ABSTRACT
The Momordica charantia (MC) fruit has been documented to possess antidiabetic properties. However, these studies were not without controversy surrounding the blood glucose-lowering ability and the mechanism of action in diabetes therapy. In an effort to evaluate such claims in the Jamaican MC species known as cerasee, aqueous extracts of the unripe fruit were studied in normal and diabetic rats. Normal male Sprague-Dawley rats were divided into groups (n = 6) orally administered distilled water, 10% dimethyl sulfoxide (DMSO) solution, the aqueous extract (400 mg/kg body weight) and glibenclamide (15 mg/kg body weight), respectively prior to assessment of fasting blood glucose (FBG) concentration. The oral glucose tolerance test (OGTT) was conducted in normoglycaemic rats orally administered distilled water, 10% DMSO solution, glibenclamide (15 mg/kg body weight) or aqueous extracts of the fruit (200 and 400 mg/kg body weight). Blood glucose concentration was also monitored in streptozotocin-induced diabetic rats administered the aqueous extract (250 mg/kg body weight) or water vehicle after an overnight fast. The aqueous extracts showed no hypoglycaemic or antidiabetic activity. However, the administration of the aqueous extracts (200 and 400 mg/kg body weight) resulted in significant improvement in glucose tolerance of glucose-primed normoglycaemic rats during the OGTT. These data suggest that the glucose-lowering mechanism of the Jamaican MC fruit species likely involves altered glucose absorption across the gastrointestinal tract.
Keywords: Cerasee, diabetes, Momordica charantia, rats
RESUMEN
Estudios realizados han documentado que la fruta de la Momordica charantia (MC) posee propiedades antidiabéticas. Sin embargo, las afirmaciones de estos estudios en torno a la capacidad de esta planta para reducir la glucosa sanguínea y a su mecanismo de acción en el tratamiento de la diabetes, no han estado libres de controversia. En un esfuerzo por evaluar dichas afirmaciones sobre la especie de MC jamaicana, conocida como cundeamor, se estudiaron extractos acuosos de la fruta aún verde en ratas normales y diabéticas. Las ratas Sprague-Dawley machos normales se dividieron en grupos (n = 6) que recibieron por vía oral agua destilada, solución de dimetilsulfóxido (DMSO) al 10%, extracto acuoso (400 mg/kg de peso corporal), y glibenclamida (15 mg/kg de peso corporal) respectivamente, antes de evaluar la concentración de glucosa en sangre en ayunas (GSA). Se realizó la prueba oral de tolerancia a la glucosa (POTG) en ratas normoglicémicas por vía oral con agua destilada, solución de DMSO al 10%, glibenclamida (15 mg/kg de peso corporal) o extractos acuosos de la fruta (200 y 400 mg/kg de peso corporal). La concentración de glucosa en sangre también fue monitoreada en ratas con diabetes inducida mediante estreptozotocina a las que se les administró extracto acuoso (250 mg/kg de peso corporal) o vehículo de agua después de un ayuno nocturno. Los extractos acuosos no demostraron ninguna actividad antidiabética o hipoglicémica. Sin embargo, la administración de los extractos acuosos (200 y 400 mg/kg de peso corporal) resultó en una mejoría significativa de la tolerancia a la glucosa en las ratas normoglicémicas primadas con glucosa durante la POTG. Estos datos sugieren que el mecanismo reductor de la glucosa de la especie de fruta jamaicana MC probablemente implica la absorción alterada de glucosa en todo el tracto gastrointestinal.
INTRODUCTION
Diabetes mellitus is a chronic syndrome characterized by high blood glucose levels due to insufficient pancreatic secretion of insulin and/or resistance to insulin produced by target cells (1). The long-term complications of this disorder, such as blindness, cardiovascular disease, kidney failure and erectile dysfunction result in a decline in the quality of life of affected individuals as well as imposing marked financial challenges (2). The more economical classes of drugs available for therapy are limited by associated unwanted effects such as hypoglycaemia, weight gain and gastrointestinal upset among other complications (3). For such reasons, many seek alternative therapy and herbal use is deemed suitable due to its natural origin, cost effectiveness and purported fewer side effects.
Momordica charantia, locally known as cerasee, is one of the many plants widely accepted as folklore treatment for diabetes mellitus in countries of Africa, Asia and the Americas (4). The Momordica charantia plant has an annual or perennial life cycle and grows up to 5 m in length. It also bears simple alternate leaves and yellow male and female flowers. The exterior of the fruit has a characteristic warty appearance and is green in colour when unripe and orange when ripe. The interior of the fruit is characterized by a seed-filled cavity enclosed by pith and a thin layer of flesh which appears white in colour when unripe and red upon ripening (3).
The Momordica charantia fruit varieties differ in size, colour and gradation of external ridges. The Jamaican variety of the plant has fruits that are smaller in size, paler and possess fewer ridges than the Trinidadian and Guyanese varieties (5). Although all parts of the plant are utilized in diabetes therapy, the fruit is predominantly used (6). Nonetheless, there is some controversy surrounding the usefulness and mechanism of action of the Momordica charantia fruit in the treatment of diabetes mellitus, with studies showing both activity and inactivity at various doses in animal models and human studies (7). Studies conducted by Karunanayake et al (8) showed that the oral administration of the Momordica charantia juice at 10 mL/kg body weight for 30 days did not result in significant decline in the blood glucose levels of streptozotocin-induced diabetic rats. Sarkar et al, however, have demonstrated significant reductions in blood glucose concentration in diabetic animal models upon Momordica charantia administration at 500 mg/kg body weight (9). Likewise, the hot water extract of the cerasee fruit, similar to the method of preparation of the plant for use in Jamaican folklore, was shown to produce hypoglycaemic activity in dogs (10). This study was therefore initiated to evaluate the blood glucose lowering potential of the fruit of the Jamaican Momordica charantia plant species in Sprague-Dawley rats.
SUBJECTS AND METHODS
The unripe Momordica charantia fruit was harvested and identified at the herbarium of The University of the West Indies; subsequent to which a specimen number (35404) was acquired.
Aqueous Momordica charantia fruit extract preparation
Fruits (500 g) were washed, cut into small pieces, added to distilled water (1 L) and blended at high speed using an electric blender, until smooth in texture. The resultant suspension was allowed to equilibrate at room temperature for an hour, shaking intermittently, and then filtered. The filtrate was frozen overnight and freeze dried for approximately five days or until a moisture-free powdered extract was obtained. This was immediately stored at −20 °C until use.
Animals
Male Sprague-Dawley rats, 300–400 g, at 24 weeks of age were obtained from the Animal House in the Department of Basic Medical Sciences at The University of the West Indies. Animals were housed under standard laboratory conditions and provided with a pellet diet (Formulab Diet 5008) and tap water ad libitum. During periods of blood sampling, rats were subjected to an overnight fast subsequent to which blood obtained from the tail vein was used to determine fasting blood glucose (FBG) levels using the Accu-Chek Active Glucometer® (Lasco Pharmaceuticals, Jamaica), similar to the method used by Rao et al (11). Ethical approval was obtained from the University Hospital of the West Indies/University of the West Indies/Faculty of Medical Sciences Ethics Committee. Animals were allowed acclimatization one week prior to conduction of tests.
Streptozotocin-induced diabetic rat model
Diabetes was induced by an intraperitoneal injection of streptozotocin (50 mg/kg body weight; Sigma Chemicals, USA) dissolved in saline, while control rats received an equivalent volume of the saline solution (12). After seven days, confirmation of the successful induction of diabetes in all rats was verified by FBG being approximately four times in excess of normal, with the average FBG concentration of the normoglycaemic rats being 4.5 ± 0.11 mmol/L while that of the diabetic rats was ≥ 19.7 ± 2.58 mmol/L. A glibenclamide challenge was performed on day 14 to ascertain the type of diabetes developed. Glibenclamide (15 mg/kg body weight; Sigma Chemicals, USA) dissolved in 10% dimethyl sulfoxide (DMSO) solution was orally administered to fasted rats and blood glucose levels determined at 30-minute intervals for three hours. Rats that were unresponsive to the glibenclamide challenge, suggesting the absence of functional pancreatic beta cells and therefore the induction of Type 1 diabetes (13), were randomly assigned to the extract-treated or vehicle-treated diabetic group (n = 6). Fasted diabetic rats were orally administered the aqueous extract (250 mg/kg body weight) or water. The blood glucose levels of the animals were assessed 30 minutes before treatment and subsequently at 30-minute intervals over 2.5 hours.
Hypoglycaemic potential of the Momordica charantia fruit extract
The FBG levels of normoglycaemic rats were measured immediately before oral administration of appropriate treatments. The test group received the aqueous extract at 400 mg/kg body weight. Glibenclamide (15 mg/kg body weight) was used as a positive control in one group, whereas water and 10% DMSO solution were given to the relevant control groups. The blood glucose concentration of animals was monitored for three hours.
Anti-hyperglycaemic potential of Momordica charantia fruit extracts
Subsequent to the determination of FBG levels in normoglycaemic rats, appropriate treatments were administered. Treatment groups orally received aqueous extracts of MC (200 and 400 mg/kg body weight) and glibenclamide (15 mg/kg body weight). Similarly, water and 10% DMSO solution were administered to corresponding control groups. The blood glucose levels of the animals were re-assessed after 30 minutes, following which a glucose load of 1.75 g/kg body weight was orally administered to all groups. The blood glucose concentration was evaluated at 30-minute intervals for 2.5 hours.
Statistical analysis
Results are expressed as means ± SEM. The student's t-test, analysis of variance (ANOVA) and the Tukey multiple range test were used to make intra- and inter-group comparisons. Statistical analyses were performed using the Sigma Plot 11.0 software. Significant levels were tested at p < 0.05.
RESULTS
Hypoglycaemic effect of aqueous Momordica charantia fruit extract in normoglycaemic rats
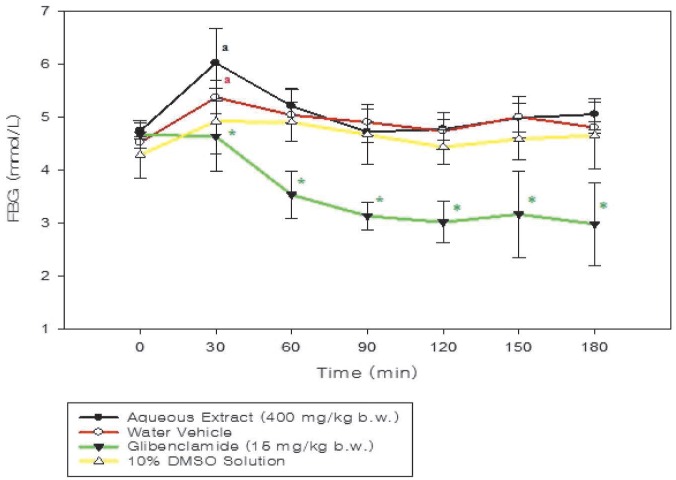
Figure 1 shows that the glibenclamide solution (15 mg/kg) significantly (p < 0.001) reduced FBG concentration in comparison to the 10% DMSO solution-treated group; however, the fruit extract did not cause a decrease in FBG concentration. There was an increase in the FBG levels of both the extract-treated (p = 0.003) and water-vehicle treated (p = 0.002) groups at the 30-minute time point.
Fig. 1. Time course graph showing the fasting blood glucose (FBG) concentration of normoglycaemic rats (n = 6) treated orally with aqueous Momordica charantia fruit extract and glibenclamide, compared to vehicle-treated rats. Significant difference between treatment and control groups is denoted by the symbol “*”, whereas change from initial value (t = 0 min) within a group is indicated by “a”.
Anti-hyperglycaemic effect of aqueous Momordica charantia fruit extract in glucose-primed normoglycaemic rats
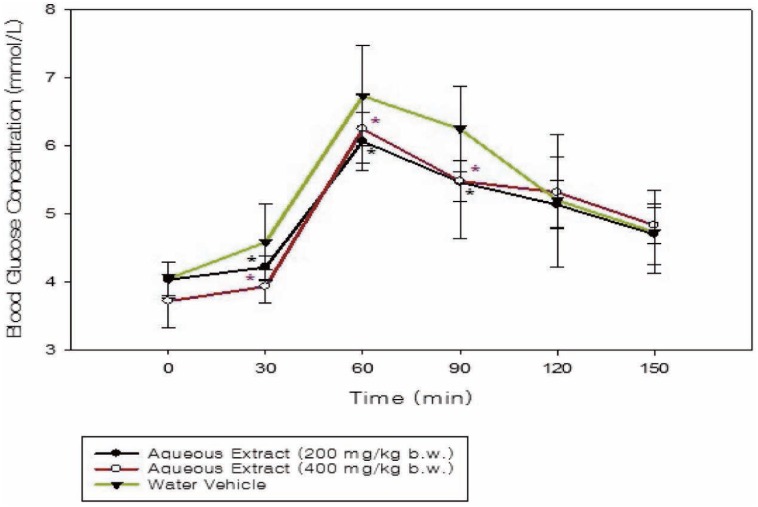
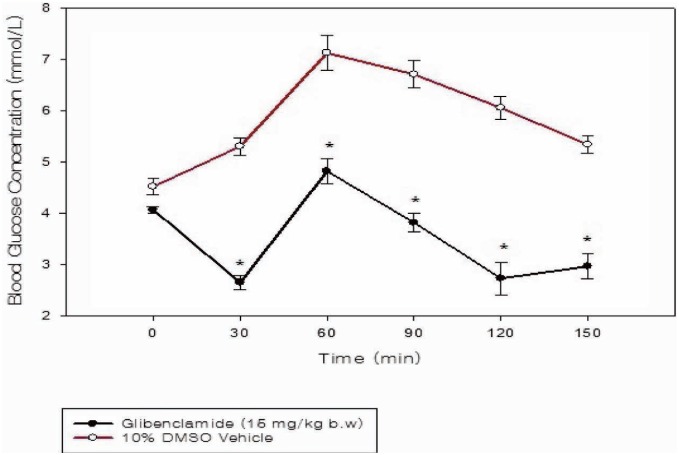
The increase in blood glucose concentrations induced by the glucose load was significantly inhibited (p = 0.032; p = 0.023) by the aqueous extract at the 200 and 400 mg/kg doses in comparison to the control group (Fig. 2). Glibenclamide also low-ered (p < 0.001) blood glucose concentration of treated rats in comparison to the vehicle treated group (Fig. 3).
Fig. 2. Time course graph showing the effect of aqueous extracts of Momordica charantia on oral glucose tolerance test (OGTT) curves of normoglycaemic rats (n = 6) compared to water vehicle-treated rats. Values are mean ± SEM. The symbol “*” represents significant difference between the treated and control groups.
Fig. 3. Time course graph showing the oral glucose tolerance test (OGTT) curves of normoglycaemic rats (n = 6) orally treated with glibenclamide compared to administration of 10% dimethyl sulfoxide (DMSO) solution. The symbol “*” represents significant difference between the treated and control groups.
Antidiabetic effect of aqueous Momordica charantia fruit extract in diabetic rats
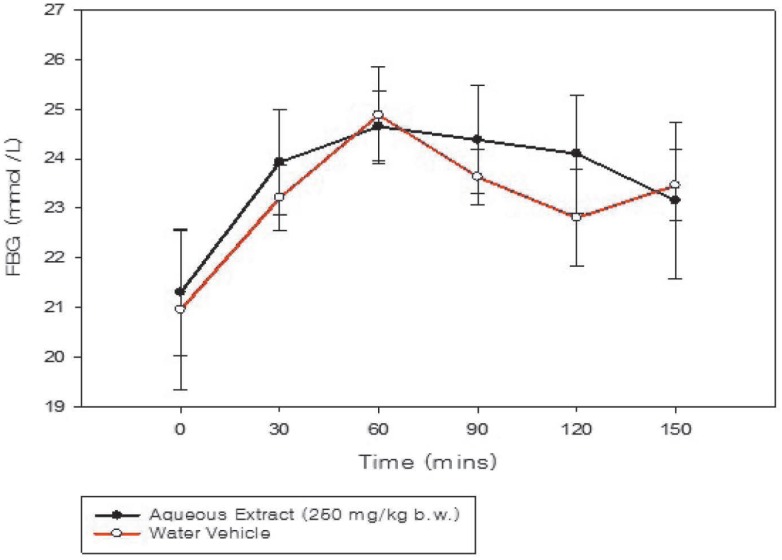
There was no significant difference in blood glucose concentration (p = 0.955) between the extract and the vehicle-treated diabetic groups (Fig. 4).
Fig. 4. Time course graph showing the fasting blood glucose (FBG) concentration of diabetic rats (n = 6) orally treated with aqueous extract of the Momordica charantia fruit compared to vehicle-treated rats.
DISCUSSION
The doses of Momordica charantia extracts reported to possess hypoglycaemic potential are extensive and range from 50– 600 mg/kg body weight (7, 14, 15). As such, median doses of 200–400 mg/kg body weight of the fruit extracts were used in this study to investigate the potential of the plant in reducing blood glucose concentration in the rats.
Glibenclamide, used as the positive control, elicited hypoglycaemic and anti-hyperglycaemic effects via a significant (p < 0.05) reduction in FBG concentration (Fig. 1) and blood glucose levels after a glucose load of 1.75 g/kg body weight during the oral glucose tolerance test [OGTT] (Fig. 3). This was within expectation as glibenclamide, belonging to the sulfonylurea class of antidiabetic drugs, acts by binding to and causing blockade of ATP-sensitive K+ channels on pancreatic beta-cells, resulting in an influx of calcium ions and therefore increased insulin secretion (16).
The Momordica charantia fruit extract lacked hypoglycaemic effect when administered to fasted normoglycaemic rats. The increases in FBG levels of the extract-treated rats (p = 0.003) at the 30-minute time point was unexpected but not deemed attributable to treatment due to a similar occurrence in the vehicle-treated groups (Figs. 1–4). Excessive sympathetic stimulation of the animals during the experimental period, evidenced by signs of excitation and heightened alertness, may have caused augmentation of the overall glycaemic response due to the metabolic role played by epinephrine and other glucoregulatory hormones (17).
Glucose-primed normoglycaemic rats treated with the aqueous extract at 200 and 400 mg/kg body weight demonstrated improvement (p < 0.05) in glucose tolerance during the OGTT (Fig. 2). The anti-hyperglycaemic effect of these doses of the aqueous extracts during the OGTT of normoglycaemic rats provided a basis for the evaluation in the diabetic model. However, administration of the aqueous extract (250 mg/kg body weight) to streptozotocin-induced Type 1 diabetic rats did not result in a significant reduction in fasting blood glucose levels (Fig. 4).
There was absence of functional pancreatic beta cells in the Type 1 diabetic rats used in this study and therefore the inability of the extract to reduce the blood glucose levels of these animals via enhanced insulin secretion. However, the Momordica charantia extracts were able to lower blood glucose levels via an alternative mechanism, evidenced by the reduction in elevated blood glucose levels of normoglycaemic glucose-primed rats.
These results support the postulation that the blood glucose lowering activity of Momordica charantia required a glucose load and in turn suggest that the action of the extract occurred via an extra-pancreatic mechanism and not by an insulin secretagogue-like effect (15). These findings of improved glucose tolerance also suggest that the active principles in the aqueous extracts used are unlikely to be peptide-based as such compounds would have been subjected to proteolytic destruction by enzymes of the digestive system, via the oral route of administration.
The implications of the anti-hyperglycaemic properties of the aqueous extracts used in this study are important. The ability to lower elevated blood glucose concentration of normoglycemic rats highlights the requirement of a glucose load for extract activity. Thus, the results of this study suggest that the inhibition of intestinal glucose absorption was possibly instrumental in the mechanism of action of these fruit extracts. This mechanism also accounts for the antidiabetic mechanism of acarbose (17).
Further investigations are necessary to confirm the proposed mechanism of anti-hyperglycaemic activity of the Momordica charantia extract.
ACKNOWLEDGEMENTS
We wish to thank Mr E Thomas and his team of technicians for valuable assistance extended. Special thanks to Professor O Simon and Dr D Pepple for much appreciated guidance throughout this study. Funding was acquired by a Research and Publication Grant provided by the Office of Graduate Studies and Research at The University of the West Indies.
REFERENCES
- 1.Schuster D, Duvuuri V. Diabetes mellitus. Clin Podiatr Med Surg. 2002;19:79–107. doi: 10.1016/S0891-8422(03)00082-X. [DOI] [PubMed] [Google Scholar]
- 2.Singh J, Cumming E, Manoharan G, Kalasz H, Adeghate E. Medicinal chemistry of the anti-diabetic effects of Momordica charantia: active constituents and modes of actions. Open Med Chem J. 2011;5:70–77. doi: 10.2174/1874104501105010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam T. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Batran S, El Gengaihi S, Shabrawy O. Some toxicological studies of Momordica charantia L. on albino rats in normal and diabetic rats. J Ethnopharmacol. 2006;108:236–242. doi: 10.1016/j.jep.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Seaforth CE, Adams CD, Sylvester Y. Aguide to the medicinal plants of Trinidad and Tobago. 1st ed. Trinidad and Tobago: CARIRI; 1986. [Google Scholar]
- 6.Burnett A, Singh P, Simon O, McKoy M. Investigation of the hypoglycaemic potential of Momordica charantia Linn (cerasee) in normal rats. West Indian Med J. 2010;59(4):36–36. [Google Scholar]
- 7.Taylor L. Technical data report for bitter melon (Momordica charantia) Herb Secrets Rainforest. 2002;2:1–101. [Google Scholar]
- 8.Karunanayake E, Jeevathayaparan S, Tennekoon K. Effect of Momordica charantia fruit juice on streptozotocin-induced diabetes in rats. J Ethnopharmacol. 1990;30:199–204. doi: 10.1016/0378-8741(90)90008-h. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar S, Pranava M, Marita R. Demonstration of the hypoglycaemic action of Momordica charantia in a validated animal model of diabetes. Pharmacol Res. 1996;33:1–4. doi: 10.1006/phrs.1996.0001. [DOI] [PubMed] [Google Scholar]
- 10.Morrison E, West M. A preliminary study of the effects of some West Indian medicinal plants on blood sugar levels in the dog. West Indian Med J. 1982;31:194–197. [PubMed] [Google Scholar]
- 11.Rao B, Sudarshan P, Rajasekhar M, Nagaraju N, Rao C. Antidiabetic activity of Terminalia pallida fruit in alloxan-induced diabetic rats. J Ethnopharmacol. 2003;85:169–172. doi: 10.1016/s0378-8741(02)00396-3. [DOI] [PubMed] [Google Scholar]
- 12.Andrade-Cetto A, Wiedenfeld H. Hypoglycemic effects of Cecropia obtusifolia on Streptozotocin diabetic rats. J Ethnopharmacol. 2001;78:145–149. doi: 10.1016/s0378-8741(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 13.Patane G, Piro S, Anello M, Rabuazzo A, Vigneri R, Purrello F. Exposure to glibenclamide increases rat beta cells sensitivity to glucose. Br J Pharmacol. 2000;129:887–892. doi: 10.1038/sj.bjp.0703131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vikrant V, Grover J, Tandon N, Rathi S, Gupta N. Treatment with extracts of Momordica charantia and Eugenia jambolana prevents hyper-glycaemia and hyperinsulinemia in fructose fed rats. J Ethnopharmacol. 2001;76:139–143. doi: 10.1016/s0378-8741(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 15.Shubhashish S, Pranava M, Marita R. Demonstration of the hypo-glycemic action of Momordica charantia in a validated animal model of diabetes. Pharmacol Res. 1996;33:1–4. doi: 10.1006/phrs.1996.0001. [DOI] [PubMed] [Google Scholar]
- 16.Gribble F, Reimann F. Sulfonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 17.Rang H, Dale M, Ritter J, Moore P. Pharmacology. In: Hunter L, editor. The endocrine pancreas and the control of blood glucose. 5th ed. Scotland, UK: Churchhill Livingstone; 2003. pp. 380–393. [Google Scholar]