ABSTRACT
Objective:
Birth palsy, otherwise known as obstetric brachial plexus paralysis (OBPP), is a closed stretch injury to the brachial plexus of nerves during the birth process resulting in varying degree of paralysis and contractures of the upper limb. The study aimed to find out the susceptibility of humans and small-bodied primates to birth palsy.
Method:
A comparative study on parturition in modern humans, hominoids, hominids, small-bodied primates and great apes was done to determine if changes in the female pelvis and neonatal head and shoulder during human evolution is the real cause for OBPP.
Results:
During evolution, the morphology of the female pelvis and birth canal changed into a narrow and twisted one and also the size of the fetal head increased. Thus, the narrow and twisted pelvis of the mother, and the relatively large head and broad shoulders of the newborn has made the birthing process of modern human and small bodied primates a precarious fine-tuned act with a very narrow margin for error. This has necessitated proper obstetric care to reduce or even at times obviate the incidence of birth injuries like OBPP.
Conclusion:
Human evolution has made human babies susceptible to birth palsy and thus is the real cause of birth palsy.
Keywords: Birth palsy, human evolution, obstetric brachial plexus paralysis (OBPP)
RESUMEN
Objetivo:
La parálisis braquial en recién nacidos, también conocida como parálisis obstétrica del plexo braquial (POPB) es una lesión cerrada causada por estiramiento de los nervios del plexo braquial durante el proceso de nacimiento, la cual trae como resultado diversos grados de parálisis y contracturas del miembro superior. El estudio persigue conocer la susceptibilidad de los seres humanos y los primates de pequeño tamaño a las parálisis braquiales en el nacimiento.
Método:
Se realizó un estudio comparativo sobre el alumbramiento en seres humanos modernos, hominoides, homínidos, primates con cuerpo pequeño, y grandes simios, para determinar si los cambios en la pelvis femenina, y en la cabeza y hombros del neonato durante la evolución humana es la verdadera causa de la POPB.
Resultados:
Durante la evolución, la morfología de la pelvis femenina y el canal de nacimiento cambió, adquiriendo una forma estrecha y retorcida, en tanto que por otra parte también aumentó el tamaño de la cabeza fetal. Por lo tanto, la pelvis estrecha y retorcida de la madre, y la cabeza relativamente grande y los hombros anchos del recién nacido han hecho del proceso de parto en los humanos modernos y los primates de cuerpo pequeño, un acto precario, optimizado, con un margen muy estrecho de error. Esto ha hecho imperativo una atención obstétrica adecuada para reducir o incluso a veces obviar la incidencia de lesiones de nacimiento como la POPB.
Conclusión:
La evolución humana ha hecho a los bebés susceptibles a la parálisis braquial obstétrica, y por ende constituye la verdadera causa de la parálisis braquial obstétrica.
INTRODUCTION
Humans differ from other mammals in many distinct ways. We are the only species who routinely walk on two legs, use a highly evolved language for communication and use highly refined tools to shape our world (1). This is possibly due to a relatively large brain, when compared to body size. We also have well positioned dexterous upper extremities, and broad shoulders having evolved from our brachiating (arm swinging locomotion) or suspensory ancestry (1). Similarly, in order to achieve a bipedal gait, the shape of the pelvis evolved to its current morphology (2).
However, the large brain, broad shoulders and bipedal gait and its corresponding changes in pelvis morphology came with a price in the form of difficult birthing process, changes in fetal morphology and neonatal development (2). Only human newborns are altricial (helpless at birth) who require a long period of dedicated parenthood (1). The birth of a human newborn also requires skilful assistance by “learned others” (the obstetrician/midwives) for the safety and well-being of mother and baby (1, 3).
We propose that the following three key changes which happened during human evolution are what made us susceptible to birth palsy:
Changes in the female pelvis for the adaptation of a bipedal gait
Changes in human head (brain) and shoulder morphology due to development of language and tool skills
Changes in the birthing process as a consequence of the above two changes
These changes are elaborated in some detail in the following paragraphs.
Parturition is the process of passage of the fetus through the birth canal. The relative size of the fetal head and shoulder with respect to maternal pelvic canal determines the ease of birth and consequently the chances of birth injuries like birth palsy (1–3).
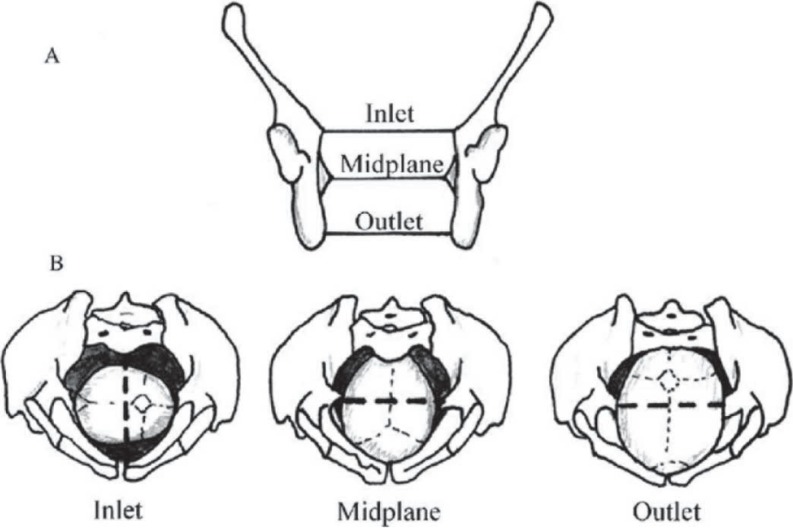
The hominoid birth canal has three relevant planes. They are inlet, the mid-plane and the outlet (1, 3) [Fig. 1].
Fig. 1. Drawings of true pelvic planes and positions of fetal head in each plane. (A) The human pelvis in coronal section, indicating the positions of the three pelvic planes: inlet, midplane and outlet. (B) The positions of the fetal head in each plane during labour. The dotted lines represent the true pelvic diameters more sexually dimorphic in each birth canal plane. (Reprinted with permission from Elsevier).
However, evolutionary differences exist in the relative dimensions of these three planes and shape of the birth canal of the various families of hominoids (3). In order to understand the changes brought about by evolution in the process of parturition that made humans susceptible to birth palsy, it is necessary to review the birth process and the changes in pelvic morphology and the fetuses of the various hominoids, viz large bodied apes, human ancestors (hominids), small bodied primates like spider monkeys, macaques etc and modern humans.
Birth in large bodied apes
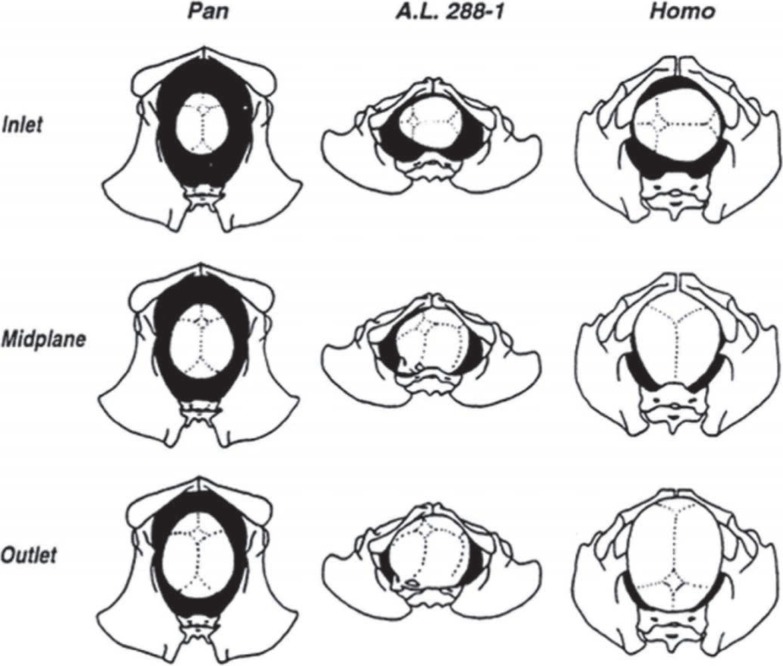
In large bodied apes such as orangutans (Pongo), gorillas (Gorilla) and chimpanzees (Pan), the three pelvic planes are longer in the sagittal (anteroposterior) dimension than in the transverse dimension (1, 3). The maximum diameter of the fetal cranium is in the sagittal plane in all primate species (1, 3). Schultz had shown that there is a wide clearance between the size of the maternal pelvic inlet and the fetal cranium in both sagittal and transverse diameters in the large bodied apes (4). Their birth canal is relatively straight (unconstrained) due to the orientation of its long axis in the sagittal dimension in all three planes (1, 3). Their fetuses also have smaller brain volume and narrow shoulders compared to wide maternal pelvis (1). The straight birth canal with the small head and narrow shoulders of the fetus leads to direct fetal descent without the need for any complex rotation of the head during parturition. This type of birth is called non-rotational parturition (1, 2) [Fig. 2]. For this reason, there is a low incidence of cephalo-pelvic disproportion and shoulder dystocia among these species (3) and hence no resultant birth palsy.
Fig. 2. Midwife's or obstetrician's ‘eye’ view of a neonatal head passing through the birth canal. In each drawing, the maternal pelvis and neonatal head are shown in inferior view, with the sacrum at the bottom of the picture and the pubic symphysis at the top. The relative dimension of the head and the pelvis at each position is to be noted. In great apes, unconstrained birth; in australopithecines and human, constrained birth. (Pan is chimpanzee, A.L 288-1 is Lucy, an australopithecine, Homo is human). (Reprinted with permission from John Wiley and Sons).
Birth in human ancestors with bipedal gait
The mammalian family to which humans belong, Hominidae, originated approximately five million years ago (5). The immediate predecessor of humans who had the characteristic hallmarks (skeletal markers) of bipedal gait is the genus Australopithecus (6, 7). Their pelvic morphology as a result of adaptation for bipedalism showed a significant change from that seen in apes and other non-human primates (1, 3). These include a shortening of the pelvis and a repositioning of the iliac blades to improve the leverage of the muscles that stabilize the pelvis during bipedal walking (3).
The knowledge of their pelvic anatomy was obtained from the two best-preserved australopithecine female specimens. One from Sterkfontein, South Africa, Sts 14 (Australopithecus africanus), and another from Hadar, Ethiopia, AL 288-1, aka Lucy [Australopithecus afarensis] (8, 9).
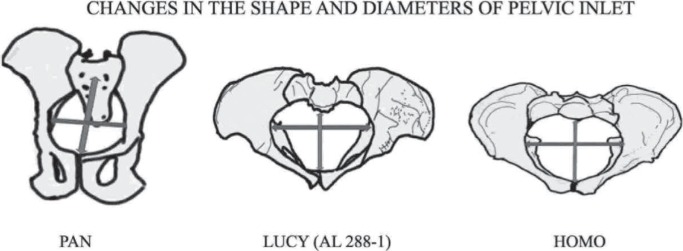
Tague and Lovejoy (10, 11) based on the anatomy of “Lucy”, have shown that the long axes of the australopithecine inlet and outlet are parallel to each other and had a constant, platypelloid shape ie narrow anteroposterior diameter, wider transverse diameter and maintain the same shape throughout its length from inlet to outlet. They suggested that there would have been no ‘bony resistance to fetal descent’ and the fetus would have moved through the passageway in a transverse orientation (3) [Figs. 2, 3]. Lucy, like the large bodied great apes, also had a non-rotational parturition even though their inlet had a maximum dimension in the transverse diameter (12). This was possible due to a small encephalization quotient [a small size of head in relation to the rest of the body] (3).
Fig. 3. View from the top of pelvic inlets of Pan, Lucy (AL 288-1) and Homo. The anteroposterior (AP) diameter is widest in Pan which changed to platypelloid in Lucy whose widest diameter is the transverse diameter due to adaptation of bipedal gait. The increase in fetal head size increased the size of birth canal of Homo mainly in anterior region but the maximum diameter still is transverse diameter. The fetal head enters the Pan pelvis in AP dimension but transversely in Lucy and Homo.
It is argued that rotation of the head within the birth canal was unnecessary, due to the small size of the fetal head, and also impossible considering the platypelloid shape of each pelvic plane (3, 10). Thus they also had non-rotational type of parturition. However, long axis of the shoulder is perpendicular to that of the fetal head. Hence, once the fetal head had passed through the pelvic outlet, the shoulder had to rotate 90 degrees (2, 3, 12) [Figs. 2, 3]. Because of the non-rotational parturition and the small size of the head and narrow shoulders, it is suggested that birth palsy did not occur in the australopithecines (3).
Birth in small bodied primates
Due to the narrow margin of clearance between the size of the maternal pelvis and the size of the fetal cranium in spider monkeys (Ateles), proboscis monkeys (Nasalis), macaques (Macaca), lesser apes or gibbons (Hylobates), their birth is reported to be difficult (3, 4). Because of their small sized body, their birth canal dimension has closer association with fetal head (1, 3). Stoller found that in small bodied primates, the fetus entered the birth canal in various positions, but then rotated to exit face first, thus revealing the “rotation” in the birth process (13). The incidence of birth palsy and neonatal deaths resulting from cephalopelvic disproportion and shoulder dystocia are not uncommon in these relatively small-bodied species and is similar to humans (3, 14, 15).
Evolution of birth in modern humans
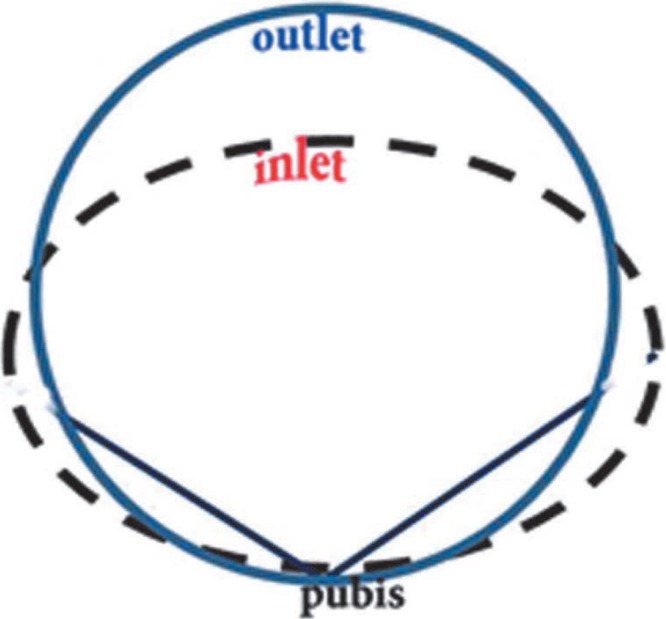
The modern pattern of parturition developed at the time of the appearance of the genus Homo (H erectus, H antecessor and H neanderthalensis etc) about 2.5 million years ago (16). It was in this genus that the size of the fetal head had increased disproportionate to body size as a result of larger brain for complex language skill and use of tool (3, 17). Consequently in the pelvis of genus Homo, apart from the width changes that were inherited from early hominids like Lucy, there were further changes in overall pelvic morphology in order to balance the body in an upright position in bipedal gait and for the passage of the large fetal head. These changes were the repositioning and change in the shape of the iliac blades and the upward rotation and shortening of the pubic rami. Thus the shape of the birth canal had changed from a broad oval in australopithecines to a more circular shape in Homo (18). The acetabulum shifted closer to the sacrum and reduced the anteroposterior diameter of the inlet while the anteroposterior diameter of the mid-plane and outlet increased relatively (18). Inter-acetabular distance had to increase to enlarge the size of the birth canal for passage of large fetal head. All the above changes caused the pelvic inlet to be larger transversely than anteroposteriorly, and the pelvic midplane and outlet to be larger anteroposteriorly than transversely (Figs. 2–4), resulting in a “twisted” birth canal with the largest dimension being first transverse and then anteroposterior (1, 3, 16, 18). The human birth canal is therefore called the constrained type ie the diameter of the birth canal is not constant throughout its length (3, 18).
Fig. 4. Constrained birth canal: the shape of human birth canal whose inlet is wider in transverse dimension but in anteroposterior dimension in midplane and outlet which makes it a twisted tube causing a rotational and constrained parturition.
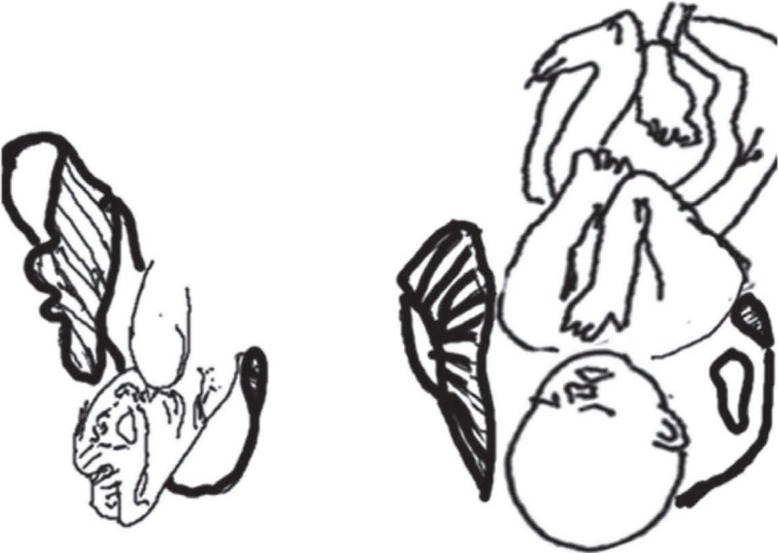
During parturition, the fetus must be oriented so that the largest dimensions of its head and shoulders align with the most spacious parts of the birth canal (1, 3). Consequently, a human fetus enters the birth canal facing sideways (mostly left occiput anterior) so that its larger anteroposterior head dimensions match up with the wider transverse dimensions of the inlet (1–3). On entering the midplane, the fetus rotates so that its head length is aligned anteroposteriorly and continues in this way until it exits the outlet. One final rotation then occurs so that the fetus' shoulders can pass anteroposteriorly all through the midplane and outlet. Typically, the fetus exits the birth canal facing posteriorly in relation to the mother, because the occiput tends to pass alongside the outlet's more spacious anterior part. This size relationship, along with a twisted birth canal shape, makes human parturition mechanically difficult and results in a unique pattern of “rotational” birth (1–3) [Fig. 1]. Sometimes, this rotation can be arrested by incongruity between canal and head/shoulder dimensions, leading to arrest of the fetal descent (3). Fetal rotation during parturition is not simply a mechanism to accommodate a large cranium in the birth canal but also a mechanism to negotiate the broad shoulder (1, 3). Hominoids (apes, australopithecines and modern humans) have broad, rigid shoulders, which are associated with adaptations of their brachiating or suspensory ancestry (1) [Fig. 5]. In modern humans, when the pelvis is platypelloid, shoulder dystocia is associated with increased mortality for both mothers and fetuses (3). Shoulder dystocia was never a problem for large-bodied great apes because of the unconstrained birth canal and the smaller head, while it is a source of difficulty that affected the early human ancestors.
Fig. 5. Exit of shoulder in baboon (left) and human (right) from maternal pelvis. The constrained human pelvis results in rotation of the head and this rotation places the shoulder in an anteroposterior dimension in inlet. This positioning produces a natural constraint to the smooth descent of the fetus at the level of the shoulder.
Rosenberg and Trevathan suggested that shoulder size may have been an important constraint during hominid birth and, along with increased fetal head size, may have been among the causes of natural selection for the more rounded pelvis (gynaecoid) of later hominids (16).
Neonatal brain development at the time of birth is 65% in monkeys (Macaca), 40% in chimpanzees and in modern humans (19). Thus the maturation of fetal human brain occurs mainly after birth (1–3). That is why human neonates are altricial, ie they are helpless at birth and require a prolonged period of dedicated parenthood (1, 3).
Modern human parturition and birth palsy
The modern human, parturition though having evolved over time, has a narrow margin of error (1, 2). Due to the similarity in the dimensions of the fetal head and the true pelvis, the human pelvis is described as “shockingly crowded” in parturition (4). It is a finely tuned relationship and any small changes in the relative size of canal or fetal parts can render the process dangerous to life and health of both the mother and fetus (3, 16, 20). The birth of a modern human therefore requires the assistance of “learned others” for safety and well-being of mother and baby. Modern anthropologists refer to this dependence on assistance as ‘obligate midwifery’ (3, 21).
The descent of the fetus can be arrested at any level during parturition (1–3). The arrested fetus must be extracted via assistance with traction, if necessary. The force applied (forceps, vacuum assistance etc) to extract an arrested fetus in a constrained human pelvic canal, especially in the late second stage of the rotational descent, would give sufficient stress to damage the delicate components of the brachial plexus, leading to birth palsy (16, 20).
The tree dwelling human ancestors had evolved in to the modern humans who, with their advanced technology and dexterity, have changed the world they live in today. The increase in brain size and development of broad shoulders, along with changes in pelvic morphology, made modern human parturition a precarious process that warranted proper assistance for the safety of mother and newborn. These complications in the modern human parturition are the results of human evolution.
The human race is not the only one that has difficulty during childbirth (1, 2, 16). The small bodied primates like monkeys and gibbons have relatively large fetal cranium and broad shoulders with narrow birth canal (3). The great apes like orangutans, chimpanzees and gorillas (the great apes are our closest living relatives) have adequate birth canals, probably as a result of their large adult body sizes and their relatively small neonates (1, 3). In all non-primate animals with wider pelvis and low quotient of encephalization and narrow pelvis, the birth canal is straight which allows easy, unconstrained, non-rotational parturition. They have a low incidence of fetal descent arrest and hence low incidence of extraction of neonates from the maternal pelvis leading to brachial plexus injury during birth (3, 20).
CONCLUSION
We put forth the argument that human evolution has made the human parturition process so precarious that birth palsy is likely in the absence of appropriate assistance. The pelvic changes passed the tests of natural selection and produced a unique reproductive biology and culture in humans. In the end, this gift of human evolution resulted in a birth process that is, without medical intervention, painful and dangerous for mothers and their young (22). Since these are consequences of changes made by human evolution, the real cause of birth palsy is human evolution. In other words, birth palsy is one of the prices we pay for evolving to the current level of mental capabilities, communication skills and bipedal gait. Thus, the birth process therefore has the “scar” of human evolution (22).
REFERENCES
- 1.Rosenberg K, Trevathan W. Birth, obstetrics and human evolution. BJOG Int J Obstet Gynaecol. 2002;109:1199–2206. doi: 10.1046/j.1471-0528.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 2.Franciscus RG. When did the modern human pattern of childbirth arise? New insights from an old neandertal pelvis. Proc Natl Acad Sci. 2009;106:9125–9126. doi: 10.1073/pnas.0903384106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg K, Trevathan W. Bipedalism and human birth: the obstetrical dilemma revisited. Evol Anthropol Issues News Rev. 1995;4:161–168. [Google Scholar]
- 4.Schultz AH. Sex differences in the pelves of primates. Am J Phys Anthropol. 1949;7:401–423. doi: 10.1002/ajpa.1330070307. [DOI] [PubMed] [Google Scholar]
- 5.Wolpoff M, Spuhler J, Smith F, Radovcic J, Pope G, Frayer D, et al. Modern human origins. Science. 1988;241:772–774. doi: 10.1126/science.3136545. [DOI] [PubMed] [Google Scholar]
- 6.Leakey MG, Feibel CS, Mcdougall I, Walker A. New four-million-year-old hominid species from Kanapoi and Allia Bay, Kenya. Nature. 1995;376:565–671. doi: 10.1038/376565a0. [DOI] [PubMed] [Google Scholar]
- 7.Leakey M, Walker A. Early hominid fossils from Africa. Sci Am. 1997;276:74–79. doi: 10.1038/scientificamerican0697-74. [DOI] [PubMed] [Google Scholar]
- 8.Broom R, Robinson JT, Schepers GWH. Sterkfontein ape-man plesianthropus. Transval Mus Mem. 1950;4:58–63. [Google Scholar]
- 9.Johanson DC, Lovejoy CO, Kimbel WH, White TD, Ward SC, Bush ME, et al. Morphology of the pliocene partial hominid skeleton (A.L. 288-1) from the Hadar formation, Ethiopia. Am J Phys Anthropol. 1982;57:403–451. [Google Scholar]
- 10.Tague RG, Lovejoy CO. The obstetric pelvis of A.L. 288-1 (Lucy) J Hum Evol. 1986;15:237–255. [Google Scholar]
- 11.Tague RG, Lovejoy CO. AL 288-291—Lucy or Lucifer: gender confusion in the pliocene. J Hum Evol. 1998;35:75–94. doi: 10.1006/jhev.1998.0223. [DOI] [PubMed] [Google Scholar]
- 12.Trevathan WR. Fetal emergence patterns in evolutionary perspective. Am Anthropol. 1988;90:674–781. [Google Scholar]
- 13.Stoller M. The obstetric pelvis and mechanism of labor in nonhuman primates. Am J Phys Anthropol. 1995;(Suppl 20):204–226. [Google Scholar]
- 14.Leutenegger W. Functional aspects of pelvic morphology in Simian primates. J Hum Evol. 1974;3:207–222. [Google Scholar]
- 15.Leutenegger W. Neonatal brain size and neurocranial dimensions in pliocene hominids: implications for obstetrics. J Hum Evol. 1987;16:291–296. [Google Scholar]
- 16.Rosenberg KR, Trevathan WR. The evolution of human birth. Sci Am. 2001;285:72–77. doi: 10.1038/scientificamerican1101-72. [DOI] [PubMed] [Google Scholar]
- 17.Leutenegger W. Encephalization and obstetrics in primates with particular reference to human evolution. In: Armstrong EFD, editor. Primate brain evolution: methods and concepts 3. 1st ed. London: Plenum Press; 1982. pp. 85–95. [Google Scholar]
- 18.Lovejoy CO. The natural history of human gait and posture. Part 1. Spine and pelvis. Gait Posture. 2005;21:95–112. doi: 10.1016/j.gaitpost.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Pilcher DL, Hammock EA, Hopkins WD. Cerebral volumetric asymmetries in non-human primates: a magnetic resonance imaging study. Laterality. 2001;6:165–179. doi: 10.1080/13576500042000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipscomb KR. Shoulder dystocia. In: Mishell DR, Brenner PF, editors. Management of common problems in obstetrics and gynecology. 3rd ed. London: Blackwell Scientific; 1994. pp. 227–232. [Google Scholar]
- 21.Buck S. The evolutionary history of the modern birth mechanism: looking at skeletal and cultural adaptations. Totem Univ West Ont J Anthropol [internet] 2011;19 Available from: http://ir.lib.uwo.ca/totem/vol19/iss1/7. [Google Scholar]
- 22.Weiner S, Monge J, Mann A. Bipedalism and parturition: an evolutionary imperative for Cesarean delivery? Clin Perinatol. 2008;35:469–478. doi: 10.1016/j.clp.2008.06.003. [DOI] [PubMed] [Google Scholar]