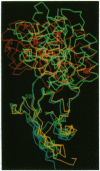
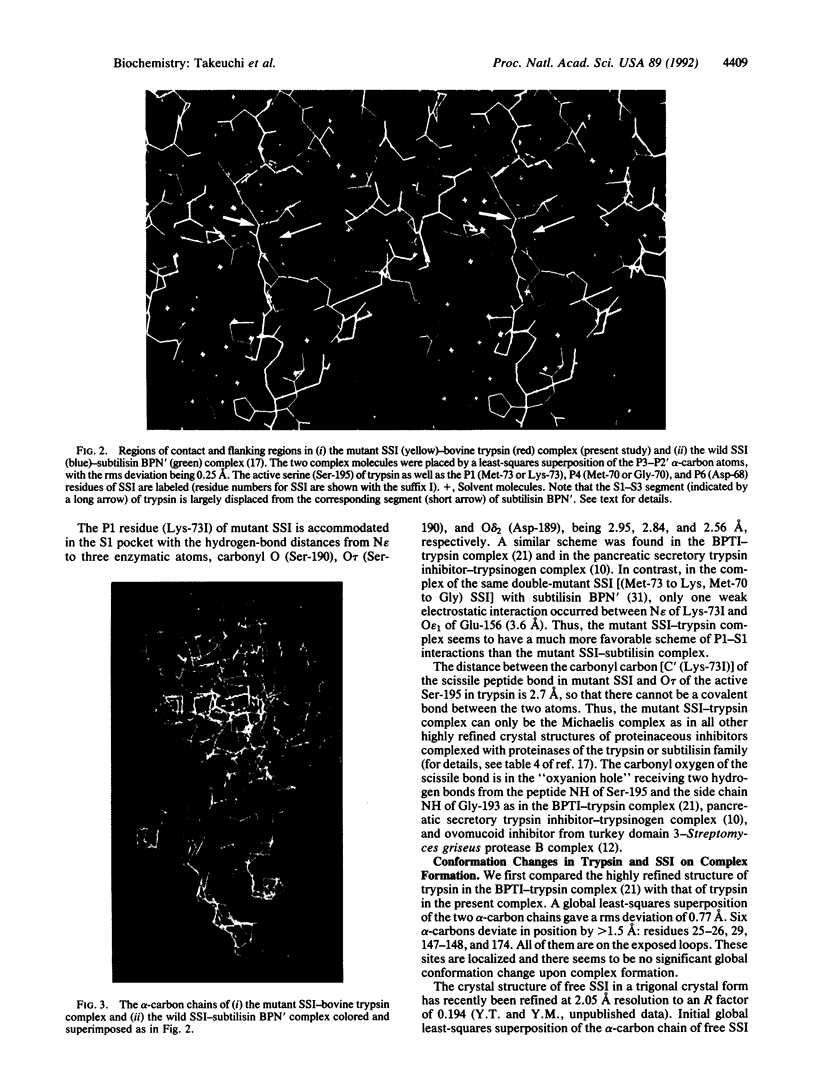
Abstract
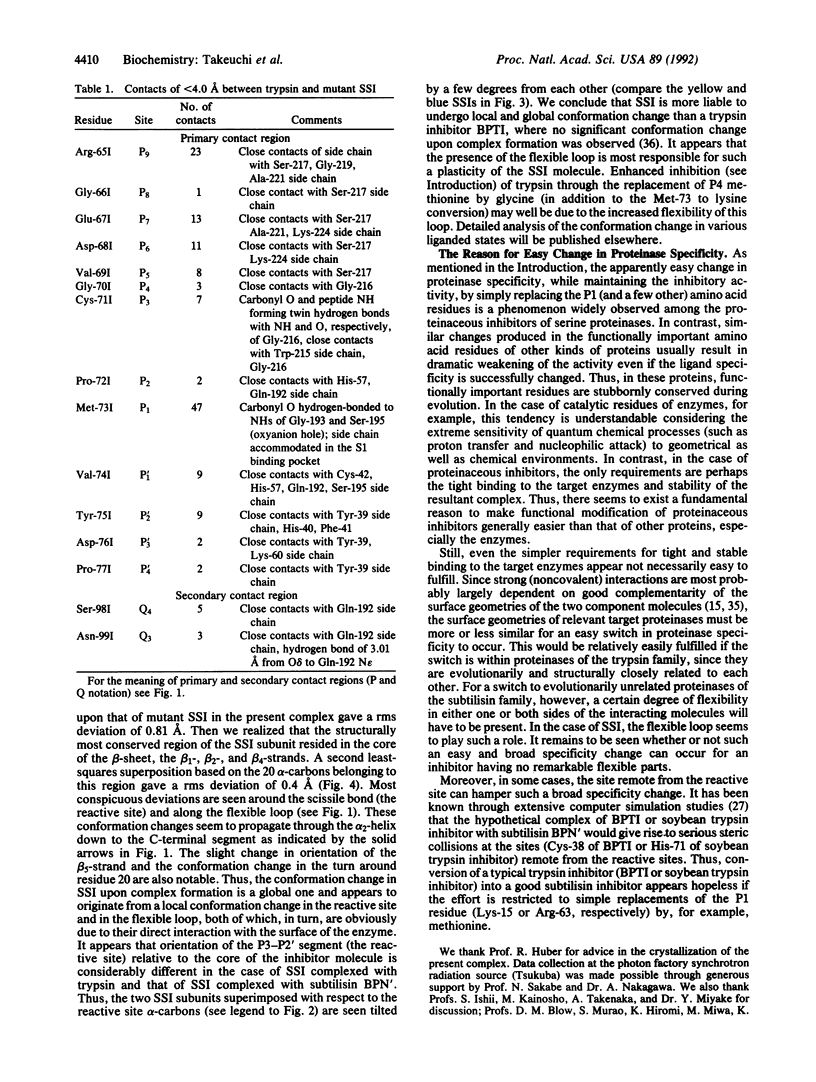
Proteinase specificity of a proteinaceous inhibitor of subtilisin (SSI; Streptomyces subtilisin inhibitor) can be altered so as to strongly inhibit trypsin simply by replacing P1 methionine with lysine (with or without concomitant change of the P4 residue) through site-directed mutagenesis. Now the crystal structure of one such engineered SSI (P1 methionine converted to lysine and P4 methionine converted to glycine) complexed with bovine trypsin has been solved at 2.6 A resolution and refined to a crystallographic R factor of 0.173. Comparing this structure with the previously established structure of the native SSI complexed with subtilisin BPN', it was found that (i) P1 lysine of the mutant SSI is accommodated in the S1 pocket of trypsin as usual, and (ii) upon complex formation, considerable conformation change occurs to the reactive site loop of the mutant SSI. Thus, in this case, flexibility of the reactive site loop seems important for successfully changing the proteinase specificity through mere replacement of the P1 residue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bode W., Schwager P., Huber R. The transition of bovine trypsinogen to a trypsin-like state upon strong ligand binding. The refined crystal structures of the bovine trypsinogen-pancreatic trypsin inhibitor complex and of its ternary complex with Ile-Val at 1.9 A resolution. J Mol Biol. 1978 Jan 5;118(1):99–112. doi: 10.1016/0022-2836(78)90246-2. [DOI] [PubMed] [Google Scholar]
- Bolognesi M., Gatti G., Menagatti E., Guarneri M., Marquart M., Papamokos E., Huber R. Three-dimensional structure of the complex between pancreatic secretory trypsin inhibitor (Kazal type) and trypsinogen at 1.8 A resolution. Structure solution, crystallographic refinement and preliminary structural interpretation. J Mol Biol. 1982 Dec 25;162(4):839–868. doi: 10.1016/0022-2836(82)90550-2. [DOI] [PubMed] [Google Scholar]
- Chen Z., Bode W. Refined 2.5 A X-ray crystal structure of the complex formed by porcine kallikrein A and the bovine pancreatic trypsin inhibitor. Crystallization, Patterson search, structure determination, refinement, structure and comparison with its components and with the bovine trypsin-pancreatic trypsin inhibitor complex. J Mol Biol. 1983 Feb 25;164(2):283–311. doi: 10.1016/0022-2836(83)90078-5. [DOI] [PubMed] [Google Scholar]
- Courtney M., Jallat S., Tessier L. H., Benavente A., Crystal R. G., Lecocq J. P. Synthesis in E. coli of alpha 1-antitrypsin variants of therapeutic potential for emphysema and thrombosis. Nature. 1985 Jan 10;313(5998):149–151. doi: 10.1038/313149a0. [DOI] [PubMed] [Google Scholar]
- Grütter M. G., Fendrich G., Huber R., Bode W. The 2.5 A X-ray crystal structure of the acid-stable proteinase inhibitor from human mucous secretions analysed in its complex with bovine alpha-chymotrypsin. EMBO J. 1988 Feb;7(2):345–351. doi: 10.1002/j.1460-2075.1988.tb02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono S., Akagawa H., Mitsui Y., Iitaka Y. Crystal structure at 2.6 A resolution of the complex of subtilisin BPN' with streptomyces subtilisin inhibitor. J Mol Biol. 1984 Sep 15;178(2):389–414. doi: 10.1016/0022-2836(84)90150-5. [DOI] [PubMed] [Google Scholar]
- Hirono S., Nakamura K. T., Iitaka Y., Mitsui Y. Crystal structure of the complex of subtilisin BPN' with its protein inhibitor Streptomyces subtilisin inhibitor. The structure at 4.3 Angstroms resolution. J Mol Biol. 1979 Jul 15;131(4):855–869. doi: 10.1016/0022-2836(79)90205-5. [DOI] [PubMed] [Google Scholar]
- Huber R., Kukla D., Bode W., Schwager P., Bartels K., Deisenhofer J., Steigemann W. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. II. Crystallographic refinement at 1.9 A resolution. J Mol Biol. 1974 Oct 15;89(1):73–101. doi: 10.1016/0022-2836(74)90163-6. [DOI] [PubMed] [Google Scholar]
- Janin J., Chothia C. Stability and specificity of protein-protein interactions: the case of the trypsin-trypsin inhibitor complexes. J Mol Biol. 1976 Jan 15;100(2):197–211. doi: 10.1016/s0022-2836(76)80148-9. [DOI] [PubMed] [Google Scholar]
- Kojima S., Kumagai I., Miura K. Effect of inhibitory activity of mutation at reaction site P4 of the Streptomyces subtilisin inhibitor, SSI. Protein Eng. 1990 May;3(6):527–530. doi: 10.1093/protein/3.6.527. [DOI] [PubMed] [Google Scholar]
- Kojima S., Obata S., Kumagai I., Miura K. Alteration of the specificity of the Streptomyces subtilisin inhibitor by gene engineering. Biotechnology (N Y) 1990 May;8(5):449–452. doi: 10.1038/nbt0590-449. [DOI] [PubMed] [Google Scholar]
- Komiyama T., Bigler T. L., Yoshida N., Noda K., Laskowski M., Jr Replacement of P1 Leu18 by Glu18 in the reactive site of turkey ovomucoid third domain converts it into a strong inhibitor of Glu-specific Streptomyces griseus proteinase (GluSGP). J Biol Chem. 1991 Jun 15;266(17):10727–10730. [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Mitsui Y., Satow Y., Watanabe Y., Hirono S., Iitaka Y. Crystal structures of Streptomyces subtilisin inhibitor and its complex with subtilisin BPN'. Nature. 1979 Feb 8;277(5696):447–452. doi: 10.1038/277447a0. [DOI] [PubMed] [Google Scholar]
- Mitsui Y., Satow Y., Watanabe Y., Iitaka Y. Crystal structure of a bacterial protein proteinase inhibitor (Streptomyces subtilisin inhibitor) at 2.6 A resolution. J Mol Biol. 1979 Jul 15;131(4):697–724. doi: 10.1016/0022-2836(79)90198-0. [DOI] [PubMed] [Google Scholar]
- Obata S., Furukubo S., Kumagai I., Takahashi H., Miura K. High-level expression in Streptomyces lividans 66 of a gene encoding Streptomyces subtilisin inhibitor from Streptomyces albogriseolus S-3253. J Biochem. 1989 Mar;105(3):372–376. doi: 10.1093/oxfordjournals.jbchem.a122671. [DOI] [PubMed] [Google Scholar]
- Obata S., Taguchi S., Kumagai I., Miura K. Molecular cloning and nucleotide sequence determination of gene encoding Streptomyces subtilisin inhibitor (SSI). J Biochem. 1989 Mar;105(3):367–371. doi: 10.1093/oxfordjournals.jbchem.a122670. [DOI] [PubMed] [Google Scholar]
- Read R. J., Fujinaga M., Sielecki A. R., James M. N. Structure of the complex of Streptomyces griseus protease B and the third domain of the turkey ovomucoid inhibitor at 1.8-A resolution. Biochemistry. 1983 Sep 13;22(19):4420–4433. doi: 10.1021/bi00288a012. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Sweet R. M., Wright H. T., Janin J., Chothia C. H., Blow D. M. Crystal structure of the complex of porcine trypsin with soybean trypsin inhibitor (Kunitz) at 2.6-A resolution. Biochemistry. 1974 Sep 24;13(20):4212–4228. doi: 10.1021/bi00717a024. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Noguchi S., Satow Y., Kojima S., Kumagai I., Miura K., Nakamura K. T., Mitsui Y. Molecular recognition at the active site of subtilisin BPN': crystallographic studies using genetically engineered proteinaceous inhibitor SSI (Streptomyces subtilisin inhibitor). Protein Eng. 1991 Jun;4(5):501–508. doi: 10.1093/protein/4.5.501. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Satow Y., Nakamura K. T., Mitsui Y. Refined crystal structure of the complex of subtilisin BPN' and Streptomyces subtilisin inhibitor at 1.8 A resolution. J Mol Biol. 1991 Sep 5;221(1):309–325. [PubMed] [Google Scholar]
- Tsunogae Y., Tanaka I., Yamane T., Kikkawa J., Ashida T., Ishikawa C., Watanabe K., Nakamura S., Takahashi K. Structure of the trypsin-binding domain of Bowman-Birk type protease inhibitor and its interaction with trypsin. J Biochem. 1986 Dec;100(6):1637–1646. doi: 10.1093/oxfordjournals.jbchem.a121872. [DOI] [PubMed] [Google Scholar]