔCT value for MMP-2 or -9 was then calculated for each age per rat by subtracting the ΔCT value of each developmental age per rat from the ΔCT value of an adult. A final average ΔΔCT value ± SEM was then calculated for each age and displayed as percent of adult values.
In situ hybridization histochemistry and analysis
Hybridization of radioactive complementary RNA (cRNA) probes (a gift from Dr. Dylan Edwards, University of East Anglia, United Kingdom) to free-floating, paraformaldehyde-perfused hippocampal tissue sections (n=5 sections per rat) at different postnatal ages (n = 4 rats per age) was used to determine the anatomical localization of MMP-2 and MMP-9 mRNAs. Details of the probes, hybridization procedures and quantitative densitometric analysis have been described previously (Das et al., 1997; Gil et al., 2002; Bozdagi et al., 2007). Specificity of these probes has been confirmed previously by Northern blot analysis (Das et al., 1997). Sense and antisense cRNA probes were transcribed from 1–2µg of purified DNA plasmid template in the presence of 35S -UTP with T7 or T3 polymerase using an RNA transcription kit (Stratagene, Cedar Creek, TX). After overnight hybridization, ribonuclease A treatment, and a series of increasingly stringent SSC washes (final wash, 0.1× SSC at 55°C for 30 minutes), sections were mounted, Nissl-counterstained, and dried. A series of sections from rats of 4–6 different ages hybridized to the same probe solution were exposed together, along with 14C plastic standards, to autoradiographic film (β-max, Amersham, Arlington Heights, IL) for 9–14 days. Film autoradiograms were then used to quantify relative intensity of probe hybridization by densitometry. Autoradiograms were scanned at high resolution, maintaining constant settings across ages. Images were then imported into ImageJ (v1.40), and aligned with high-resolution scanned images of the Nissl-staining on the same sections from which the autoradiograms were derived. Based on cytoarchitecture, sampling boxes of constant dimensions were then placed over stratum (st.) pyramidale (CA1 and CA3), st. radiatum (CA1), and the dentate gyrus granule cell layer (3–6 sampling boxes per region). Optical density readings were taken and converted to absolute values of radioactivity (nCi/g) by reference to optical density readings of the radioactive standards, used to generate a standard curve of known amounts of radioactivity. Control sections processed with sense probes showed no probe hybridization (data not shown).
Immunolabeling
Brains of animals of each age (n = 3–5 animals per age) were sectioned at 50 µm on a vibratome and blocked for 1 hour in 3% normal goat serum (Jackson Laboratory, Germantown, NY) at room temperature. Sections were then incubated in one or more of the following primary antibodies or sera (Table 1): rabbit polyclonal anti-MMP-2 or MMP-9 (1:500, Torrey Pines Biolabs, Houston, TX); mouse monoclonal anti-MMP-9 (1:500, NeuroMab Facility, Davis, CA); mouse monoclonal anti-postsynaptic density 95 (PSD-95) (1:500, Affinity BioReagents, Rockford, IL); mouse monoclonal anti-L1CAM (1:50, Abcam) or mouse monoclonal anti-synaptophysin (1:1000, Sigma-Aldrich, Saint Louis, MO). Primary antibody binding was visualized using species-appropriate, direct fluorophore-conjugated secondary antibodies (Alexa conjugates, 1:400; Jackson ImmunoResearch, West Grove, PA). Sections were analyzed by confocal microscopy. Double- or triple-labeled material (e.g. Figs. 6–8) was captured in single-optical sections.
Table 1.
Primary Antibodies
| Antibody | Host, isotype | Immunogen | Source | Cat. # | Clone # | Lot # |
|---|---|---|---|---|---|---|
| MMP-2 | Rb | aa 115–447, 72 kDa rat MMP-2 | Torrey Pines Biolabs | TP220 | n/a | 012316 |
| MMP-9 | Rb | aa 107–463, 92 kDa rat MMP-9 | Torrey Pines Biolabs | TP221 | n/a | 120515 |
| MMP-9 | Ms, IgG2a | aa 1–708 rat MMP-9 (full-length) | NeuroMab Facility | 75—100 | L51/82 | n/a |
| PSD-95 | Ms, IgG2a | purified rat PSD-95 | Affinity BioReagents | MA1-045 | 6G6-1C9 | n/a |
| Synaptophysin | Ms, IgG 1 | rat retina synaptosome | Sigma-Aldrich | S5768 | SVP-38 | n/a |
| L1/Ng-CAM | Ms, IgG1 | C-terminus of chicken NgCAM | Abcam (M. Grumet) | ab24345 | 2C2 | n/a |
Rb, rabbit; Ms, mouse
Antibody characterization (Table 1)
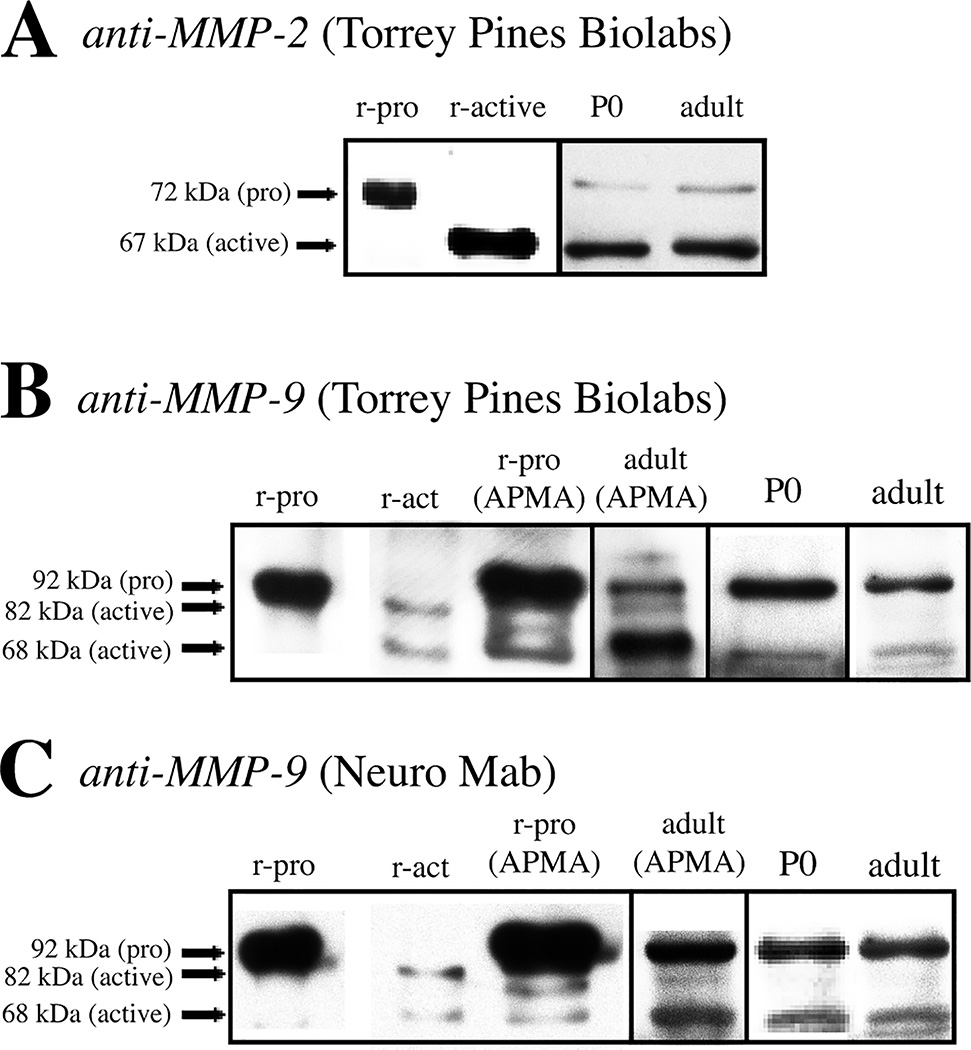
The rabbit polyclonal MMP-2 antiserum was raised against a recombinant catalytic domain of the 72 kDa form of rat MMP-2. The rabbit polyclonal MMP-9 antiserum was raised against a recombinant catalytic domain of the 92 kDa form of rat MMP-9. The mouse monoclonal MMP-9 antibody was raised against a fusion protein of full-length rat MMP-9. The specificity of the rabbit polyclonal MMP-2 and -9 antisera has been detailed previously (Nagy et al., 2006). Briefly, by immunoblotting, each antiserum recognizes human recombinant pro- and active-forms of the appropriate MMP (MMP-2 or MMP-9) against which it was raised, but does not cross-react with recombinant pro- or active-forms of the other (inappropriate) MMP. Additionally, bands of the appropriate molecular mass are detected by blotting hippocampal lysates prepared from adult (Bozdagi et al., 2007; Nagy et al., 2007) or young (3–6 week-old) rats (Nagy et al., 2006). We confirmed and extended such characterization here (Fig. 1) using immunoblotting methods that have been detailed previously (Nagy et al., 2006; Nagy et al., 2007). MMP-2 exists as a 72 kDa pro- (inactive) form and a 67 kDa active-form, and human recombinant versions of these proteins (Calbiochem, San Diego, CA) are recognized by the MMP-2 antisera by immunoblotting (Fig. 1A, left side). Further, immunoblotting whole-hippocampal lysates prepared from P0 or P60 adult rats (n=4 animals per age) shows that the pro- and active-forms of MMP-2 are recognized developmentally as well as in adulthood (Fig. 1A, right side). MMP-9 exists as a 92 kDa pro-form and two active forms of 82 and 68 kDa (Sternlicht and Werb, 2001). Both the rabbit polyclonal MMP-9 antiserum (Fig. 1B) and the mouse monoclonal MMP-9 antibody (Fig. 1C) recognize human recombinant versions of the pro- and the two active-forms of the protein. Immunoblots of P0 or adult rat whole-hippocampal lysates show that each recognizes the pro-form of MMP-9, however only a band corresponding to the 68 kDa active-form is detectable (Fig. 1B, C). To further verify the specificity of the active forms of MMP-9, we pre-treated recombinant pro-MMP-9 and adult rat hippocampal lysates with 1 mM of p-Aminophenylmercuric Acetate (APMA; Sigma-Aldrich, St. Louis, MO) for 2 hrs at 37°C, then stopped the reaction by adding 25 mM EDTA prior to immunoblotting. APMA is an organomercurial compound that induces some autoactivation of the pro form. Under these conditions, both the MMP-9 antiserum and antibody recognize the two active forms of MMP-9 (Fig. 1B, C). This suggests that in brain, the 68 kDa active-MMP-9 is the predominant active form during development and in adulthood.
Fig. 1. Characterization of MMP-antisera and antibodies.
Immunoblots showing antisera and antibody specificity by probing human recombinant pro and active MMP-2 (A) and human recombinant active and pro MMP-9 (B, C). Antisera for MMP-2 recognized the pro form of the protein (72 kDa) and the active form (67 kDa). The MMP-9 antisera from Torrey Pines (B) and the antibody from Neuro Mab (C) both recognized one recombinant pro band at 92 kDa and two recombinant active bands at 82 and 68 kDa. Pro-recombinant MMP-9 and lysates were activated with the organomercurial compound p-Aminophenylmercuric Acetate (APMA) that induces some autoactivation of MMPs. Immunoblots of the activated product showed both active forms at the same molecular weights as recombinant active MMP-9, as well as some remaining pro form that was not activated. However, in hippocampal lysates, both the MMP-9 antisera (B) and antibody (C) recognized the 92 kDa pro-form, but only the 68 kDa active form, suggesting that the latter is the major active form of MMP-9 in both developing (P0) and adult hippocampus.
The L1CAM antibody was raised against chicken NgCAM and recognizes a C-terminal portion that is conserved with mammalian L1CAM (Friedlander et al., 1994). By western blot of early postnatal mouse hippocampal lysates, the antibody recognizes full-length (~220 kDa) L1CAM (Montag-Sallaz et al., 2002; Tian et al., 2007). This antibody does not cross-react with mouse CHL1 (Montag-Sallaz et al., 2002). The PSD-95 and synaptophysin antibodies have been characterized extensively as detailed on the antibody database maintained by the Journal of Comparative Neurology. In general, the immunolabeling patterns of L1CAM, PSD-95 and synaptophysin that we obtained were entirely consistent with the many published accounts using these same antibodies under similar experimental conditions that we used here.
In-situ gelatin substrate zymography
Fluorescent in-situ gelatin substrate zymography was used to localize proteolytic (gelatinolytic) activity, and was carried out using procedures previously detailed (Gawlak et al., 2009). Briefly, animals (n = 3–4 rats per age) were perfused with an alcohol fixative made of one volume of methanol and three volumes of 95% ethanol. Brains were stored in this solution for 6 hours in 4°C and then stored overnight at −20°. Brains were subsequently washed in 99.8% ethanol and infiltrated with polyester wax by taking specimens through a graded series of ethanol/polyester wax solutions (Fisher Scientific Company, Pittsburg, PA) at increasing temperatures until reaching the final solution of 100% wax at 42°C. After embedding in 100% polyester wax, brains were cut on a microtome into 6 µm-thick sections through the dorsal hippocampus. Sections were mounted on silane-coated slides, dewaxed in absolute ethanol at 37°C for 5 minutes, submerged in PBS and then pre-incubated in water for 37°C for 40 min. The slides were then overlaid with the fluorogenic substrate Dye-Quenched (DQ) gelatin (15 µg/mL; Molecular Probes, Eugene, OR) in buffer supplied by the manufacturer for 40 min at 37°C. Slides were then washed in PBS and fixed in 4% paraformaldehyde at room temperature for 15 min. Some sections were coverslipped at this point and examined by confocal microscopy. Other sections were subsequently immunofluorescently labeled for MMP-2 or MMP-9 singly, or double-labeled with PSD-95 or synaptophysin, using species-appropriate, fluorescently-conjugated secondary antibodies (Jackson Labs). Specificity of the gelatinolytic reaction was verified in control experiments by incubating sections with the broad-spectrum MMP inhibitor GM6001 (500 µM; Millipore, Billerica, MA) prior to and during exposure to DQ-gelatin. Under these conditions no gelatinolytic activity was observed (data not shown) as expected (Gawlak et al., 2009).
Photomicrographs and figure layout
Acquired microscope images were imported into Adobe Photoshop CS5.1 (Adobe Systems, San Jose, CA) where minimal adjustments in contrast, brightness and sharpness were made if necessary. Bar graphs were assembled in Adobe Illustrator (CS5.1). For the in situ hybridization analyses shown in Figs. 3 and 4, the quality of the Nissl staining in the counterstained sections was adequate for assigning the positions of the sampling boxes used for quantification, as described above, however the enzymatic treatment of the sections required for the in situ protocol significantly diminished the quality of the Nissl staining for photographic purposes. Thus, the Nissl stained section shown in Fig. 3A is an untreated, surrogate section taken from a P0 rat to convey lamination and to illustrate how we used cytoarchitecture to guide placement of sampling boxes on the film autoradiogram. Final figure layout was achieved with Adobe InDesign CS5.5.
Statistical analysis
Comparisons across ages were made with one-way ANOVA and Scheffé’s post-hoc tests; pair-wise comparisons between each developmental age and adults were made with unpaired Student’s t-tests with a Bonferroni correction for multiple comparisons. A minimum criterion of p < 0.05 (after correction) was used to determine statistical significance. In all figures, data are mean values ± SEM.
RESULTS
MMP-2 and -9 mRNA levels peak during first postnatal week
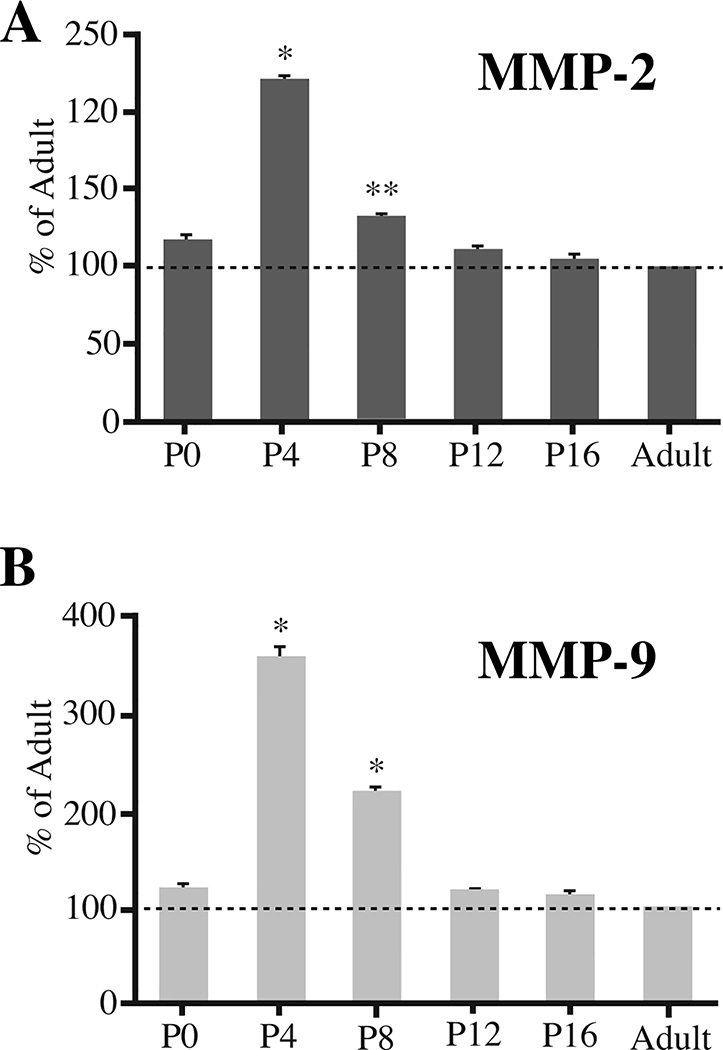
Quantitative real-time RT-PCR (qRT-PCR) was used to evaluate changes in overall levels of MMP-2 (Fig. 2A) and MMP-9 (Fig. 2B) transcripts in whole-hippocampus from P0 through adulthood. At P0, levels of MMP-2 transcripts were comparable to adult values, but by P4 they had increased about 2-fold (220.7 ± 2.1% of adult levels). By P8, levels of MMP-2 transcripts were lower in comparison with those at P4, but remained significantly higher than those in adulthood (132.2 ± 1.6% of adult levels). By P12 and thereafter, MMP-2 transcript levels returned to adult values. Developmental changes in MMP-9 transcript levels followed a similar profile to those described for MMP-2. Levels of MMP-9 transcripts at P0 were comparable to those in adults; by P4, transcript levels were about 3.5 fold higher than those in adults (355.3 ± 12.7%), dropping by P8 (216.8 ± 1.1% of adult levels) and returning to adult-levels by P12 and thereafter.
Fig. 2. Developmental changes in expression levels of MMP-2 and MMP-9 in hippocampus.
Relative transcript levels of MMP-2 (A) and MMP-9 (B) and GAPDH (as a standard internal control) were evaluated by quantitative real-time RT-PCR (qRT-PCR). Both MMPs show peak expression levels at P4 in comparison with adult levels (*p<0.001). Transcript levels decline somewhat by P8, but remain significantly higher than those of adults (*p<0.001 or **p<0.01).
Distribution of MMP-2 and -9 transcripts in developing hippocampal subfields
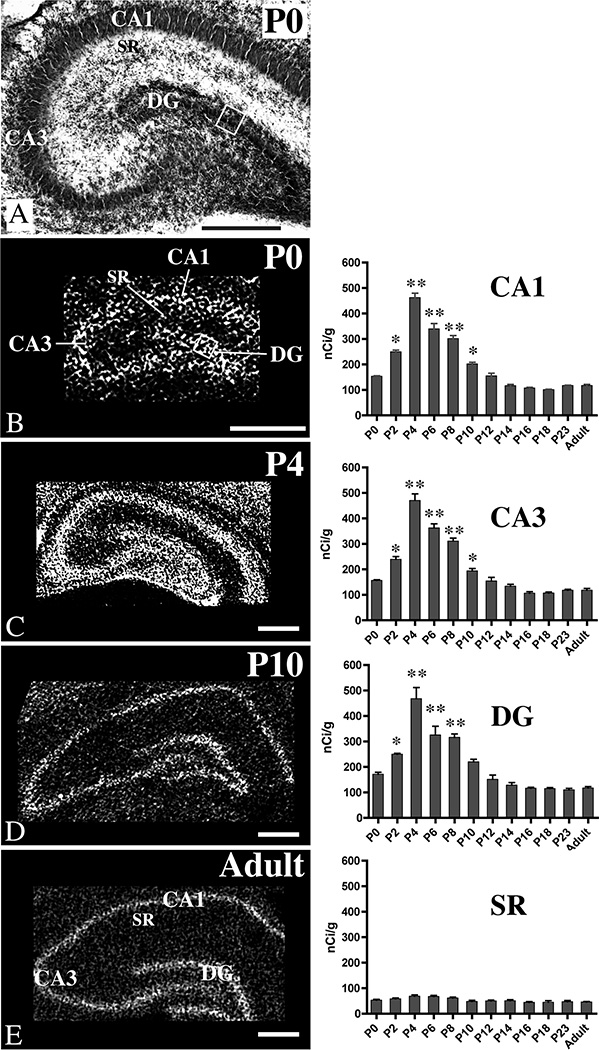
In situ hybridization histochemistry using transcript-specific, radioactive cRNA probes was used to evaluate the distribution of MMP-2 and -9 mRNAs in the subfields of hippocampus during postnatal development (Figs. 3, 4). At each age examined, hybridization of MMP-2 and MMP-9 probes was concentrated in the pyramidal cell layer (CA1 and CA3) and the DG granule cell layer (or the anlage of the DG granule cell layer at P0 (see Fig. 3A), since this layer, which is first evident morphologically ~E20 (Bayer, 1980b), forms definitively largely after P0). Film densitometry revealed that in each of the subfields of hippocampus (CA1, CA3 and DG), relative levels of MMP-2 probe hybridization at P0 were comparable to those in adulthood, rose sharply to peak at P4, then declined gradually to adult levels by ~P12–P14 (Fig. 3). In contrast, there were no changes in intensity of MMP-2 probe hybridization in CA1 stratum radiatum over the ages examined (Fig. 3).
Fig. 3. Distribution of MMP-2 mRNAs in developing and adult hippocampus.
Nissl-stained section through hippocampus taken at P0 (A) and representative film autoradiographs (left column, B–E) of hippocampal sections taken at P0 through adulthood. The autoradiographs (B–E) show patterns of probe hybridization to MMP-2 mRNAs in CA1, CA3, stratum radiatum (SR) and the dentate gyrus (DG). Autoradiographs have been inverted, so hybridization signal is white. Scale bars = 250 µm (A), 500 µm (B–E). The graphs (right column) show developmental changes in relative intensity levels of probe hybridization in pyramidal cell body layers (CA1 and CA3), the granule cell layer (DG) or its anlage (at P0), and CA1 stratum radiatum (SR). Densitometric readings obtained from sampling boxes (3–6 per region) were converted to measures of radioactivity (nCi/g) by reference to 14C standards. Placement of the sampling boxes used for optical density measurements was guided by cytoarchitecture visualized on Nissl-stained sections. An example of one sampling box is illustrated by the white boxes overlying the anlage of the DG granule cell layer in the P0 sections shown in A and B. *p<0.01 or **p<0.001 vs adults.
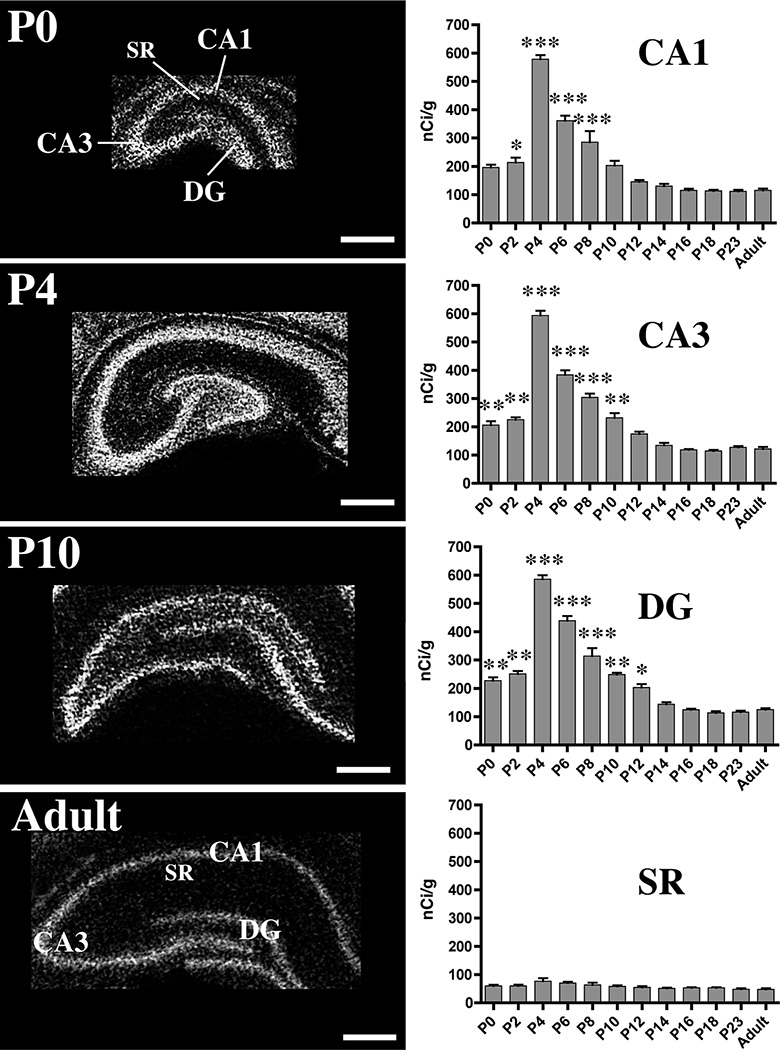
Fig. 4. Distribution of MMP-9 mRNAs in developing and adult hippocampus.
Representative film autoradiographs (left column) of hippocampal sections taken from P0 through adulthood show patterns of probe hybridization to MMP-9 mRNAs in CA1, CA3, stratum radiatum (SR) and the dentate gyrus (DG). Autoradiographs have been inverted such that hybridization signal is white. Scale bars = 500 µm. The graphs (right column) show developmental changes in relative intensity levels of probe hybridization in pyramidal cell body layers (CA1 and CA3), the granule cell layer (DG) or its anlage at P0, and CA1 stratum radiatum (SR). Densitometric readings were converted to measures of radioactivity (nCi/g) by reference to 14C standards. *p<0.05, **p<0.01, or ***p<0.001 vs. adults. Other conventions as in Figure 3.
The developmental pattern of probe hybridization to MMP-9 mRNAs was similar, but not identical to that of MMP-2 (Fig. 4). At P0, intensity of probe hybridization in CA3 and the anlage of the DG granule cell layer was slightly, but significantly, higher in comparison with adult levels. In all subfields, the intensity of probe hybridization in cell-dense layers of each subfield increased rapidly to peak at P4, then declined gradually to adult-like levels by ~P12–P14. There were no changes in intensity of MMP-9 probe hybridization in CA1 stratum radiatum over the ages examined (Fig. 4). Taken together, the developmental profile of MMP-2 and -9 transcript levels shown by probe hybridization is consistent with changing transcript levels shown by qRT-PCR.
Localization of MMP-2 and -9 immunoreactivity in developing hippocampus
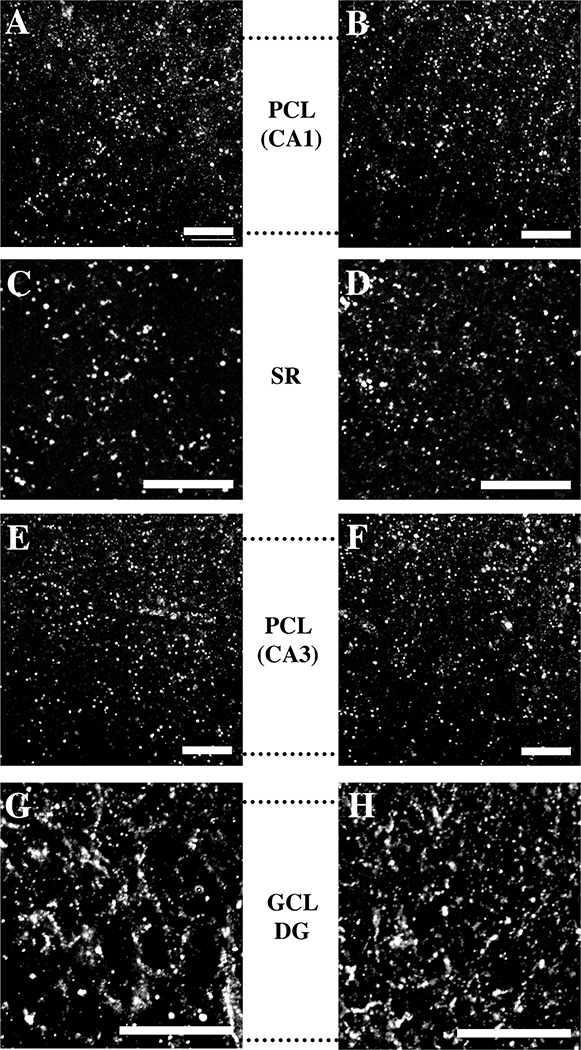
We localized MMP-2 and MMP-9 in developing hippocampus by immunolabeling with MMP-specific antisera or antibodies that recognize both the pro (inactive) and active forms of the proteins (Fig. 1). MMP-2 and -9 immunoreactivity was present in each of the hippocampal subfields at all ages examined, in a pattern that was largely similar across ages. Figure 5 shows representative examples of the immunolocalization pattern in each subfield at P8. MMP-2 and -9 immunoreactive profiles were punctate; in pyramidal cell layers of CA1 and CA3 (Fig. 5A, B, E, F) and the granule cell layer of the DG (Fig. 5G, H), such immunoreactive puncta outlined unlabeled cell somata. Some immunolabeled puncta in the DG were larger in comparison with the CA fields, and in some instances, had the appearance of short fragments of processes (Fig. 5G, H). In the neuropil of CA1 stratum radiatum (Fig. 5C, D), immunoreactive puncta were densely and evenly distributed.
Fig. 5. Localization of MMP-2 and -9 immunoreactivity in developing hippocampus.
Immunolabeling of MMP-2 (left column, A, C, E, G) and MMP-9 (right column, B, D, F, H) in hippocampal sections at P8 in area CA1 and CA3 pyramidal cell layers (PCL, A, B, E, F) and area CA1 stratum radiatum (SR, C, D). Immunolabeling was also present in the granule cell layer (GCL, G, H) of the dentate gyrus (DG). Dotted lines correspond to the positions of laminar borders. Scale bars = 20 µm.
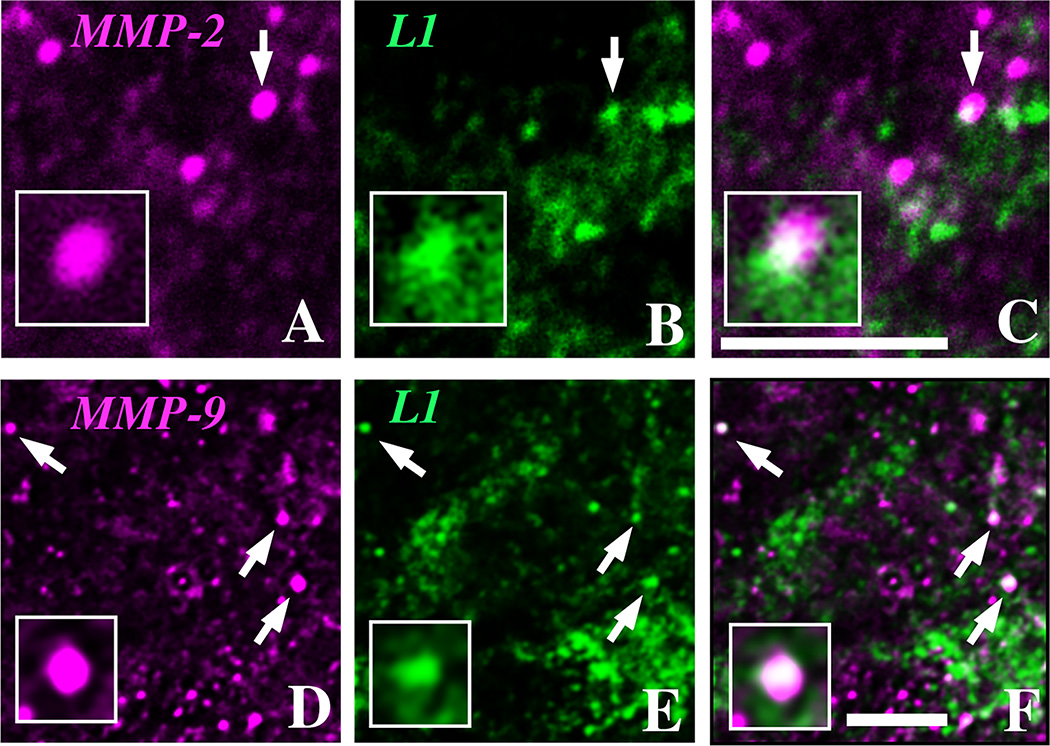
Previous studies suggest that MMPs can localize to growing axons or other processes during development (McFarlane, 2003). Thus, we next investigated whether MMP-2 and/or -9 immunolabeling corresponded to developing processes during early postnatal hippocampal development. We used L1CAM, a member of the Ig superfamily of cell adhesion molecules, as a marker of growing axons, terminals and other processes (Demyanenko et al., 1999; Schuster et al., 2001; Mintz et al., 2003; Nakamura et al., 2006) and examined sections at P4, a stage when mossy fibers and other intrinsic pathways are growing in the developing hippocampus (Amaral and Dent, 1981). We found that, as reported previously, L1CAM immunolabeling was present throughout all fields of hippocampus (data not shown). Qualitative inspection of high-power confocal images showed that some, but not all, L1CAM-immunolabeled structures were co-labeled with antisera to MMP-2 (Fig. 6A–C) or MMP-9 (Fig. 6D–F).
Fig. 6. MMPs codistribute with L1CAM.
MMP-2 (A, magenta) and MMP-9 (D, magenta) immunopositive puncta co-distribute in single optical sections with axonal growth marker L1CAM (B, E, green) in area CA1 in P4 hippocampal sections shown in single images (arrows, A, B, D, E) and as overlays (arrows, C, F), where white pixels indicate regions of overlap of the two labels. Higher-power insets show examples of co-distribution. Scale bars = 5 µm.
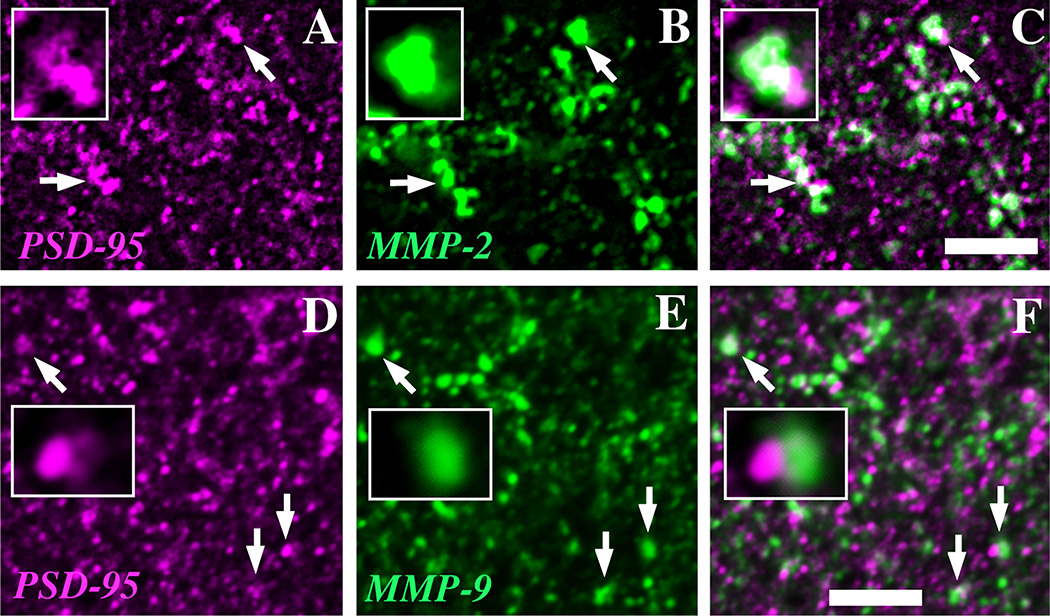
MMP-9 is localized in part to perisynaptic regions in adult hippocampus (Nagy et al., 2006; Bozdagi et al., 2007; Gawlak et al., 2009). Accordingly, we used PSD-95 as a marker of excitatory synapses (Kennedy, 1997) and examined area CA1 for codistribution with MMP-2 or -9 at P8, the beginning of the peak period of synaptogenesis in developing area CA1 (Steward and Falk, 1991). Figure 7 shows that some PSD-95 immunolabeled puncta codistributed with MMP-2 (arrows, Fig. 7A–C) or MMP-9 (arrows, Fig. 7D–F), or in other cases, showed direct apposition (insets, Fig. 7D–F). Taken together, these data are consistent with the idea that MMP-2 and -9 are associated, at least in part, with growing processes and developing synapses in early postnatal hippocampus.
Fig. 7. MMPs codistribute with PSD-95 in developing hippocampus.
Some puncta immunopositive for the postsynaptic marker PSD-95 (A, D, magenta) codistribute in single optical sections with immunolabeling for MMP-2 (B, green) and MMP-9 (E, green) within the neuropil of area CA1 in P8 hippocampal sections shown in single images (arrows, A, B, D, E) and as overlays (arrows, C, F), where white pixels indicate region of overlap of the two labels. Higher power insets show patterns of co-distribution (insets, top row) or where MMP-9 and PSD-95 puncta show direct apposition (insets, bottom row). Scale bars = 5 µm.
Discrete foci of proteolysis codistribute with MMPs and synaptic markers in developing hippocampus
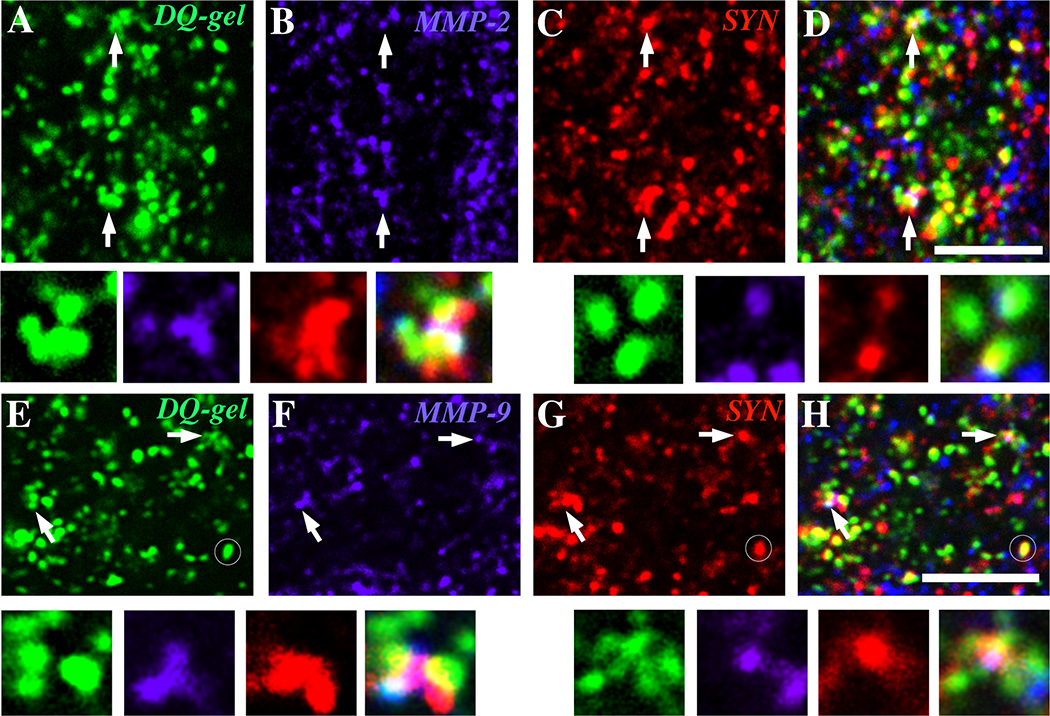
In adult rat hippocampus, MMP-9 immunoreactivity codistributes with focal spots of gelatinolytic activity, suggesting that MMPs contribute in part to such proteolytic activity (Bozdagi et al., 2007; Gawlak et al., 2009). To investigate this in developing hippocampus, we combined high-resolution in-situ zymography (Gawlak et al., 2009) with immunolabeling for synaptic markers and MMP-2 or -9. At all developmental ages examined, proteolytic (gelatinolytic) activity was observed by high-resolution DQ-gelatin zymography in each hippocampal subfield, particularly within the neuropil (arrows, Fig. 8A, E). Such activity appeared mostly punctate, although occasionally some interspersed cellular processes were also labeled. Many, but not all, of these gelatinolytic puncta co-distributed with immunolabeling for MMP-2 (Fig. 8A, B) or MMP-9 (Fig. 8E, F). Conversely, some MMP immunoreactive profiles were not gelatinolytic. Using a triple-labeling strategy, we determined that many, but not all, of the gelatinolytic/MMP puncta co-distributed with immunolabeling for the presynaptic vesicle marker synaptophysin (arrows, Fig. 8, and insets). Conversely, some gelatinolytic puncta that codistributed with synaptophysin lacked MMP immunoreactivity (circles, Fig. 8E, G, H).
Fig. 8. Foci of proteolysis codistribute with MMPs and synaptic markers.
Gelatinolytic puncta visualized by in situ gelatin-substrate zymography (arrows, A, E) co-distribute in single optical sections with MMP-2 (arrows, B) and MMP-9 (arrows, F) immunopositive puncta (blue) and the presynaptic marker synaptophysin (arrows, C, G, red) in neuropil of area CA1 in P8 hippocampal sections. Higher power insets show patterns of co-distribution or apposition of the three labels. There are numerous MMP-2 and MMP-9 puncta that are not gelatinolytic, which presumably represent inactive forms, as well as gelatinolytic puncta that codistribute with synaptophysin, but not with MMPs (circles in E, G, H), suggesting that proteases other than MMP-2 and -9 contribute to gelatinolysis. Scale bars = 5 µm.
DISCUSSION
We utilized a combination of biochemical and anatomical techniques to investigate expression and localization patterns of MMP-2 and -9 and their presumptive proteolytic activities during early postnatal development of rat hippocampus, a time of considerable cellular remodeling as neuronal architecture and connectivity is becoming established (Zimmer and Haug, 1978; Bayer, 1980b; Amaral and Dent, 1981). We found that levels of both MMP transcripts peak in all hippocampal subfields by P4, then decline to adult levels by ~P12. Moreover, a combination of in situ zymography and immunolocalization revealed numerous foci of MMP-positive gelatinolytic activity, some of which codistributed with markers of synapses and growing axons or other processes. Together, these observations indicate that MMP-2 and -9 are at the right place and time to drive active remodeling of the local pericellular microenvironment necessary for proper development of axonal pathways, lamination and synaptic connections in hippocampus.
Peak expression during first postnatal week
Our qRT-PCR and in situ hybridization analyses showed that levels of both MMP-2 and -9 transcripts peaked during the first postnatal week before declining to adult levels ~P10–12. This time-course of peak MMP expression is generally similar to that reported for several MMPs, including MMP-2 and -9, within developing rodent cerebellum (Vaillant et al., 1999; Aizawa et al., 2001; Ayoub et al., 2005), superior colliculus (Oliveira-Silva et al., 2007) and other brain regions (Jaworski, 2000; Ulrich et al., 2005). Because MMP activity generally can be regulated at the levels of transcription, translation and post-translation (Sternlicht and Werb, 2001), levels of MMP proteolytic activity cannot always be accurately predicted by levels of mRNA. Nevertheless, when combined with our western blot, immunolocalization and in situ zymographic analyses, MMP-2 and -9 protein levels appear abundant and the enzymes are likely to be proteolytically active during this early period of enhanced mRNA expression.
The first postnatal week in rodents is a period of considerable growth and differentiation of the hippocampal formation that includes cellular migration, programmed cell death, axonal and dendritic growth and the onset of a major wave of spine and synapse formation (Zimmer and Haug, 1978; Bayer, 1980b; Gould et al., 1991; Steward and Falk, 1991; Ferrer et al., 1994). There is evidence in other developing systems that proteolysis by MMPs and related family members contributes to such developmental events (Rivera et al., 2010). For example, in cerebellum, perturbing MMP-9-mediated proteolysis by neutralizing antibodies or by deletion of the MMP-9 gene delays migration of granule cell precursors (Vaillant et al., 2003; Ayoub et al., 2005). Similarly, ablation of the gene encoding the disintegrin metalloproteinase ADAM10 in neural progenitor cells leads to profoundly disturbed patterns of neuronal migration and lamination in mouse neocortex (Jorissen et al., 2010). Molecularly, such aberrant cellular positioning following perturbation of MMP proteolysis may reflect critical, early roles of MMPs in processing the secreted glycoprotein Reelin. Reelin is necessary for proper neuronal migration and lamination in hippocampus and other cortical structures (Tissir and Goffinet, 2003) and undergoes significant extracellular proteolytic processing by several different metalloproteinases for full bioactivity in vivo (Lambert de Rouvroit et al., 1999; Jossin et al., 2007; Tinnes et al., 2011; Hisanaga et al., 2012; Krstic et al., 2012; Tinnes et al., 2013).
There is a period of apoptosis in the hippocampus during the first postnatal week (Gould et al., 1991; Ferrer et al., 1994). The peak hippocampal expression of MMP-9 during this period may reflect control of such programmed cell death, since recent evidence indicates that MMP-9 can regulate apoptosis through proteolytic processing of laminin, which in turn affects signaling cascades triggered by laminin-β1 integrin interactions that affect neuronal survival (Murase and McKay, 2012).
Peak MMP expression levels during the first postnatal week also correspond to a period of major axonal growth and targeting in hippocampus as intrinsic and extrinsic circuitry is establishing (Fricke and Cowan, 1977; Loy et al., 1977; Singh, 1977; Amaral and Dent, 1981). Metalloproteinases, including MMPs, have been shown previously to enable axon pathfinding and process outgrowth in a variety of developmental models (McFarlane, 2003; Hehr et al., 2005). These data are consistent with our finding that both MMP-2 and -9 immunoreactivity in early postnatal hippocampus codistributed with profiles labeled by L1CAM, although we did not attempt here to identify the origin of such L1CAM-labeled processes. The mechanisms underlying MMP-mediated modulation of process outgrowth include both permissive and proactive events (Rivera et al., 2010). MMPs can cleave inhibitory extracellular matrix (ECM) proteins such as chondroitin sulfate proteoglycans (CSPGs) (Nakamura et al., 2000) that are expressed in hippocampus and constrain fiber trajectory (Wilson and Snow, 2000; Butler et al., 2004), thereby enabling a permissive environment for axonal growth and targeting. MMPs can also activate or terminate signaling cascades that modulate axon growth and targeting through the proteolytic processing of cell-surface or ECM-harbored molecules (Galko and Tessier-Lavigne, 2000). For example, classic Cadherins influence guidance of hippocampal mossy fibers during this developmental window (Bekirov et al., 2008; Williams et al., 2011) and MMP-mediated ectodomain cleavage of Cadherins has been characterized (Marambaud et al., 2002; Monea et al., 2006; Warren et al., 2012). Similarly, the role of Ephrins and their Eph receptors in the regulation of axonal outgrowth during hippocampal development is well-described (Martinez et al., 2005), and interactions between these molecules can be terminated by metalloprotease-mediated cleavage of either, affecting axon growth and repulsion (Hattori et al., 2000; Ethell and Ethell, 2007; Lin et al., 2008). Reelin, which is processed by MMPs as noted above, is also required for ingrowth of afferents from the entorhinal cortex (Del Rio et al., 1997), and thus MMP-2 and/or -9 may be regulating availability of active forms of Reelin to modulate such afferent ingrowth to the dentate gyrus. Finally, the BDNF receptor TrkB, which when inhibited pharmacologically impairs hippocampal mossy fiber growth (Tamura et al., 2006), becomes activated by MMPs through proteolytic conversion of pro-BDNF to mature BDNF (Hwang et al., 2005). While all or any of these speculative molecular targets of MMP-2 and/or -9 are likely to be important contributors to proper establishment of hippocampal circuitry, it is noteworthy that there are no overt defects in hippocampal circuitry reported for mice in which genes encoding either MMP-2 or -9 have been ablated (Itoh et al., 1997; Nagy et al., 2006). This likely reflects redundancy in the molecular targets of the approximately 20 or more different MMPs and related metalloproteinases that are expressed in brain (Huntley, 2012), as well as the possibility of developmental compensation in levels of remaining MMPs (Esparza et al., 2004).
MMP-mediated proteolysis at developing synapses
A major period of synaptogenesis and concurrent elaboration of dendritic spines starts in the hippocampus towards the end of the first postnatal week (Amaral and Dent, 1981; Steward and Falk, 1991; Fiala et al., 1998) as MMP mRNA expression levels begin to taper towards adult values. Using hippocampus from animals aged P8 as representative of the onset of this rapid period of synapse and spine acquisition, we showed that foci of proteolytic activity (gelatinolysis) codistributed with immunolocalization of MMP-2 and -9 as well as synaptic markers, a pattern that is also typical of adult hippocampus (Bozdagi et al., 2007; Gawlak et al., 2009). These data support the conclusion that these particular MMPs are proteolytically active in and around synapses as they are forming. There were, however, gelatinolytic puncta that lacked MMP immunolocalization, indicating that other gelatinolytic proteases may also be contributing. Net MMP proteolytic activity is ultimately controlled by the balance between levels of MMPs and the TIMPs, which are also developmentally regulated in brain (Fager and Jaworski, 2000).
Functionally, ~P8 is an age just prior to the developmental onset of L-LTP induced by naturalistic theta-burst stimulation (TBS) of CA1 synapses (Cao and Harris, 2012). At more mature CA1 synapses, TBS activates MMP-9, while synaptic delivery of proteolytically-active MMP-9 drives synaptic potentiation and dendritic spine enlargement concurrently through β1-integrin-mediated signaling in spine heads (Wang et al., 2008), where β1-integrins are concentrated (Mortillo et al., 2012). Thus, during early postnatal development, perisynaptically localized MMP-mediated proteolysis may be signaling through β1-integrins to drive functional synapse maturation (Chavis and Westbrook, 2001) or the onset of adult-like properties of synapse and spine plasticity. Mechanistically, MMP-integrin signaling could be affecting compartmentalization and trafficking of surface glutamate receptors (Michaluk et al., 2009), degrading perisynaptic ECM proteins such as CSPGs that act to constrain spine development and plasticity (Orlando et al., 2012), or modulating transsynaptic adhesive interactions that control synaptic strength (Peixoto et al., 2012).
Acknowledgments
Grant Sponsor: National Institute of Mental Health grant MH075783.
We are grateful for the services of the DNA core in the Department of Genetics and Genomic Sciences, and thank Ms. Noreen Mall in the Biorepository Cooperative and Histology Service Shared Resource Facility at Mount Sinai School of Medicine for help with tissue processing.
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors declare no known conflicts of interest.
ROLE OF AUTHORS: P.K.A. and G.W.H. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: P.K.A., G.W.H.; Acquisition of data: P.K.A.; Analysis and interpretation of data: P.K.A., G.W.H.; Drafting of the manuscript: P.K.A., G.W.H.
LITERATURE CITED
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Ayoub AE, Cai TQ, Kaplan RA, Luo J. Developmental expression of matrix metalloproteinases 2 and 9 and their potential role in the histogenesis of the cerebellar cortex. J Comp Neurol. 2005;481:403–415. doi: 10.1002/cne.20375. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980a;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol. 1980b;190:115–134. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- Bekirov IH, Nagy V, Svoronos A, Huntley GW, Benson DL. Cadherin-8 and N-cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus. 2008;18:349–363. doi: 10.1002/hipo.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Nagy V, Kwei KT, Huntley GW. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J Neurophysiol. 2007;98:334–344. doi: 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CD, Schnetz SA, Yu EY, Davis JB, Temple K, Silver J, Malouf AT. Keratan sulfate proteoglycan phosphacan regulates mossy fiber outgrowth and regeneration. J Neurosci. 2004;24:462–473. doi: 10.1523/JNEUROSCI.3040-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Harris KM. Developmental regulation of the late phase of long-term potentiation (L-LTP) and metaplasticity in hippocampal area CA1 of the rat. J Neurophysiol. 2012;107:902–912. doi: 10.1152/jn.00780.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Das SK, Yano S, Wang J, Edwards DR, Nagase H, Dey SK. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse uterus during the peri-implantation period. Dev Genet. 1997;21:44–54. doi: 10.1002/(SICI)1520-6408(1997)21:1<44::AID-DVG5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature. 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Tsai AY, Maness PF. Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J Neurosci. 1999;19:4907–4920. doi: 10.1523/JNEUROSCI.19-12-04907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza J, Kruse M, Lee J, Michaud M, Madri JA. MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. FASEB. 2004;18:1682–1691. doi: 10.1096/fj.04-2445com. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Fager N, Jaworski DM. Differential spatial distribution and temporal regulation of tissue inhibitor of metalloproteinase mRNA expression during rat central nervous system development. Mech Dev. 2000;98:105–109. doi: 10.1016/s0925-4773(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Tortosa A, Blanco R, Martin F, Serrano T, Planas A, Macaya A. Naturally occurring cell death in the developing cerebral cortex of the rat. Evidence of apoptosis-associated internucleosomal DNA fragmentation. Neurosci Lett. 1994;182:77–79. doi: 10.1016/0304-3940(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke R, Cowan WM. An autoradiographic study of the development of the entorhinal and commissural afferents to the dentate gyrus of the rat. J Comp Neurol. 1977;173:231–250. doi: 10.1002/cne.901730203. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko MJ, Tessier-Lavigne M. Function of an axonal chemoattractant modulated by metalloprotease activity. Science. 2000;289:1365–1367. doi: 10.1126/science.289.5483.1365. [DOI] [PubMed] [Google Scholar]
- Gawlak M, Gorkiewicz T, Gorlewicz A, Konopacki FA, Kaczmarek L, Wilczynski GM. High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience. 2009;158:167–176. doi: 10.1016/j.neuroscience.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Gil OD, Needleman L, Huntley GW. Developmental patterns of cadherin expression and localization in relation to compartmentalized thalamocortical terminations in rat barrel cortex. J Comp Neurol. 2002;453:372–388. doi: 10.1002/cne.10424. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Naturally occurring cell death in the developing dentate gyrus of the rat. J Comp Neurol. 1991;304:408–418. doi: 10.1002/cne.903040306. [DOI] [PubMed] [Google Scholar]
- Gundelfinger ED, Frischknecht R, Choquet D, Heine M. Converting juvenile into adult plasticity: a role for the brain's extracellular matrix. Eur J Neurosci. 2010;31:2156–2165. doi: 10.1111/j.1460-9568.2010.07253.x. [DOI] [PubMed] [Google Scholar]
- Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26:8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- Hehr CL, Hocking JC, McFarlane S. Matrix metalloproteinases are required for retinal ganglion cell axon guidance at select decision points. Development. 2005;132:3371–3379. doi: 10.1242/dev.01908. [DOI] [PubMed] [Google Scholar]
- Hisanaga A, Morishita S, Suzuki K, Sasaki K, Koie M, Kohno T, Hattori M. A disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4) cleaves Reelin in an isoform-dependent manner. FEBS Lett. 2012;586:3349–3353. doi: 10.1016/j.febslet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JJ, Park MH, Choi SY, Koh JY. Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J Biol Chem. 2005;280:11995–12001. doi: 10.1074/jbc.M403172200. [DOI] [PubMed] [Google Scholar]
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- Jaworski DM. Developmental regulation of membrane type-5 matrix metalloproteinase (MT5-MMP) expression in the rat nervous system. Brain Res. 2000;860:174–177. doi: 10.1016/s0006-8993(00)02035-7. [DOI] [PubMed] [Google Scholar]
- Jorissen E, Prox J, Bernreuther C, Weber S, Schwanbeck R, Serneels L, Snellinx A, Craessaerts K, Thathiah A, Tesseur I, Bartsch U, Weskamp G, Blobel CP, Glatzel M, De Strooper B, Saftig P. The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. J Neurosci. 2010;30:4833–4844. doi: 10.1523/JNEUROSCI.5221-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Gui L, Goffinet AM. Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J Neurosci. 2007;27:4243–4252. doi: 10.1523/JNEUROSCI.0023-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- Krstic D, Rodriguez M, Knuesel I. Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators. PloS one. 2012;7:e47793. doi: 10.1371/journal.pone.0047793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert de Rouvroit C, de Bergeyck V, Cortvrindt C, Bar I, Eeckhout Y, Goffinet AM. Reelin, the extracellular matrix protein deficient in reeler mutant mice, is processed by a metalloproteinase. Exp Neurol. 1999;156:214–217. doi: 10.1006/exnr.1998.7007. [DOI] [PubMed] [Google Scholar]
- Lin KT, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J Biol Chem. 2008;283:28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy R, Lynch G, Cotman CW. Development of afferent lamination in the fascia dentata of the rat. Brain Res. 1977;121:229–243. doi: 10.1016/0006-8993(77)90149-4. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Otal R, Sieber BA, Ibanez C, Soriano E. Disruption of ephrin-A/EphA binding alters synaptogenesis and neural connectivity in the hippocampus. Neuroscience. 2005;135:451–461. doi: 10.1016/j.neuroscience.2005.06.052. [DOI] [PubMed] [Google Scholar]
- McFarlane S. Metalloproteases: carving out a role in axon guidance. Neuron. 2003;37:559–562. doi: 10.1016/s0896-6273(03)00089-8. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J Neurosci. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz CD, Dickson TC, Gripp ML, Salton SR, Benson DL. ERMs colocalize transiently with L1 during neocortical axon outgrowth. J Comp Neurol. 2003;464:438–448. doi: 10.1002/cne.10809. [DOI] [PubMed] [Google Scholar]
- Monea S, Jordan BA, Srivastava S, DeSouza S, Ziff EB. Membrane localization of membrane type 5 matrix metalloproteinase by AMPA receptor binding protein and cleavage of cadherins. J Neurosci. 2006;26:2300–2312. doi: 10.1523/JNEUROSCI.3521-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag-Sallaz M, Schachner M, Montag D. Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Mol Cell Biol. 2002;22:7967–7981. doi: 10.1128/MCB.22.22.7967-7981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortillo S, Elste A, Ge Y, Patil SB, Hsiao K, Huntley GW, Davis RL, Benson DL. Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic beta1-integrin. J Comp Neurol. 2012;520:2041–2052. doi: 10.1002/cne.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, McKay RD. Matrix metalloproteinase-9 regulates survival of neurons in newborn hippocampus. J Biol Chem. 2012 doi: 10.1074/jbc.M111.297671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn Mem. 2007;14:655–664. doi: 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, Miura R, Yamaguchi Y, Okada Y. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275:38885–38890. doi: 10.1074/jbc.M003875200. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tamura H, Horinouchi K, Shiosaka S. Role of neuropsin in formation and maturation of Schaffer-collateral L1cam-immunoreactive synaptic boutons. J Cell Sci. 2006;119:1341–1349. doi: 10.1242/jcs.02862. [DOI] [PubMed] [Google Scholar]
- Okulski P, Jay TM, Jaworski J, Duniec K, Dzwonek J, Konopacki FA, Wilczynski GM, Sanchez-Capelo A, Mallet J, Kaczmarek L. TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol Psych. 2007;62:359–362. doi: 10.1016/j.biopsych.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Oliveira-Silva P, Jurgilas PB, Trindade P, Campello-Costa P, Perales J, Savino W, Serfaty CA. Matrix metalloproteinase-9 is involved in the development and plasticity of retinotectal projections in rats. Neuroimmuno. 2007;14:144–149. doi: 10.1159/000110638. [DOI] [PubMed] [Google Scholar]
- Olson ML, Meighan PC, Brown TE, Asay AL, Benoist CC, Harding JW, Wright JW. Hippocampal MMP-3 elevation is associated with passive avoidance conditioning. Regul Pept. 2008;146:19–25. doi: 10.1016/j.regpep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O. Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci. 2012;32:18009–18017. doi: 10.1523/JNEUROSCI.2406-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp AM, Nyilas R, Szepesi Z, Lorincz ML, Takacs E, Abraham I, Szilagyi N, Toth J, Medveczky P, Szilagyi L, Juhasz G, Juhasz G. Visible light induces matrix metalloproteinase-9 expression in rat eye. J Neurochem. 2007;103:2224–2233. doi: 10.1111/j.1471-4159.2007.04917.x. [DOI] [PubMed] [Google Scholar]
- Peixoto RT, Kunz PA, Kwon H, Mabb AM, Sabatini BL, Philpot BD, Ehlers MD. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron. 2012;76:396–409. doi: 10.1016/j.neuron.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci. 2010;30:15337–15357. doi: 10.1523/JNEUROSCI.3467-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster T, Krug M, Stalder M, Hackel N, Gerardy-Schahn R, Schachner M. Immunoelectron microscopic localization of the neural recognition molecules L1, NCAM, and its isoform NCAM180, the NCAM-associated polysialic acid, beta1 integrin and the extracellular matrix molecule tenascin-R in synapses of the adult rat hippocampus. J Neurobiol. 2001;49:142–158. doi: 10.1002/neu.1071. [DOI] [PubMed] [Google Scholar]
- Singh SC. The development of olfactory and hippocampal pathways in the brain of the rat. Anatomy and embryology. 1977;151:183–199. doi: 10.1007/BF00297480. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Falk PM. Selective localization of polyribosomes beneath developing synapses: a quantitative analysis of the relationships between polyribosomes and developing synapses in the hippocampus and dentate gyrus. J Comp Neurol. 1991;314:545–557. doi: 10.1002/cne.903140311. [DOI] [PubMed] [Google Scholar]
- Tamura M, Koyama R, Ikegaya Y, Matsuki N, Yamada MK. K252a, an inhibitor of Trk, disturbs pathfinding of hippocampal mossy fibers. Neuroreport. 2006;17:481–486. doi: 10.1097/01.wnr.0000208997.23448.ea. [DOI] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnes S, Ringwald J, Haas CA. TIMP-1 inhibits the proteolytic processing of Reelin in experimental epilepsy. FASEB. 2013;27:2542–2552. doi: 10.1096/fj.12-224899. [DOI] [PubMed] [Google Scholar]
- Tinnes S, Schafer MK, Flubacher A, Munzner G, Frotscher M, Haas CA. Epileptiform activity interferes with proteolytic processing of Reelin required for dentate granule cell positioning. FASEB. 2011;25:1002–1013. doi: 10.1096/fj.10-168294. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Gerhauser I, Seeliger F, Baumgartner W, Alldinger S. Matrix metalloproteinases and their inhibitors in the developing mouse brain and spinal cord: a reverse transcription quantitative polymerase chain reaction study. Dev Neurosci. 2005;27:408–418. doi: 10.1159/000088455. [DOI] [PubMed] [Google Scholar]
- Vaillant C, Didier-Bazes M, Hutter A, Belin MF, Thomasset N. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci. 1999;19:4994–5004. doi: 10.1523/JNEUROSCI.19-12-04994.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant C, Meissirel C, Mutin M, Belin MF, Lund LR, Thomasset N. MMP-9 deficiency affects axonal outgrowth, migration, and apoptosis in the developing cerebellum. Molecular and cellular neurosciences. 2003;24:395–408. doi: 10.1016/s1044-7431(03)00196-9. [DOI] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KM, Reeves TM, Phillips LL. MT5-MMP, ADAM-10, and N-cadherin act in concert to facilitate synapse reorganization after traumatic brain injury. J Neurotrauma. 2012;29:1922–1940. doi: 10.1089/neu.2012.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Wilke SA, Daggett A, Davis E, Otto S, Ravi D, Ripley B, Bushong EA, Ellisman MH, Klein G, Ghosh A. Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron. 2011;71:640–655. doi: 10.1016/j.neuron.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MT, Snow DM. Chondroitin sulfate proteoglycan expression pattern in hippocampal development: potential regulation of axon tract formation. J Comp Neurol. 2000;424:532–546. doi: 10.1002/1096-9861(20000828)424:3<532::aid-cne10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Haug FM. Laminar differentiation of the hippocampus, fascia dentata and subiculum in developing rats, observed with the Timm sulphide silver method. J Comp Neurol. 1978;179:581–617. doi: 10.1002/cne.901790309. [DOI] [PubMed] [Google Scholar]