ABSTRACT
Objective:
In the last decade, dental implants have emerged as a crucial modality and serve as an individual form of therapy for dental failure. However, disparities in host responses have led to peri-implantitis and implant failure. The pathological mechanisms driving peri-implantitis remain largely unknown. In this study, we evaluated the role of oxidative stress and advanced glycation end products (AGEs) in the progression of peri-implantitis and dental implants failure, compared with chronic periodontal disease.
Subjects and Methods:
Three patient groups (peri-implantitis, chronic periodontal disease and control), each with 10 subjects (7M/3F) and average age ranging from 40–60 years were selected for analysis. Salivary oxidative stress and tissue AGE levels were analysed by probing for reactive oxygen species (ROS) and Maillard reaction-related fluorescence, respectively.
Results:
We observed significant increase (> 2-fold) in oxidative stress and AGE levels in patients with peri-implantitis and chronic periodontal disease compared to controls, with chronic periodontal disease having the highest levels. In addition, we observed a strong positive correlation (r = 0.94) between oxidative stress and AGE levels in the patients.
Conclusion:
We propose that increased AGE levels and oxidative stress, although not the only pathway, are significant mediators in the pathogenesis of peri-implantitis. Altering them may potentially be used in combination with other modalities to manage peri-implantitis.
Keywords: Advanced glycation, end products, dental implants, oxidative stress, peri-implantitis
RESUMEN
Objetivo:
En la última década, los implantes dentales han surgido como una modalidad crucial y sirven como forma individual de terapia para el fracaso dental. Sin embargo, las disparidades en las respuestas de los receptores han llevado a la peri-implantitis y al fracaso del implante. Los mecanismos patológicos que conducen a la peri-implantitis, se desconocen en gran medida. En este estudio, evaluamos el papel del estrés oxidativo y los productos finales de la glicación avanzada (PGA) en la progresión de la peri-implantitis y el fracaso de los implantes dentales, en comparación con la enfermedad periodontal crónica.
Sujetos y métodos:
Tres grupos de pacientes (peri-implantitis, enfermedad periodontal crónica y control) cada uno con 10 sujetos (7H/3M) y edad promedio de 40 a 60 años, fueron seleccionados para el análisis. El estrés oxidativo salival y los niveles PGA del tejido se analizaron mediante el sondeo de especies reactivas del oxígeno (ERO) y fluorescencia por reacción de Maillard, respectivamente.
Resultados:
Observamos un aumento significativo (> 2 veces) en el estrés oxidativo y los niveles PGA de los pacientes con peri-implantitis y enfermedad periodontal crónica en comparación con los controles, correspondiendo los niveles más altos a la enfermedad periodontal crónica. Además, observamos una fuerte correlación positiva (r = 0.94) entre el estrés oxidativo y los niveles de PGA en los pacientes.
Conclusión:
Proponemos que el aumento de los niveles de PGA y el estrés oxidativo, aunque no son la única vía, constituyen mediadores importantes en la patogénesis de la peri-implantitis. El alterarlos puede potencialmente utilizarse en combinación con otras modalidades para tratar la peri-implantitis.
INTRODUCTION
Many clinical dental implants have been established and are used either individually or in combination with other treatment modalities for dental failure. Despite the implants having a high survival rate and osseointegration, over a five-year period, ~ 15% of dental implants manifest peri-implant inflammatory reactions and crestal bone loss, which eventually lead to implant failure (1). It is speculated that host factors determine whether implants osseointegrate or cause inflammatory responses leading to peri-implantitis.
Provided with good procedural techniques, at least three post installation host responses are proposed for endosseous implants: (a) functional bone tissue development with osseointegration, (b) connective tissue formation around implant causing osseointegration failure and (c) acute or chronic inflammation causing early implant failure (2). These responses are determined by a wide range of factors such as, genetic predisposition, smoking, dietary habits, blood pressure and glucose levels, and differences in them set the platform for the onset of peri-implantitis (3). Given the variability between the patients and the wide range of host response modulating factors, identifying a unifying molecular pathogenic manifestation would aid understanding of disease progression and development of therapeutics.
A role for oxidative stress – imbalance between reactive oxygen species (ROS) manifestation and host ability to detoxify or repair the damage – has been well documented in many disease pathogenesis (4). In normal conditions, ROS are produced as natural products of aerobic metabolism and are removed by cellular defence mechanisms involving enzymatic and non-enzymatic systems. In contrast, in disease conditions, an imbalance is manifested and causes a hyper-inflammatory condition, and leads to cellular damage (4). In addition to ROS, increased advanced glycation end products (AGEs) – heterogeneous products formed from non-enzymatic protein glycation reactions – are also usually manifested in oxidative stress conditions. Previous studies have shown that AGE increase can be a cause of development of or mediating factor for many systemic diseases and can cause a wide range of pathological effects such as inducing inflammatory cytokines, and enhancing oxidative stress (5). More importantly, recent studies suggest that AGE binds to a specific receptor for AGE (RAGE) and can induce chronic inflammation through nuclear factor-kappa B pathway – a pathway associated with diverse effects (6). Altogether, increased ROS and AGE represent two markers of pathognomonic oxidative stress, and can cause hyper-inflammation and permanent tissue damage.
Although the significance of oxidative stress is known in many other diseases including oral disease such as chronic periodontal disease (7, 8), it is unclear whether there is a similar role for oxidative stress in dental implant related peri-implantitis. In this study, we investigated the levels of oxidative stress and AGE in peri-implantitis, chronic periodontal disease and healthy controls for comparison. We selected patients with similar age group, smoking habits and matching diagnosis as per disease groups, and evaluated oxidative stress through lipid peroxidation and AGE analysis by Maillard reaction-related fluorescence.
SUBJECTS AND METHODS
Patient information, tissue collection and processing were conducted as per the institute's ethical guidelines and under the supervision of the Department of Stomatology, Beijing Chao-Yang Hospital, an affiliate of Capital Medical University, Beijing, PR China. There were three patients groups (Table). All the patients were selected based on their clean medical history for any other systemic diseases, sharing similar smoking habits and for not taking any prescribed medications in the last six months. In addition, based on the availability of patients, the gender distribution was kept uniform between the groups, and hence the final patient counts in each group were set as 10 patients (seven males and three females) per group for the study.
Table. Summary of patient groups used in the study.
| Groups | Features | Age distribution (mean ± standard deviation) |
|---|---|---|
| 1.Peri-implantitis | Implant failed within six months | 40–60 years (50.6 ± 4.6) |
| 2. Periodontal disease | Patients diagnosed for chronic periodontal disease | 50–55 years (52.2 ± 2.9) |
| 3. Control samples | Healthy individuals without any dental complaints | 37–51 years (45.0 ±5.8) |
Salivary enzyme collection
Saliva was collected as a preoperative procedure in the morning. The patients were mouth-washed and then saliva was collected using Salimetrics® collection system (Salimetrics UK – Oral Swab – Swab Storage Tube). The collected samples were processed further as described below for oxidative stress evaluation.
Oxidative stress analysis
Saliva samples were centrifuged at 6000 g for 10 minutes to remove any particles and the clear salivary supernatant was used for analysis. Oxidative stress was measured as a read-out of lipid peroxidation measurement (ROS measurement), using thiobarbituric acid reactive substances (TBARS) assay (BioAssay Systems, CA, USA) following manufacturer's instructions.
Tooth/Implant collection and processing
The collection and processing method was followed as described previously (9). Briefly, patients were administered with local anaesthesia (mepivacaine 2% and adrenaline 1/100 000 – Scandonest® 2%) and implant (for group 1)/tooth (for groups 2 and 3) were collected in a phosphate buffered solution [PBS; pH 7.4] (Sigma-Aldrich, St Louis, MO, USA). A total of 10 teeth (one tooth per patient) was collected from each group. For subsequent tissue sample extraction, the tooth/implant was incubated in 37 °C water bath for five minutes, sliced with a lancet to collect apical and coronal tissues. The collected tissues were used as the samples for AGE analysis.
Advanced glycation end product analysis
Advanced glycation end product was measured from tissue samples collected from tooth. Briefly, tissue samples were hydrolysed five times in 0.25M oxalic acid and then dialysed against PBS. Total protein content was determined by ultraviolet absorbance at 280 nm and then calculated from a standard curve. Total AGE was measured as Maillard reaction-related fluorescence, a representative of AGE formation. Measurements were made with excitation and emission wavelength of 360 and 450 nm, respectively (10, 11), and with quinine sulfate (1µM in 0.1N H2SO4) as a standard. The levels of AGE were expressed as arbitrary fluorescence units per milligram of protein.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 6). Data were analysed using one-way analysis of variance (ANOVA) and statistical significance was set at minimal value of p < 0.05. All values are listed as mean ± standard deviation.
RESULTS
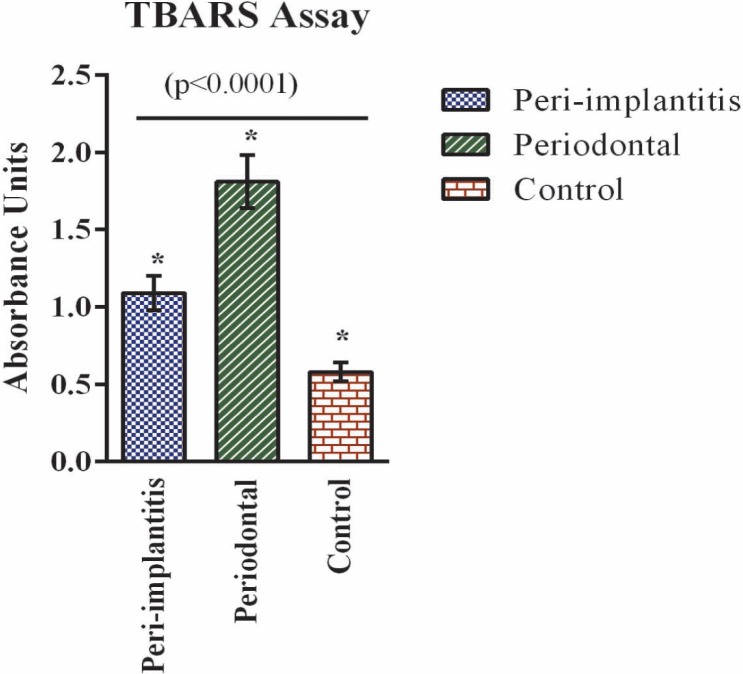
Per the needs of the study and based on the availability of the sample types for analysis, we evaluated oxidative stress through lipid peroxidation analysis. Lipid peroxidation refers to oxidative degradation of lipids mediated through free radical chain reactions between ROS and unsaturated lipids (12). The end products are reactive aldehydes such as malondialdehyde and can readily be measured using common assays such as TBARS assay (13). Lipid peroxidation analysis as evaluated through TBARS assay from salivary samples showed a significant increase (> 2-fold) in both peri-implantitis and chronic periodontal disease compared to healthy control samples (Fig. 1). In addition, data indicate that the lipid peroxidation levels in chronic periodontal disease were two-fold higher than the levels in peri-implantitis.
Fig. 1. Oxidative stress analysis. Oxidative stress was analysed by probing for reactive oxygen species. Reactive oxygen species was measured in saliva samples through thiobarbituric acid reactive substances assay for the three groups – peri-implantitis, periodontal disease and control. Values are provided as mean ± standard deviation (n = 10 each). Statistical significance was observed between all groups (*p < 0.0001).
Similarly, AGE analyses are usually done through protein gel staining methods or through analysing Maillard reaction-related fluorescence (11, 14). Given that fluorescence gives total sum-value from all the glycated proteins, we chose to measure AGE levels through Maillard reaction-related fluorescence. Our data indicate increased AGE accumulation in peri-implantitis and chronic periodontal disease patients compared to controls (Fig. 2). Similar to ROS levels, the AGE levels in periodontal disease were ~ 1.5-fold higher than in peri-implantitis.
Fig. 2. Advanced glycation end products analysis. Advanced glycation end products levels were measured in tissue samples through Maillard reaction-related fluorescence for all three groups – peri-implantitis, periodontal disease and control. Values are provided as mean ± standard deviation (n = 10 each). Statistical significance was seen between all groups (*p < 0.0001).
Finally, we also checked if the ROS and AGE levels were inter-dependent of each other through Pearson correlation analysis of all patients' TBARS and AGE assay results. Our analysis showed strong correlation between the AGE and ROS levels (Pearson coefficient, r = 0.94; p < 0.0001) in all the patients, implying a positive association between the ROS, AGE levels and the patient condition (Fig. 3).
Fig. 3. Correlation analysis. Thiobarbituric acid reactive substances (TBARS) assay units of patients were plotted against corresponding advanced glycation end products (AGE) assay units to evaluate the correlation between them. Two-tailed Pearson correlation analysis was performed (r = 0.94; p < 0.0001).
DISCUSSION
Previous research data suggest that increased ROS can induce AGE production and can cause irreversible deposition, which leads to inflammation and tissue damage (15). It is also known that AGE deposition in the extracellular matrix can increase ROS production and cause inflammation and tissue damage (16). This mutual facilitative role of ROS and AGE would result in a synergistic loop-effect and can overall accelerate tissue damage. Their contribution in many oral inflammatory diseases including periodontal diseases has been previously described (4–6). In accordance, our data have demonstrated elevated AGE and ROS in samples from patients with periodontal disease. However, here we have also highlighted their involvement in peri-implantitis conditions by demonstrating their increased levels in patients' saliva and tissue samples.
Given this observation of both ROS and AGE increase in peri-implantitis as seen in periodontal diseases, it is well worth exploring therapeutic intervention by modulating the oxidative stress and AGE levels. A recent small case series on peri-implantitis has also shown similar evidence for a possible link between oxidative stress and peri-implantitis, and suggested treatment options (14). However, most current clinical treatment options adapted from periodontal diseases have been ineffective for peri-implantitis and can be attributed to their histological and pathological differences, such as infiltration levels and microbial diversity (17–19). Hence, understanding the molecular mechanism associated with periodontal disease and peri-implantitis as reported here is of critical importance, and would help us to differentiate pathological features between these conditions and manage peri-implantitis effectively. Our data indicate that both ROS and AGE increased in both peri-implantitis and periodontal disease compared to the healthy group, with periodontal disease having the highest values. Although, this is in agreement with the conclusion of increased oxidative stress from another study (14), it differs in the levels of markers when compared with chronic periodontal disease. We observed that both lipid peroxidation and AGE were higher in chronic periodontal disease than peri-implantitis, while AGE was higher in peri-implantitis than periodontal disease in another study (14). The significant difference between peri-implantitis and chronic periodontal disease irrespective of the marker analysis implies that the underlying pathological features causing the stress to increase ROS and AGE may be different for both the diseases and could lead to varied treatment responses. Although this difference could be in part explained by the chronic condition of periodontal disease that has major stress manifestation, the previous failure of treatment strategies adapted from periodontal disease to peri-implantitis warrants caution.
Nevertheless, host differences such as genetic background, smoking and diet could also be influencing the outcomes of the study; despite that, the results from both studies clearly indicate a role for oxidative stress in the progression of peri-implantitis. However, our data differ as we compare periodontal disease and peri-implantitis. The significant difference between them suggests the existence of specific causes or mechanisms for the progression of peri-implantitis that differ from periodontal disease. Future studies, directed towards understanding the causative and molecular mechanism specific to peri-implantitis, oxidative stress and the role in promoting peri-implantitis, are required and would aid to translate the antioxidative stress therapy to patients with peri-implantitis.
CONCLUSION
Overall, our study highlights a significant role for oxidative stress in the less understood peri-implantitis conditions. In addition, by comparative analysis, we delineate to exhibit a relatively milder stress manifestation than in periodontal disease, and targeting it specifically could be beneficial for peri-implantitis therapeutic interventions.
REFERENCES
- 1.Att W, Stappert C. Implant therapy to improve quality of life. Quintessence Int. 2003;34:573–581. [PubMed] [Google Scholar]
- 2.Lemons JE. Biomaterials, biomechanics, tissue healing, and immediate function dental implants. J Oral Implantol. 2004;30:318–424. doi: 10.1563/0712.1. [DOI] [PubMed] [Google Scholar]
- 3.Cook SD, Dalton JE. Biocompatibility and biofunctionality of implanted materials. Alpha Omegan. 1992;85:41–47. [PubMed] [Google Scholar]
- 4.Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM, et al. Free radical biology of the cardiovascular system. Clin Sci. 2012;6:73–91. doi: 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- 5.Pietropaoli D, Monaco A, Del Pinto R, Cifone MG, Marzo G, Giannoni M. Advanced glycation end products: possible link between metabolic syndrome and periodontal diseases. Int J Immunopathol Pharmacol. 2012;6:9–17. doi: 10.1177/039463201202500102. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt AM, Stern DM. Hyperinsulinemia and vascular dysfunction: the role of nuclear factor-kappaB, yet again. Circ Res. 2000;6:722–724. doi: 10.1161/01.res.87.9.722. [DOI] [PubMed] [Google Scholar]
- 7.Pietropaoli D, Tatone C, D'Alessandro AM, Monaco A. Possible involvement of advanced glycation end products in periodontal diseases. Int J Immunopathol Pharmacol. 2010;6:683–691. doi: 10.1177/039463201002300301. [DOI] [PubMed] [Google Scholar]
- 8.Soory M. Redox status in periodontal and systemic inflammatory conditions including associated neoplasias: antioxidants as adjunctive therapy? Infect Disord Drug Targets. 2009;6:415–427. doi: 10.2174/187152609788922582. [DOI] [PubMed] [Google Scholar]
- 9.Takatsu M, Uyeno S, Komura J, Watanabe M, Ono T. Age-dependent alterations in mRNA level and promoter methylation of collagen alpha1(I) gene in human periodontal ligament. Mech Ageing Dev. 1999;6:37–48. doi: 10.1016/s0047-6374(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 10.Atanasova M, Konova E, Betova T, Baydanoff S. Non-enzymatic glycation of human fibrillin-1. Gerontology. 2009;55:73–81. doi: 10.1159/000157436. [DOI] [PubMed] [Google Scholar]
- 11.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci USA. 1984;81:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyton KZ, Kensler TW. Oxidative mechanism in carcinogenesis. Br Med Bull. 1993;49:523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Ann Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Pietropaoli D, Ortu E, Severino M, Ciarrocchi I, Gatto R, Monaco A. Glycation and oxidative stress in the failure of dental implants: a case series. BMC Res Notes. 2013;6:296–296. doi: 10.1186/1756-0500-6-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 16.Topkas E, Keith P, Dimeski G, Cooper-White J, Punyadeera C. Evaluation of saliva collection devices for the analysis of proteins. Clin Chim Acta. 2012;6:1066–1070. doi: 10.1016/j.cca.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Renvert S, Roos-Jansaker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35(Suppl 8):305–315. doi: 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 18.Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol. 2011;38(Suppl 11):188–202. doi: 10.1111/j.1600-051X.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 19.Lang NP, Berglundh T, on behalf of Working Group 4 of the Seventh European Workshop on Periodontology Periimplant diseases: where are we now?—Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontal. 2011;38(Suppl 11):178–181. doi: 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]