Abstract
The ability to correlate experimental ion mobility data with candidate structures from theoretical modeling provides a powerful analytical and structural tool for the characterization of biomolecules. In the present paper, a theoretical workflow is described to generate and assign candidate structures for experimental trapped ion mobility and H/D exchange (HDX-TIMS-MS) data following molecular dynamics simulations and statistical filtering. The applicability of the theoretical predictor is illustrated for a peptide and protein example with multiple conformations and kinetic intermediates. The described methodology yields a low computational cost and a simple workflow by incorporating statistical filtering and molecular dynamics simulations. The workflow can be adapted to different IMS scenarios and CCS calculators for a more accurate description of the IMS experimental conditions. For the case of the HDX-TIMS-MS experiments, molecular dynamics in the “TIMS box” accounts for a better sampling of the molecular intermediates and local energy minima.
Keywords: ion mobility spectrometry, molecular dynamic simulations, candidate structure generation, collision cross section, structural motifs
Introduction
Ion mobility spectrometry (IMS) when combined with theoretical modeling has proven to be a versatile technique for conformational analysis of intermediate and equilibrium structures of molecular ions by measuring the ion-neutral, collision cross-section (CCS).[1–9] That is, high resolution IMS-MS provides rapid separation of isomers [10–13], conformers [14–16], and species of differing chemical class [17, 18] (based on differences in functional groups, polarities, and atomic compositions), which is advantageous for the rapid characterization and screening of complex mixtures. Ion mobility measurements have been used to explore molecular dynamics and follow structural changes occurring on the millisecond time scale by comparison to CCS of candidate structures under controlled conditions (e.g., reactive/inert, polar/nonpolar bath gas at different temperatures).[10, 19, 20] A variety of theoretical models have been developed to calculate CCS in monoatomic and polyatomic bath gases (e.g., the rigid sphere model and the polarization limit, 12-6-4 and 12-4 potential models and forms thereof, [21–23] non specular scattering models [24] and collision integral based models as the projection approximation,[2, 25] the exact hard sphere scattering [26] and the trajectory methods [27–29]). However, a major difficulty in simulating processes occurring in IMS experiments is the long time scale over which structural rearrangements may occur (approximately a few milliseconds) whereas molecular dynamics simulations are typically limited to nanoseconds.[30–32] Although a number of enhanced sampling methods have been developed to reduce the computation time, further improvements are needed to efficiently correlate the theoretical results with experimental data. During the development of contemporary IMS analyzers (e.g., periodic focusing DC ion guide [33–35], segmented quadrupole drift cell [36], multistage IMS [37–39], field asymmetric IMS [40], and transient wave ion guide [41, 42]), a common pursuit has been to increase IMS resolving power, ion transmission and sensitivity.[13, 43–50] We have recently introduced the trapped ion mobility spectrometer (TIMS) [51–53]. One of the main advantages of TIMS is the possibility to perform molecular ion kinetics experiments and hydrogen deuterium back-exchange (HDX) experiments simultaneously over long time scales (milliseconds to few seconds), thus providing complimentary information on the evolution of molecular ions in the gas phase. This experimental advantage requires the use of alternative theoretical methods in order to follow the isomerization kinetics (or folding pathways) in order to efficiently propose candidate structures.
In the present paper, we describe a theoretical predictor for candidate structure assignment from HDX-TIMS-MS data of biomolecule-related conformational space. The proposed methodology can be extended to candidate structural assignments from CCSs obtained using other variants of IMS separations.
Theoretical Methods
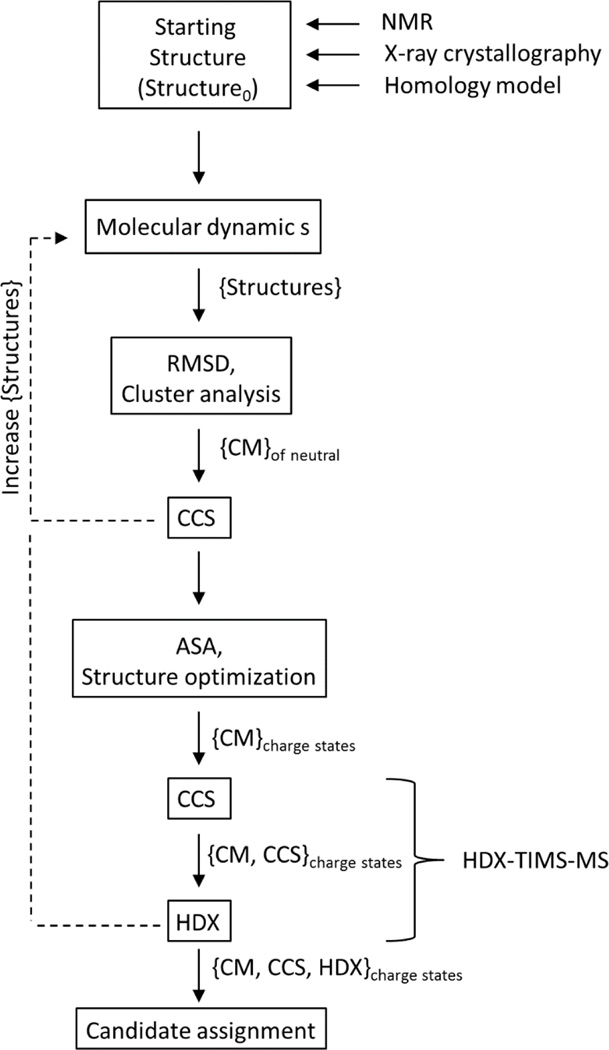
The basis for the theoretical predictor consist on a combination of molecular dynamics, enhanced sampling and statistical clustering of candidate structures (see Scheme 1). Molecular dynamics simulations are used to generate a pool of candidate structures {structures} that encompasses the CCS range observed experimentally regardless of the charge state (e.g., native, unfolded states, and intermediate transition states are populated). Multiple molecular dynamics simulation packages can be used to generate the candidate structure pool; for the case of the data presented here YASARA software (www.yasara.org) was used. Experimental conditions are mimicked by populating the simulation cell (or “TIMS box”) with the IMS bath molecules (or atoms). Simulated annealing protocols using a NVT thermostat (fixed number of particles N, volume V, and temperature T) with a T-damping temperature routine[54] at different temperatures are used during the generation of the candidate structure pool {structures}. Although, charge state distribution are typically observed in the study of biomolecules using soft ionization techniques (e.g. nano ESI and ESI), the starting structure, structure0, used in the “TIMS box” does not takes into consideration individual charge states or charge localization. Instead, known three-dimensional structures or homology models are utilized to provide the initial coordinate system for structure0. Although there are several methods to calculate theoretical CCS from a geometry file, MOBCAL was utilized in the data shown (more details can be found in the supporting material) [26].
Scheme 1.
Candidate structure generation workflow for experimental CCS and HDX data. RMSD and ASA correspond to root-mean-square deviation and accessible surface area, respectively.
The size of the candidate pool {structures} varies with the size of the biomolecule; that is, the larger the system, the larger the size of the {structures} pool in order to investigate kinetic intermediates of the protein and to cover a large number of local energy minima. The statistical processing codes written in R (http://www.r-project.org) and the software used to interpret the structural pools have been described in the supporting material. Statistical sampling is utilized to address the diversity of the {structures} by measuring the root-mean-square deviation (RMSD) and classifying them using hierarchical clustering algorithm. If the diversity is low, more simulations are required (see Scheme 1). A code capable of generating the all-vs-all RMSD matrix of the {structures} pool is included in the supplementary material. Briefly, a pair-wise RMSD comparison of superimposed coordinates (vi and wi) of all atoms following a three dimensional alignment is utilized to provide a numerical assessment of structural similarity/diversity between two structures with the same formula:
| (1) |
Hierarchical clustering permits the grouping of conformations on the basis of structural similarity within the cluster (to be defined apriori as a function of the size of the molecule of interest). That is, candidates comprising a given cluster are more structurally similar than those grouped in any other cluster. Each cluster is represented by an identity vector {CM} as well as a center of mass structure (CM). The CM corresponds to the structure that is most-equally distant in RMSD to all members of the given cluster.
Once the CM structures are identified, charge assignment is performed by scoring the accessible surface area based on the basicity/acidity of the chemical groups. In the case of peptides, proteins and protein complexes, charge assignment is based on the score of the amino acid residues from each tri-dimensional structure. [55, 56]. For example, solvent accessibility and the pka of the acidic (aspartic acid and glutamic acid) and basic (arginine, histidine and lysine) amino acid residues are primarily utilized to assign the protonation and de-protonation sites. It is known that charge localization can influence electrostatic interactions and therefore the conformational dynamics of molecular ions [57]. To account for the charge state influence on the CCS, energy optimization steps are performed after the charge site assignments for all CM candidate structures. It should be pointed out that this protocol reduces significantly the computation time (in contrast to single MDs for each charge) and no more than 10% change in CCS has been observed before and after the geometry optimization when the charges are included. In addition to CCS filtering in the workflow proposed, other experimental data can be added to evaluate the number of candidate structures. For example, in the case of HDX-TIMS-MS, the number of CM can be tailored as a function of the number (or rates) of exchanges as a function of the trapping time.
Results and Discussion
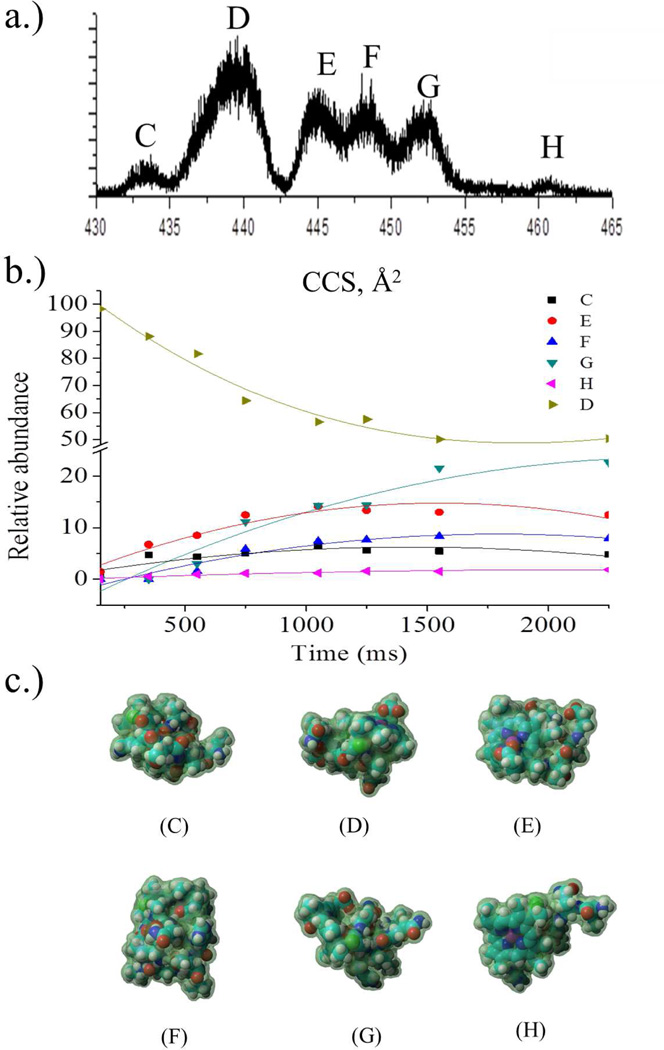
Trapping ions in the gas-phase based on their mobility permits the study of isomerization kinetics and folding pathways of biomolecules. Figure 1 illustrates the information that can be obtained from TIMS-MS analysis for the case of the [M+2H]+2 charge state of microperoxidase-11 (MP-11). MP11 is an eleven amino acid peptide that contains a covalently bound heme group and presents several conformations in the gas-phase as a function of the charge state (see Figure 1a). The relative abundance of the IMS bands changes with the trapping time (millisecond-second time scale) and permits the study of the isomerization/folding/unfolding pathways. To better understand this dynamics, candidate structures are necessary for each IMS band in order to better predict a kinetic pathway (see example of [M+2H]+2 as a function of time, Figure 1b). Current molecular dynamic simulations do not permit the study of these intermediate transitions due to the long time scale over which the process occurs. However, this limitation can be overcome using the theoretical prediction workflow presented here. That is, a “TIMS box” is used to generate the pool of {structures} that characterizes the experimental conformational space of the biomolecule and charges are assigned a posteriori followed by a geometry optimization (see figure 1c). Charge assignment for the MP-11 [M+2H]+2 charge state was determined on the basis of side-chain pka and all the possible combinations. That is, structures proposed in figure 1c combine the proton localization on the N-terminal (Val), lysine side chain and C-terminal.
Figure 1.
a) Typical IMS projection plot of the [M+2H]+2 charge state of microperoxidase 11 (MP-11) b) kinetic curves corresponding to the conformational dynamics (150ms–2250ms) of the MP-11 [M+2H]+2 charge state conformers identified, and c) the most stable candidate structures for the IMS bands observed for MP-11 peptide [M+2H]+2 charge state.
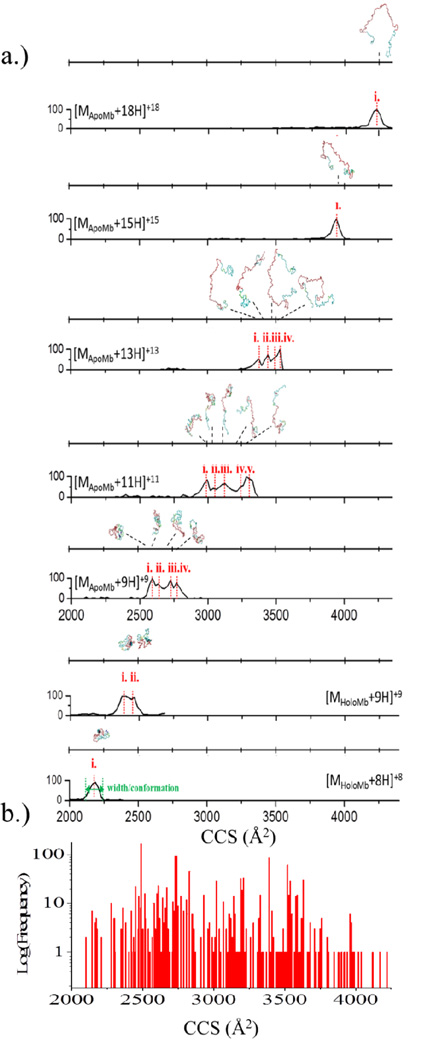
Analogously, kinetic intermediates of proteins can be studied with HDX-TIMS-MS methodology. A measurement of conformational changes by means of CCS, in combination with H/D back exchange, provides complimentary experimental information for candidate structure assignment. The potential of the theoretical predictor is illustrated for the case of myoglobin HDX-TIMS-MS measurements (see Figure 2). Inspection of the IMS bands for the apo and holo-forms of myoglobin as a function of charge state shows that as the charge state increases, the CCS increases (see Figure 2a). In addition, the number of IMS bands (or conformations) also changes with the number of charge states. By using the theoretical prediction workflow, candidate structures can be proposed in a single simulation covering the full range of CCSs observed (Figure 2a). The myoglobin {structures} pool was clustered according to similarity (e.g., RMSD values); here, each cluster has been characterized by the CCS of the CM. Figure 2b depicts the frequency of cluster assignment. The consistency in cluster size has been used as a measurement of “quality” of the molecular dynamics simulations to characterize the conformational dynamics of the experimental data. CM structures of the energetically more stable structures can be used to propose the protein unfolding mechanism and can be used as candidate structures for mobility conformer bands.
Figure 2.
a) IMS projection plots and candidate structures for holo and apo myoglobin as a function of the charge state. c) Number of CM structures as a function of their CCSs of the {structure} pool used to generate the candidate structures.
A practical criteria based on the IMS band width is typically useful to define the number of CM structures needed to describe the experimental IMS data. For example, the IMS bands corresponding to [Mholo+8H]+8 and [MApo+18H]+18 showed similar width (single IMS bands), and can be used as a criteria of the instrument response (or unit IMS band) to fit the data of the intermediate states where multiple IMS bands are observed. Native, compact structures have been shown to have fewer conformational changes than the kinetic intermediates and denatured states [57, 58]. In the case of myoglobin, an increase in CCS translates in additional unfolding of the protein (see Figure 2a). Unfolding of the protein, rather than an overall expansion of the bulk while maintaining a folded state can be determined on the basis of the number of H/D exchanges identified for each charge state. An increase in the number of H/D exchanges recorded for each successive charge state confirms additional unfolding marked by the exposure of additional exchangeable hydrogens. As described in the theoretical predictor workflow (see Scheme 1), information obtained by HDX experiments can be used to interrogate the {CM} candidate structure assignments to more probable conformations. In general, HDX-TIMS-MS experiments provide two complimentary pieces of information, CCS and ASA groups, both of which lead improved predictions of candidate conformations for proteins.
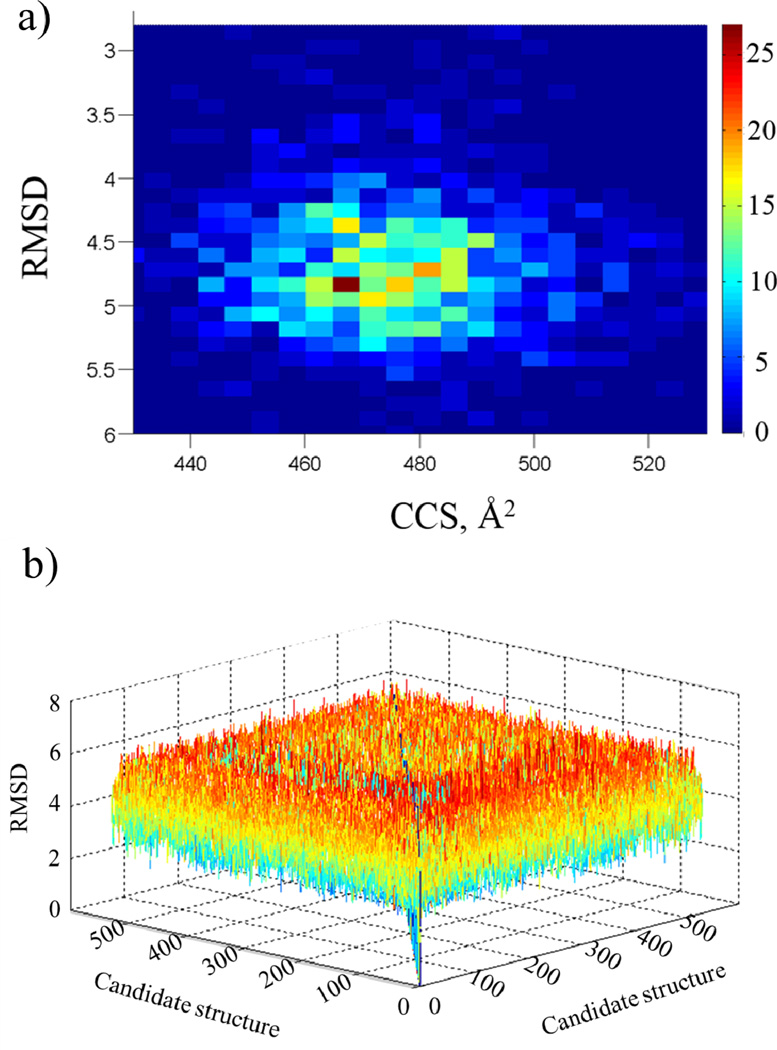
The statistical filtering is used as a method to assess the ability of the {structures} pool to characterize the conformational space of a biomolecule. An assessment of the {structures} pool can be made based on the RMSDs and CCSs values (see example in Figure 3a). That is, a comparison of the {structures} pool to structure0 allows for a critical evaluation of the MD settings and number of structures necessary in the {structures} pool. The range of CCS and RMSD that can be encountered varies with the size of the molecule under study. For example, the RMSD matrix obtained for a peptide in comparison to that of a protein will vary to a lesser degree. That is, as a result of the larger protein size and the larger number of transitions that can be encountered the range of RMSDs is much broader for a given CCS. In addition, the range of the resulting all-vs-all RMSD matrix for all candidate structures illustrates the extent of structural variation obtained in the MD simulations (see Figure 3b). The variation of RMSD values within the all-versus-all matrix, as presented visually in figure 3b, can be used to assess the ability of molecular dynamics simulations to characterize the experimentally observed conformational space. Structural variations can be maximized with multiple {CM} potential candidates per unit of IMS band (or CCS range) to populate different intermediate transitions. That is, statistical filtering of molecular dynamics simulations is an effective tool to readily assess theoretical calculations for IMS experiments.
Figure 3.
a) Distribution of RMSD relative to structure0 as a function of the CCS for MP11 candidate structures. b) Three-dimensional plot depicting the all-versus-all RMSD matrix of the {structures} pool for MP11 candidate structures.
Conclusion
The advantage of a theoretical prediction workflow to assign candidate structures for biomolecules using IMS and HDX measurements has been presented. The described methodology yields a low computational cost and simple workflow by incorporating statistical filtering and molecular dynamics simulations. The advantages of the proposed methodology for peptides and proteins studies have been illustrated when using complementary IMS and HDX experimental data. The inclusion of HDX data obtained under the same mobility conditions improves the ability to filter and make candidate structure assignments. In addition, the ability study isomerization kinetics of biomolecules aides in identifying prominent structural motifs that can persist over time.
The workflow can be adapted to different IMS scenarios and CCS calculators for a more accurate description of the experimental conditions. For the case of the HDX-TIMS-MS experiments, molecular dynamics in the “TIMS box” accounts for a better sampling of the molecular intermediates and local energy minima.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (Grant No. R00GM106414) and FFL Bruker Daltonic, Inc. fellowship. The authors would like to acknowledge the Instructional & Research Computing Center (IRCC) at Florida International University for providing high performance computing resources that have contributed to the research results reported within this research.
Footnotes
Supplemental Material
Supplemental material includes custom-built scripts written in R code (www.R-project.org) to conduct the RMSD calculations and the hierarchical clustering analysis to identify CM conformations. Test input and output files are provided.
References
- 1.Dugourd P, Hudgins RR, Jarrold MF. High-resolution ion mobility studies of sodium chloride nanocrystals. Chemical Physics Letters. 1997;267:186–192. [Google Scholar]
- 2.Gert von H, Ming-Teh H, Paul RK, Michael TB. Structures of carbon cluster ions from 3 to 60 atoms: Linears to rings to fullerenes. J. Chem. Phys. 1991;95:3835–3837. [Google Scholar]
- 3.Scott CD, Ugarov M, Hauge RH, Sosa ED, Arepalli S, Schultz JA, Yowell L. Characterization of Large Fullerenes in Single-Wall Carbon Nanotube Production by Ion Mobility Mass Spectrometry. J. Phys. Chem. C. 2007;111:36–44. [Google Scholar]
- 4.Becker C, Qian K, Russell DH. Molecular Weight Distributions of Asphaltenes and Deasphaltened Oils Studied by Laser Desorption Ionization and Ion Mobility Mass Spectrometry. Anal. Chem. 2008;80:8592–8597. doi: 10.1021/ac801473f. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Lima FA, Becker C, Gillig K, Russell WK, Nascimento MAC, Russell DH. Experimental and Theoretical Studies of (CsI)nCs+ Cluster Ions Produced by 355 nm Laser Desorption Ionization. The Journal of Physical Chemistry A. 2008;112:11061–11066. doi: 10.1021/jp8047086. [DOI] [PubMed] [Google Scholar]
- 6.Young D, Douglas KM, Eiceman GA, Lake DA, Johnston MV. Laser desorption– ionization of polycyclic aromatic hydrocarbons from glass surfaces with ion mobility spectrometry analysis. Analytica Chimica Acta. 2002;453:231–243. [Google Scholar]
- 7.Lanucara F, Holman SW, Gray CJ, Eyers CE. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nature Chemistry. 2014;6:281–294. doi: 10.1038/nchem.1889. [DOI] [PubMed] [Google Scholar]
- 8.Shvartsburg AA, Li FM, Tang KQ, Smith RD. Characterizing the structures and folding of free proteins using 2-D gas-phase separations: Observation of multiple unfolded conformers. Anal Chem. 2006;78:3304–3315. doi: 10.1021/ac060283z. [DOI] [PubMed] [Google Scholar]
- 9.Ruotolo BT, Benesch JLP, Sandercock AM, Hyung SJ, Robinson CV. Ion mobility-mass spectrometry analysis of large protein complexes. Nat Protoc. 2008;3:1139–1152. doi: 10.1038/nprot.2008.78. [DOI] [PubMed] [Google Scholar]
- 10.Kanu AB, Hill HH., Jr Identity confirmation of drugs and explosives in ion mobility spectrometry using a secondary drift gas. Talanta. 2007;73:692–699. doi: 10.1016/j.talanta.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 11.Schenk ER, Mendez V, Landrum JT, Ridgeway ME, Park MA, Fernandez-Lima F. Direct Observation of Differences of Carotenoid Polyene Chain cis/trans Isomers Resulting from Structural Topology. Analytical Chemistry. 2014;86:2019–2024. doi: 10.1021/ac403153m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierson NA, Chen L, Russell DH, Clemmer DE. Cis–Trans Isomerizations of Proline Residues Are Key to Bradykinin Conformations. Journal Of The American Chemical Society. 2013;135:3186–3192. doi: 10.1021/ja3114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merenbloom SI, Glaskin RS, Henson ZB, Clemmer DE. High-Resolution Ion Cyclotron Mobility Spectrometry. Analytical Chemistry. 2009;81:1482–1487. doi: 10.1021/ac801880a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer HA, Marini JT, Stone EG, Ruotolo BT, Gillig KJ, Russell DH. The Structure of Gas-Phase Bradykinin Fragment 1–5 (RPPGF) Ions: An Ion Mobility Spectrometry and H/D Exchange Ion-Molecule Reaction Chemistry Study. J. Am. Soc. Mass Spectrom. 2005;16:893–905. doi: 10.1016/j.jasms.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Schenk ER, Ridgeway ME, Park MA, Leng F, Fernandez-Lima F. Isomerization Kinetics of AT Hook Decapeptide Solution Structures. Analytical Chemistry. 2014;86:1210–1214. doi: 10.1021/ac403386q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molano-Arevalo JC, Hernandez DR, Gonzalez WG, Miksovska J, Ridgeway ME, Park MA, Fernandez-Lima F. Flavin Adenine Dinucleotide structural motifs: from solution to gas-phase. Anal Chem. 2014 doi: 10.1021/ac5023666. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruotolo GFVBT, Thomson LM, Woods AS, Gillig KJ, Russell DH. J. Proteome Res. 2002;1:303. doi: 10.1021/pr025516r. [DOI] [PubMed] [Google Scholar]
- 18.May JC, Goodwin CR, Lareau NM, Leaptrot KL, Morris CB, Kurulugama RT, Mordehai A, Klein C, Barry W, Darland E, Overney G, Imatani K, Stafford GC, Fjeldsted JC, McLean JA. Conformational Ordering of Biomolecules in the Gas Phase: Nitrogen Collision Cross Sections Measured on a Prototype High Resolution Drift Tube Ion Mobility-Mass Spectrometer. Analytical Chemistry. 2014;86:2107–2116. doi: 10.1021/ac4038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zilch LW, Kaleta DT, Kohtani M, Krishnan R, Jarrold MF. Folding and Unfolding of Helix-Turn-Helix Motifs in the Gas Phase. J. Am. Soc. Mass Spectrom. 2007;18:1239–1248. doi: 10.1016/j.jasms.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasciotti M, Lalli PM, Heerdt G, Steffen RA, Corilo YE, Sá GFd, Daroda RJ, Reis FdAM, Morgon NH, Pereira RCL, Eberlin MN, Klitzke CF. Int. J. Ion Mobil. Spec. 2013;16:117. [Google Scholar]
- 21.McDaniel EW, Mason EA. Mobility and diffusion of ions in gases. New York, New York: John Wiley and Sons, Inc.; 1973. [Google Scholar]
- 22.Steiner WE, English WA, Hill HH. J. Phys. Chem. A. 2006;110:1836. doi: 10.1021/jp056158t. [DOI] [PubMed] [Google Scholar]
- 23.Kim HI, Johnson PV, Beegle LW, Beauchamp JL, Kanik I. J. Phys. Chem. A. 2005;109:7888. doi: 10.1021/jp051274h. [DOI] [PubMed] [Google Scholar]
- 24.Larriba C, Hogan CJ. Ion Mobilities in Diatomic Gases: Measurement versus Prediction with Non-Specular Scattering Models. The Journal of Physical Chemistry A. 2013;117:3887–3901. doi: 10.1021/jp312432z. [DOI] [PubMed] [Google Scholar]
- 25.Bleiholder C, Wyttenbach T, Bowers MT. A novel projection approximation algorithm for the fast and accurate computation of molecular collision cross sections (I). Method. International Journal of Mass Spectrometry. 2011;308:1–10. [Google Scholar]
- 26.Shvartsburg AA, Jarrold MF. An exact hard-spheres scattering model for the mobilities of polyatomic ions. Chemical Physics Letters. 1996;261:86–91. [Google Scholar]
- 27.Mesleh MF, Hunter JM, Shvartsburg AA, Schatz GC, Jarrold MF. Structural Information from Ion Mobility Measurements: Effects of the Long-Range Potential. J. Phys. Chem. 1996;100:16082–16086. [Google Scholar]
- 28.Kim HI, Kim H, Pang ES, Ryu EK, Beegle LW, Loo JA, Goddard WA, Kanik I. Anal. Chem. 2009;81:8289. doi: 10.1021/ac900672a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campuzano I, Bush MF, Robinson CV, Beaumont C, Richardson K, Kim H, Kim HI. Structural Characterization of Drug-like Compounds by Ion Mobility Mass Spectrometry: Comparison of Theoretical and Experimentally Derived Nitrogen Collision Cross Sections. Analytical Chemistry. 2011;84:1026–1033. doi: 10.1021/ac202625t. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Lima FA, Wei H, Gao YQ, Russell DH. On the Structure Elucidation Using Ion Mobility Spectrometry and Molecular Dynamics. The Journal of Physical Chemistry A. 2009;113:8221–8234. doi: 10.1021/jp811150q. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Lima FA, Blase RC, Russell DH. A study of ion-neutral collision cross42 section values for low charge states of peptides, proteins, and peptide/protein complexes. International Journal of Mass Spectrometry. 2010;298:111–118. doi: 10.1016/j.ijms.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schenk E, Nau F, Fernandez-Lima F. Theoretical predictor for candidate structure assignment from IMS data of biomolecule-related conformational space. International Journal of Ion Mobility Spectrometry. 2014 doi: 10.1007/s12127-015-0165-0. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillig KJ, Russell DH. A periodic field focusing ion mobility spectrometer. WO0165589. The Texas A & M University System; Patent Cooperation Treaty Int. Appl. 2001:36.
- 34.Gillig KJ, Ruotolo BT, Stone EG, Russell DH. An electrostatic focusing ion guide for ion mobility-mass spectrometry. Int. J. Mass Spectrom. 2004;239:43–49. [Google Scholar]
- 35.Silveira JA, Gamage CM, Blase RC, Russell DH. Gas-phase ion dynamics in a periodic-focusing DC ion guide. International Journal of Mass Spectrometry. 2010;296:36–42. [Google Scholar]
- 36.Guo Y, Wang J, Javahery G, Thomson BA, Siu KWM. Ion Mobility Spectrometer with Radial Collisional Focusing. Analytical Chemistry. 2004;77:266–275. doi: 10.1021/ac048974n. [DOI] [PubMed] [Google Scholar]
- 37.Koeniger SL, Merenbloom SI, Valentine SJ, Jarrold MF, Udseth HR, Smith RD, Clemmer DE. An IMS-IMS Analogue of MS-MS. Anal. Chem. 2006;78:4161–4174. doi: 10.1021/ac051060w. [DOI] [PubMed] [Google Scholar]
- 38.Kurulugama RT, Nachtigall FM, Lee S, Valentine SJ, Clemmer DE. Overtone mobility spectrometry: Part 1. Experimental observations. Journal of the American Society for Mass Spectrometry. 2009;20:729–737. doi: 10.1016/j.jasms.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glaskin RS, Valentine SJ, Clemmer DE. A Scanning Frequency Mode for Ion Cyclotron Mobility Spectrometry. Analytical Chemistry. 2010;82:8266–8271. doi: 10.1021/ac1017474. [DOI] [PubMed] [Google Scholar]
- 40.Kolakowski BM, Mester Z. Review of applications of high-field asymmetric waveform ion mobility spectrometry (FAIMS) and differential mobility spectrometry (DMS) Analyst. 2007;132:842–864. doi: 10.1039/b706039d. [DOI] [PubMed] [Google Scholar]
- 41.Pringle SD, Giles K, Wildgoose JL, Williams JP, Slade SE, Thalassinos K, Bateman RH, Bowers MT, Scrivens JH. An investigation of the mobility separation of some peptide and protein ions using a new hybrid quadrupole/travelling wave IMS/oa-ToF instrument. Int. J. Mass Spectrom. 2007;261:1–12. [Google Scholar]
- 42.Bush MF, Hall Z, Giles K, Hoyes J, Robinson CV, Ruotolo BT. Collision Cross Sections of Proteins and Their Complexes: A Calibration Framework and Database for Gas-Phase Structural Biology. Analytical Chemistry. 2010;82:9557–9565. doi: 10.1021/ac1022953. [DOI] [PubMed] [Google Scholar]
- 43.Dugourd P, Hudgins RR, Clemmer DE, Jarrold MF. High-resolution ion mobility measurements. Review of Scientific Instruments. 1997;68:1122–1129. [Google Scholar]
- 44.Kemper PR, Dupuis NF, Bowers MT. A new, higher resolution, ion mobility mass spectrometer. International Journal of Mass Spectrometry. 2009;287:46–57. [Google Scholar]
- 45.Blase RC, Silveira JA, Gillig KJ, Gamage CM, Russell DH. Increased ion transmission in IMS: A high resolution, periodic-focusing DC ion guide ion mobility spectrometer. International Journal of Mass Spectrometry. 2011;301:166–173. [Google Scholar]
- 46.May J, Russell D. A Mass-Selective Variable-Temperature Drift Tube Ion Mobility-Mass Spectrometer for Temperature Dependent Ion Mobility Studies. Journal of the American Society for Mass Spectrometry. 2011;22:1134–1145. doi: 10.1007/s13361-011-0148-2. [DOI] [PubMed] [Google Scholar]
- 47.Kemper PR, Bowers MT. A hybrid double-focusing mass spectrometer--high-pressure drift reaction cell to study thermal energy reactions of mass-selected ions. Journal of the American Society for Mass Spectrometry. 1990;1:197–207. [Google Scholar]
- 48.Wu C, Siems WF, Asbury GR, Hill HH. Electrospray Ionization High-Resolution Ion Mobility Spectrometry–Mass Spectrometry. Analytical Chemistry. 1998;70:4929–4938. doi: 10.1021/ac980414z. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Clemmer DE. Characterizing Oligosaccharides Using Injected-Ion Mobility/Mass Spectrometry. Analytical Chemistry. 1997;69:2504–2509. doi: 10.1021/ac9701344. [DOI] [PubMed] [Google Scholar]
- 50.Jarrold MF, Constant VA. Silicon cluster ions: Evidence for a structural transition. Phys. Rev. Lett. 1991;67:2994. doi: 10.1103/PhysRevLett.67.2994. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Lima FA, Kaplan DA, Park MA. Note: Integration of trapped ion mobility spectrometry with mass spectrometry. Rev. Sci. Instr. 2011;82:126106. doi: 10.1063/1.3665933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Lima F, Kaplan D, Suetering J, Park M. Gas-phase separation using a trapped ion mobility spectrometer. Int. J. Ion Mobility Spectrom. 2011;14:93–98. doi: 10.1007/s12127-011-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez DR, DeBord JD, Ridgeway ME, Kaplan DA, Park MA, Fernandez-Lima F. Ion dynamics in a trapped ion mobility spectrometer. Analyst. 2014;139:1913–1921. doi: 10.1039/c3an02174b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez-Lima FA, Wei H, Gao YQ, Russell DH. On the Structure Elucidation Using Ion Mobility Spectrometry and Molecular Dynamics. J Phys Chem A. 2009;113:8221–8234. doi: 10.1021/jp811150q. [DOI] [PubMed] [Google Scholar]
- 55.Bogatyreva NS, Ivankov DN. The relationship between the solvent-accessible surface area of a protein and the number of native contacts in its structure. Mol Biol+ 2008;42:932–938. [PubMed] [Google Scholar]
- 56.Kaltashov IA, Mohimen A. Estimates of protein surface areas in solution by electrospray ionization mass spectrometry. Anal Chem. 2005;77:5370–5379. doi: 10.1021/ac050511+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Susa AC, Mortensen DN, Williams ER. Effects of Cations on Protein and Peptide Charging in Electrospray Ionization from Aqueous Solutions. J Am Soc Mass Spectr. 2014;25:918–927. doi: 10.1007/s13361-014-0864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall Z, Politis A, Bush MF, Smith LJ, Robinson CV. Charge-State Dependent Compaction and Dissociation of Protein Complexes: Insights from Ion Mobility and Molecular Dynamics. J Am Chem Soc. 2012;134:3429–3438. doi: 10.1021/ja2096859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.