ABSTRACT
Objective:
To characterize the frequency, incidence and severity of dengue fever in Suriname and to detect historic clusters of disease by integrating epidemiological data into a spatial visualization platform.
Methods:
The frequency, incidence and severity of all reported dengue fever (DF) and dengue haemorrhagic fever (DHF) cases in Suriname from 2001 to 2012 were calculated and stratified by demographic factors. Using a geographic information systems (GIS) platform, we visualized the distribution of DF cases and used Moran's I to detect autocorrelation. Furthermore, a retrospective spatial Poisson probability model was used to identify local clusters of DF within Suriname. Local clusters were divided into neighbourhoods and individual DF cases were mapped to the street level.
Results:
In Suriname, cases of DF emerge in cyclical patterns (three to five years) with seasonal peaks following the short and the long rainy season. Chi-squared analysis indicated a statistically significant (p < 0.05) difference between age group, ethnicity and district and the onset of DHF. The spatial analysis detected spatial autocorrelation and four statistically significant (p < 0.05) clusters were identified in the two most populated districts of Paramaribo and Wanica.
Conclusion:
In Suriname, identification of demographic and environmental risk factors that contribute to the development of DHF is essential to determine how preventive action can be more effectively allocated. The integration of epidemiological data into a GIS platform allowed for the identification of historic epidemiological clusters of dengue which will be used to guide environmental health studies in Suriname.
Keywords: Dengue fever, spatial distribution, Suriname
RESUMEN
Objetivo:
Caracterizar la frecuencia, incidencia y severidad del dengue en Surinam, y detectar los agrupamientos (clusters) históricos de la enfermedad mediante la integración de los datos epidemiológicos en una plataforma de visualización espacial.
Métodos:
La frecuencia, incidencia y severidad de todos los casos de fiebre de dengue (FD) y fiebre hemorrágica de dengue (FHD) reportados en Surinam desde 2001 a 2012, fueron calculados y estratificados de acuerdo con factores demográficos. Utilizando una plataforma de sistemas de información geográfica (SIG), visualizamos la distribución de los casos de FD, y usamos la I de Moran para detectar la autocorrelación. Además, se usó un modelo de probabilidad de Poisson para el análisis espacial retrospectivo para identificar los agrupamientos locales de FD dentro de Surinam. Dichos agrupamientos locales fueron divididos en barrios, y los casos individuales de FD fueron mapeados a nivel de la calle.
Resultados:
En Surinam, los casos de FD emergen en patrones cíclicos (tres a cinco años) con picos estacionales tras la corta y la larga estación de lluvias. El análisis de chi-cuadrado indicó una diferencia estadísticamente significativa (p < 0.05) entre el grupo de edad, la etnicidad y el distrito, y la aparición de la FHD. El análisis espacial detectó la autocorrelación espacial y cuatro agrupamientos estadísticamente significativos de (p < 0.05) fueron identificados en los dos distritos más poblados de Paramaribo y Wanica.
Conclusión:
En Surinam, la identificación de los factores de riesgo demográficos y ambientales que contribuyen al desarrollo de la FHD, es esencial para determinar cómo las medidas preventivas pueden distribuirse mejor. La integración de los datos epidemiológicos en una plataforma SIG hizo posible la identificación de clusters epidemiológicos históricos de dengue, y será utilizada para guiar los estudios de salud ambiental en Surinam.
INTRODUCTION
Over the last 50 years, the incidence of dengue fever (DF) worldwide has increased by 50-fold and 2.5 billion people currently live in dengue-endemic regions. An estimated 50 million cases of DF occur worldwide every year (1). Currently, dengue is hyperendemic in most of the countries of the World Health Organization's (WHO) Region of the Americas, including Suriname (2). Over the past 30 years, an increased level of urbanization and successful re-infestation of Aedes aegypti, the primary vector of dengue transmission, have contributed to the rapid spread of dengue in Suriname (3). The first endemic cases of DF occurred in 1981, with a total of 22 laboratory confirmed cases and an estimated 10% of the population of Paramaribo suffering from DF-like symptoms (4). An annual resurgence of DF and dengue haemorrhagic (DHF) fever began in 1997 (5) and the last registered dengue outbreak occurred toward the end of the rainy season in 2012. This annual resurgence of DF and DHF cases is influenced by demographic and environmental factors that have not been previously studied in Suriname. Thus, it is imperative to identify which factors exhibit patterns associated with the spatial and temporal distribution of DF and DHF. This study aims to 1) characterize the frequency, incidence and severity of dengue cases in Suriname from 2001 to 2012 and 2) integrate clinical and demographic information of all dengue cases to identify historic clusters of DF in Suriname.
The dengue virus (DENV) is a pathogenic human virus that belongs to the genus Flavivirus (family Flaviviridae). Four different types of DENV (DENV type 1 through 4, DENV1–4), which result in distinguishable serological strains, are capable of causing DF in humans (6). DENV-1 became the first documented serotype circulating in Suriname and subsequently DENV-4 was reported in 1981 (7). DENV4 was reported in Suriname in 1994, followed by DENV1 in 1998, DENV2 in 1999 and DENV3 in 2001 (8, 9). Moreover, different serotypes have co-circulated within the population: DENV1, DENV2 and DENV3 were isolated from the patients during the largest dengue outbreak in Suriname in 2005.
The Surinamese Bureau of Public Health (Bureau Openbare Gezondheidzorg – BOG), under the Ministry of Health, is the national institute in charge of dengue surveillance and control. In 2001, epidemiological surveillance of dengue began by having hospitals and regional clinics report all cases of DF and DHF to the BOG. The reported dengue cases include demographic (age, gender, ethnicity) and clinical (date of onset, hospital admission and discharge and laboratory test results) information. The surveillance and vector control practices of the BOG are essential in curbing the disease. Additionally, the application of innovative spatio-temporal visualization techniques, such as geographic information system (GIS), is becoming more commonplace in efforts to implement better surveillance and control techniques against vector-related diseases. Geographic information system software can be utilized not only to identify if cases of DF and DHF cluster, but also to visualize how they emerge overtime and if those cases are also related to environmental or demographic drivers (10). Thus, integrating GIS technology to improve disease surveillance enables more efficient public health planning by identifying target areas in which more resources should be invested.
The objective of this study is to complete the initial steps for the integration of epidemiological data into a spatial visualization platform to map dengue risk areas in Suriname. The findings will be used to inform an interdisciplinary study that integrates epidemiological data to guide environmental health research in Suriname.
METHODS
Data sources and case classification
Dengue is a reportable disease in Suriname. Since 2001, all suspected cases of DF reported to the BOG have been assigned a unique reference number and compiled into a database that includes demographic and clinical information and an address coded according to the Surinamese General Bureau of Statistics (ABS) codebook. Researchers did not have access to these personal identifiers. The database was screened and DF cases were selected for analysis based on positive serology test or on virus/nucleic acid isolation, respectively. Dengue haemorrhagic fever cases were selected based on clinical diagnosis (according to the WHO criteria) when no diagnostic test was recorded. However, due to the generic nature of DF symptoms, cases of DF based solely on clinical diagnosis were classified as suspected and excluded from the analysis. In this study, cases of DHF are not counted as cases of DF, therefore, the term ‘dengue cases’ refers to both cases of DF and DHF. The study protocol was approved by the Tulane University School of Public Health and Tropical Medicine Institutional Review Board (IRB) and the Ministry of Health, Suriname.
Data analysis:
The annual frequency of total cases of dengue, DF and DHF for the entire country from 2001 through 2012 was calculated. Annual dengue severity was measured as the number of DHF cases compared to total cases of dengue. Frequency distributions were also calculated by age group, gender, ethnicity and district. These categorical variables were used to compare the frequency of DF vs DHF by Chi-squared test using SPSS® Statistics version 19. Suriname is divided into 10 subdivisions, also known as districts. Annualized dengue incidence rates were obtained using mid-year population estimates based on 2004 and 2012 ABS census information. District incidence rates were obtained using total dengue cases and district population estimates from 2002 through 2012 (no case by district data are available for 2001).
Spatial analysis
To identify global patterns of dengue cases, we calculated Moran's I as a global index for spatial autocorrelation using GeoDa™ (http://geodacenter.asu.edu). Cases were weighed according to distance and the number of nearest neighbourhoods was set to four. For the randomization, 999 permutations were selected. To detect local clusters of probable cases of dengue, we deployed the Kuldorff's spatial scan statistics using SatScan software version 9.1.1 (www.satscan.org).
We used a retrospective spatial Poisson probability model for discrete data to detect significant high rate (hotspots) and low rate (coldspot) clusters with geographic overlap. The number of replications was set to 999 times with the maximum spatial cluster size set at 20%. The most likely clusters (primary clusters) and other clusters (secondary clusters) that were detected within Suriname were visualized using ArcGIS v10.2 (http://www.esri.com). The relative risk (rr), log-likelihood ratio (LLR) and p-value for each displayed cluster was recorded. Neighbourhood borders within the Paramaribo district were drawn and exported as a polygon shapefile and the district's streets were added in as a layer. There were no neighbourhood borders available for other districts. The address codes provided in the BOG dengue database allowed each case of dengue to be localized to a street within a resort. To protect patient privacy, the BOG address codes provide the street name and the two intersecting streets for each case of dengue but no house number. Therefore, the cases of dengue were mapped half-way on the street of the two intersecting streets.
RESULTS
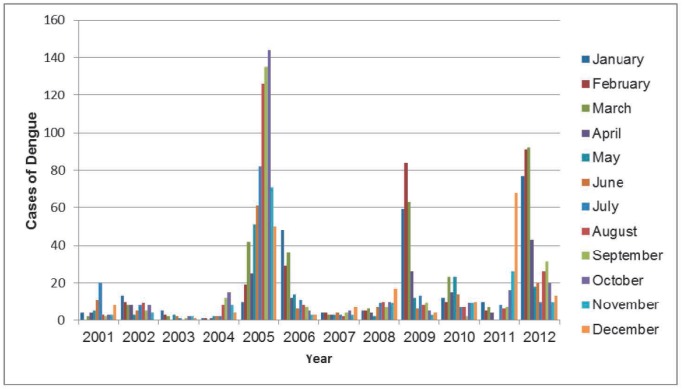
There were a total of 2393 cases of dengue, of which 366 progressed into DHF as reported to the BOG between 2001 and 2012. Figure 1 illustrates a temporal pattern of emergence in cases of dengue since 2001 with a higher proportion of annual cases during the months of August to October and December to February.
Fig. 1. Annual frequency of dengue cases by month. Dengue cases reported between January 2001 and December 2012 were stratified by month. There is a temporal pattern of emergence of dengue cases with the number of cases peaking after both the short and long rainy season in February and October, respectively. The biggest dengue outbreak occurred in 2005.
In addition, the incidence of dengue peaked during the 2005 epidemic with 16.4 cases per 10 000 people (Table 1). To date, 2005 has the greatest incidence of dengue cases but only 11% of all cases progressed into DHF. During the 2009 epidemic, 38% of cases progressed into DHF but the number of people affected was much lower at 5.1 cases per 10 000 (Table1).
Table 1. Annualized cases of dengue, dengue fever (DF) and dengue haemorrhagic fever (DHF).
| Year | Dengue n (%) |
DF n (%) |
DHF n (%) |
Incidence* | Severity (%) |
Serotype |
|---|---|---|---|---|---|---|
| Total | 2399 (100) | 2033 (100) | 366 (100) | 3.94 | 15.2 | |
| 2001 | 65 (2.7) | 56 (2.7) | 9 (2.4) | 1.4 | 13.8 | 3 |
| 2002 | 81 (3.4) | 77 (3.8) | 4 (1.1) | 1.7 | 4.9 | 3 |
| 2003 | 22 (0.9) | 22 (1.1) | 0 (–) | 0.5 | 0 | 2 |
| 2004 | 56 (2.3) | 53 (2.6) | 3 (0.8) | 1.1 | 5.3 | 3 |
| 2005 | 816 (34.0) | 723 (35.6) | 93 (25.4) | 16.4 | 11.4 | 2, 3 |
| 2006 | 182 (7.6) | 166 (8.2) | 16 (4.4) | 3.6 | 8.8 | 2 |
| 2007 | 45 (1.9) | 38 (1.9) | 7 (1.9) | 0.9 | 15.5 | 2 |
| 2008 | 91 (3.8) | 70 (3.4) | 21 (5.7) | 1.4 | 23.1 | –** |
| 2009 | 292 (12.2) | 179 (8.8) | 113 (30.9) | 5.1 | 38.7 | -** |
| 2010 | 141 (5.9) | 121 (5.9) | 20 (5.5) | 2.5 | 14.2 | 1, 2, 4 |
| 2011 | 157 (6.5) | 135 (6.6) | 22 (6.0) | 2.8 | 14.0 | 2, 4 |
| 2012 | 451 (18.8) | 393 (19.3) | 58 (19.3) | 7.7 | 12.9 | 1, 2, 4 |
per 10 000 people
No serotype information available
Therefore, the 2009 epidemic was much worse in severity compared to 2005. The frequency of dengue cases, DF and DHF stratified by age group, gender and ethnicity are shown in Table 2.
Table 2. Frequency, severity and incidence of dengue, dengue fever (DF) and dengue haemorrhagic fever (DHF) cases by demographics.
| Dengue‡
(%) |
DF‡
n(%) |
DHF (%) |
Severity (%) |
|
|---|---|---|---|---|
| Total | 2393 (100) | 2027 (100) | 366 (100) | 15.3 |
| Age (year)γ* | ||||
| >1 | 85 (3.5) | 70 (3.4) | 15 (4.1) | 17.6 |
| 1–14 | 675 (28.2) | 552 (27.2) | 123 (33.6) | 18.2 |
| 15–29 | 679 (28.4) | 598 (29.5) | 81 (22.1) | 11.9 |
| 30–44 | 504 (21.1) | 435 (21.5) | 69 (18.8) | 13.7 |
| 45–59 | 302 (12.6) | 242 (11.9) | 60 (16.4) | 19.9 |
| 60+ | 144 (6.0) | 127 (6.3) | 17 (4.6) | 11.8 |
| Genderγ | ||||
| Male | 1357 (56.7) | 1147 (56.6) | 210 (57.4) | 15.5 |
| Female | 1028 (42.9) | 873 (43.1) | 155 (42.3) | 15.1 |
| Ethnicityγ* | ||||
| Creole | 295 (12.3) | 256 (12.6) | 39 (10.6) | 13.2 |
| Hindustani | 922 (38.5) | 788 (38.8) | 134 (36.6) | 14.5 |
| Javanese | 389 (16.2) | 314 (15.5) | 75 (20.5) | 19.3 |
| Chinese | 259 (10.8) | 226 (11.1) | 33 (9.0) | 12.7 |
| European | 42 (1.7) | 30 (1.5) | 12 (3.3) | 28.6 |
| Indigenous | 58 (2.4) | 51 (2.5) | 7 (1.9) | 12.1 |
| Maroon | 59 (2.5) | 49 (2.4) | 10 (2.7) | 16.9 |
Cases without district information were excluded from the total
Dengue cases with missing information were excluded from Chi-square analysis
Chi-squared test p < 0.05
There is no variation in severity between genders. Severity is higher in certain age groups (> 1, 1–14 and 45–59 years) and ethnicities (Hindustani and Javanese). Chi-squared analysis indicates no significant difference in cases of DF and DHF by gender. However the difference between DF and DHF cases was statistically significant for age group and ethnicity [p < 0 05] (Table 2) indicating that certain demographic factors influence the progression into DHF within our study population.
Global and local clusters
The frequency, incidence and severity of dengue cases from 2002 to 2012 are shown in Table 3. The districts of Nickerie and Coronie, and Marowijne and Brokopondo were grouped together for Chi-squared analysis. Frequency, incidence and severity were highest in the two most urbanized districts of Paramaribo and Wanica (Table 3).
Table 3. Frequency, severity and incidence of dengue, dengue fever (DF) and dengue haemorrhagic fever (DHF) cases by district.
| Dengue‡
n(%) |
DF‡
n (%) |
DHF n (%) |
Severity (%) |
Incidence** | |
|---|---|---|---|---|---|
| Total* | 2393 (100) | 2027 (100) | 366 (100) | 15.3 | 3.9 |
| District*** | |||||
| Paramaribo | 1286 (57.7) | 1085 (57.6) | 201 (58.4) | 15.6 | 4.7 |
| Wanica | 481 (21.6) | 388 (20.6) | 93 (27.0) | 19.3 | 4.8 |
| Para | 64 (2.9) | 54 (2.8) | 10 (2.9) | 15.6 | 3.0 |
| Commewijne | 109 (4.9) | 92 (4.9) | 17 (4.9) | 15.6 | 3.8 |
| Saramaca | 68 (3.0) | 57 (3.0) | 11 (3.2) | 16.2 | 3.8 |
| Nickerie/Coronie | 176 (7.9) | 172 (9.2) | 4 (1.2) | 2.3 | 4.0 |
| Marowijne/Brokopondo | 27 (1.2) | 23 (1.2) | 4 (1.2) | 14.8 | 0.8 |
| Sipaliwi | 17 (0.7) | 13 (0.7) | 4 (1.2) | 23.5 | 0.4 |
Cases without district information were excluded from the total
No information by district available for 2001
per 10 000 person-year
Chi-squared test p < 0.05
Furthermore, Global Moran's I suggest that there is spatial autocorrelation, or clustering, of dengue cases (Moran's I = 0.052, z > 1.96, p = 0.001) at the resort level within Suriname. To identify where the population had the highest relative risk (rr) of dengue cases in Suriname, we identified the two most likely (highest likelihood ratio-LLR) and statistically significant (p < 0.05) clusters (also referred to as hotspots) by resort between 2002 to 2012 with a maximum spatial cluster size of the total population set at 20% (Table 4). Additionally, the two lowest rr clusters (or coldspots) were identified using the same procedure. The most likely hotspots and coldspots were classified as primary while all other identified clusters were classified as secondary.
Table 4. Cluster analysis of dengue cases in Suriname.
| Cluster | Rate | Cluster centre | Relative risk | Likelihood ratio | p-value |
|---|---|---|---|---|---|
| Primary | High | Blauwgrond | 26.03 | 150.6 | 0.000 |
| Secondary | High | Munder | 19.9 | 93.4 | 0.000 |
| Primary | Low | Latour | 0.18 | 110.9 | 0.000 |
| Secondary | Low | Koewarasan | 0.41 | 38.6 | 0.000 |
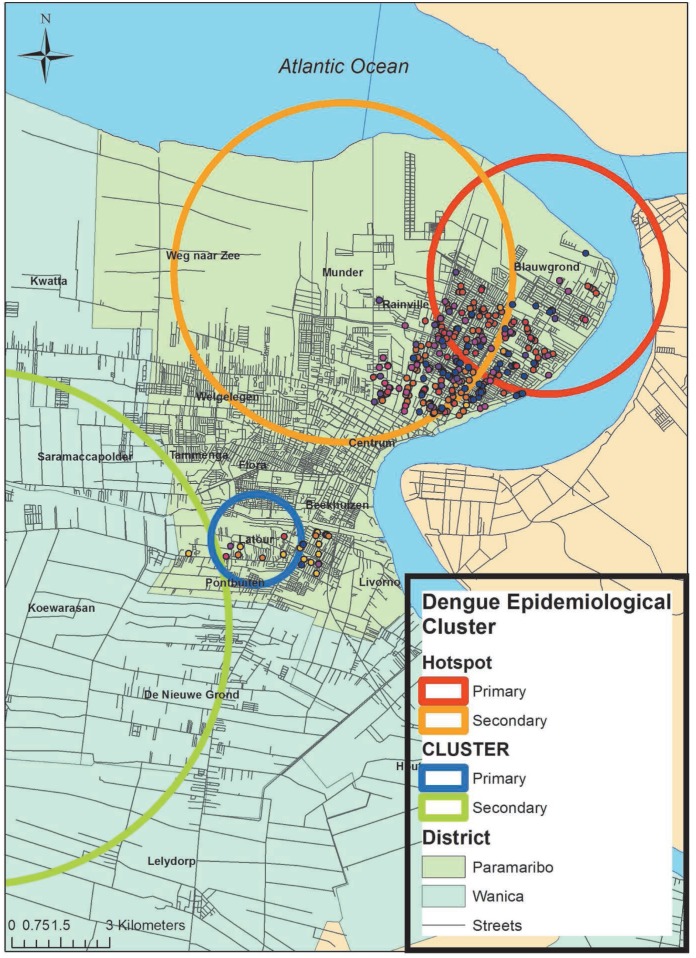
The rr, LLR and p-value are displayed in Table 4. The centroid and radius of the clusters were used to visualize the clusters in Suriname using ArcGIS v10.2. Both primary and secondary hotspots and coldspots were located in resorts within Paramaribo and Wanica districts (Fig. 2). The resorts within the hotspots included Blauwgrond, Rainville and Munder located on the northern side of district Paramaribo while Koewarasan and Latour made up the coldspots in a more southern region of this district. The resorts that are closer to each other tend to have similar annualized incidence rates. Neighbourhood boundaries, streets and cases of dengue within the two primary clusters better illustrate the dispersion of cases in the identified high and low epidemiological rate areas (Fig. 2).
Fig. 2. Spatial distribution of dengue cases for Paramaribo and Wanica, Suriname. Four clusters: one primary (red circle) and one secondary (orange circle) hotspot and one primary (blue circle) and one secondary (green circle) coldspot were detected within Paramaribo and Wanica districts based on dengue cases distribution from 2002 to 2012. Only dengue cases within the resorts of the primary hotspot (Blauwgrond and Rainville) and primary coldspot (Latour) are displayed on the map. Dots indicate a single case of dengue.
DISCUSSION
In Suriname, the number of cases of dengue peaks following the end of the long and short rainy season in August and February, respectively. Since 2001, dengue epidemics have emerged every three to four years, which has also been documented for other countries (11). The cyclical emergence of dengue is influenced by climate conditions and climatological phenomena such as El Nino-Southern Oscillation, evident by the large 2005 epidemic in Suriname (12–14). In addition to an increase in dengue cases, frequency analysis indicated that the severity of dengue has increased since 2001, most notably during the smaller but more severe epidemics of 2009 and 2012. An increase in severity is expected because since 2001, all four serotypes of dengue have circulated or co-circulated within the population (Fig. 1). Unfortunately, there is no record of the dengue serotype circulating during the 2009 epidemic. Such information is important because the introduction of a new serotype into a non-naïve population increases the incidence and number of DHF cases during a dengue outbreak (15). Over time, the immune status of the population changes as more people become infected with one of the four dengue viruses, increasing their susceptibility of developing DHF. An increase in cases of DHF strains the healthcare system because more people require hospitalization during a dengue epidemic. This was evident during the 2012 epidemic which exhausted national healthcare capabilities and prompted the Ministry of Health to open an emergency hospital. Additionally, the serotypes (16, 17), the sequential order in which the serotypes are encountered within a population (18–20) and the time elapsed between primary and secondary infections (15, 21) are associated with increased pathogenicity and the development of DHF. Therefore, it can be expected that as more people become exposed to the dengue virus, the disease dynamics within the Surinamese population will change. These changes require a better characterization of the demographic factors that influence the development of DHF in Suriname.
Our results indicate that certain ethnicities (Javanese and Hindustanis) and age groups (1–14 years) occur in a disproportionate number of cases of DHF. These demographic characteristics have been identified as contributing risk factors for the development of DHF (22–24). The Surinamese population is characterized by an ethnic diversity profile unlike any other country in South America. Therefore, these findings are the first step to examine demographic risk factors that contribute to the development of DHF within such a unique demographic composition.
Innovative spatio-temporal visualization techniques, such as GIS, are becoming more commonplace in efforts to implement better surveillance and control techniques against dengue fever. These techniques have been used to identify DF and DHF spatial clustering, where clusters emerge and how demographic factors change over space and time (10). Here, the results of the global spatial analysis revealed that there was significant clustering of cases of dengue in Suriname. The identified hotspots are located in areas where there is a historically higher relative risk of dengue compared to the rest of the population. Similarly, the coldspots are located in areas where the relative risk of dengue is much lower.
The data are limited to cases of dengue from hospitals and clinics in Suriname reported to the BOG between 2001 and 2012. Asymptomatic or flu-like DF cases are frequently under-reported, potentially causing dengue incidence to be under-estimated. Additionally, serology tests have a low specificity and sensitivity to acute dengue infection or to distinguish between primary and secondary infections of dengue (25, 26). Therefore, data do not provide information on previous dengue infections, which are an important determinant in the development of DHF. However, the data analysis does provide epidemiological knowledge that can be used as a tool to design resource-efficient environmental and entomological studies. Such studies would provide more information about the local dispersion of the Aedes aegypti mosquito and dengue virus transmission. This study represents the first steps to identify demographic and environmental factors that influence the transmission of dengue fever and the development of DHF in Suriname.
ACKNOWLEDGEMENTS
This study is in collaboration with the Bureau of Public Health, Suriname. A special thanks to Dr Beatrix Jubithana, epidemiologist of the Bureau of Public Health, for her assistance with this project.
REFERENCES
- 1.World Health Organization . Dengue: guidelines for diagnosis, treatment, prevention and control. New edition. Geneva: WHO Press; 2009. [PubMed] [Google Scholar]
- 2.San Martin JL, Brathwaite O, Zambrano B, Solorzano JO, Bouckenooghe A, Dayan GH, et al. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brathwaite DO, San Martin JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg. 2012;87:584–598. doi: 10.4269/ajtmh.2012.11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinheiro FP. Dengue in the Americas 1980 – 1987. Epidemiol Bull. 1989;10:1–8. [PubMed] [Google Scholar]
- 5.World Health Organization . DengueNet [Internet] Washington (DC): World Health Organization; [[cited 2013 Feb 2]]. Available from http://apps.who.int/globalatlas/default.asp. [Google Scholar]
- 6.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson ME, Chen LH. Dengue in the Americas. Dengue Bull. 2002;26:44–61. [Google Scholar]
- 8.Pan American Health Organization . Number of reported cases of dengue and dengue hemorrhagic fever (DHF), Region of the Americas (by country and subregion) [Internet] Washington (DC): Regional Offices for the Americas of the World Health Organization; [[cited 2014 Feb 2]]. Available from http://amro.who.int/english/ad/dpc/cd/dengue-cases-2008.htm. [Google Scholar]
- 9.Caribbean Epidemiology Centre Report on communicable diseases for weeks 1–8, 2012. CAREC Surveillance Report. 2012;32:1–28. [Google Scholar]
- 10.Duncombe J, Clements A, Hu W, Weinstein P, Ritchie S, Espino FE. Geographical information systems for dengue surveillance. Am J Trop Med Hyg. 2012;86:753–755. doi: 10.4269/ajtmh.2012.11-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halide H, Ridd P. A predictive model for dengue hemorrhagic fever epidemics. Int J Environ Health Res. 2008;18:253–265. doi: 10.1080/09603120801966043. [DOI] [PubMed] [Google Scholar]
- 12.Kovats RS, Bouma MJ, Hajat S, Worrall E, Haines A. El Niṅo and health. Lancet. 2003;362:1481–1489. doi: 10.1016/S0140-6736(03)14695-8. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon AS, Bush ABG, Smoyer-Tomic KE. Dengue epidemics and the El Nino Southern Oscillation. Clim Res. 2001;19:35–43. [Google Scholar]
- 14.Hopp MJ, Foley JA. Worldwide fluctuations in dengue fever cases related to climate variability. Clim Res. 2003;25:85–94. [Google Scholar]
- 15.Diaz-Quijano FA, Waldman EA. Factors associated with dengue mortality in Latin America and the Caribbean, 1995–2009: an ecological study. Am J Trop Med Hyg. 2012;86:328–334. doi: 10.4269/ajtmh.2012.11-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, et al. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–251. doi: 10.1006/viro.1997.8504. [DOI] [PubMed] [Google Scholar]
- 17.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 18.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 19.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 20.Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 21.Guzman MG, Kouri G, Valdes L, Bravo J, Vazquez S, Halstead SB. Enhanced severity of secondary dengue-2 infections: Death rates in 1981 and 1997 Cuban outbreaks. Rev Panam Salud Publica. 2002;11:223–227. doi: 10.1590/s1020-49892002000400003. [DOI] [PubMed] [Google Scholar]
- 22.de la Sierra B, Kouri G, Guzman MG. Race: a risk factor for dengue hemorrhagic fever. Arch Virol. 2007;152:533–542. doi: 10.1007/s00705-006-0869-x. [DOI] [PubMed] [Google Scholar]
- 23.Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis. 2002;6:118–124. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 24.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, et al. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–1489. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaterij S, Allen JC, Chow A, Leo YS, Ooi EE. Evaluation of the NS1 rapid test and the WHO dengue classification schemes for use as bedside diagnosis of acute dengue fever in adults. Am J Trop Med Hyg. 2011;84:224–228. doi: 10.4269/ajtmh.2011.10-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Souza VA, Tateno AF, Oliveira RR, Dominques RB, Araujo ES, Kuster GW, et al. Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J Clin Virol. 2007;39:230–233. doi: 10.1016/j.jcv.2007.04.005. [DOI] [PubMed] [Google Scholar]