Abstract
Susceptibility to stress-linked psychological disorders, including post-traumatic stress disorder and depression, differs between men and women. Dysfunction of medial prefrontal cortex (mPFC) has been implicated in many of these disorders. Chronic stress affects mPFC in a sex-dependent manner, differentially remodeling dendritic morphology and disrupting prefrontally mediated behaviors in males and females. Chronic restraint stress induces microglial activation, reflected in altered microglial morphology and immune factor expression, in mPFC in male rats. Unstressed females exhibit increased microglial ramification in several brain regions compared to males, suggesting both heightened basal activation and a potential for sex-dependent effects of stress on microglial activation. Therefore, we assessed microglial density and ramification in the prelimbic region of mPFC, and immune-associated genes in dorsal mPFC in male and female rats following acute or chronic restraint stress. Control rats were left unstressed. On the final day of restraint, brains were collected for either qPCR or visualization of microglia using Iba-1 immunohistochemistry. Microglia in mPFC were classified as ramified, primed, reactive, or amoeboid, and counted stereologically. Expression of microglia-associated genes (MHCII, CD40, IL6, CX3CL1, and CX3CR1) was also assessed using qPCR. Unstressed females showed a greater proportion of primed to ramified microglia relative to males, alongside heightened CX3CL1-CX3CR1 expression. Acute and chronic restraint stress reduced the proportion of primed to ramified microglia and microglial CD40 expression in females, but did not significantly alter microglial activation in males. This sex difference in microglial activation could contribute to the differential effects of stress on mPFC structure and function in males versus females.
Keywords: Microglia, Microglial Morphology, Prefrontal Cortex, Acute Stress, Chronic Stress, Sex Differences
1. Introduction
Susceptibility to multiple stress-linked psychological disorders differs between men and women, with women nearly twice as likely to suffer from post-traumatic stress disorder and major depression (Solomon and Herman, 2009). Various stress-linked structural and functional changes in medial prefrontal cortex (mPFC) have been implicated in these disorders (Price and Drevets, 2010), and stress can produce depressive-like behaviors and cognitive deficits (Cerqueira et al., 2007). Chronic stress affects mPFC in a sex-dependent manner, differentially remodeling neurons (Garrett and Wellman, 2009) and disrupting prefrontally-mediated behaviors, such as spatial working memory (Bowman et al., 2003), in males and females.
Emerging evidence suggests that acute and chronic stress induce microglial cell-mediated immune activation and inflammation in the brain (Walker et al., 2013). Microglia are the resident immune cells of the central nervous system (CNS). In their basal state, ramified microglia use numerous thin, dynamic processes to monitor the microenvironment, dendritic spines, synaptic contacts, and perisynaptic astrocytic clefts (Salter and Beggs, 2014). Upon activation, microglia transition through several morphological states (Ransohoff and Perry, 2009). In the primed state, activated processes thicken, elongate, and reorient toward neuronal and astroglial signals. Activated microglia regulate synaptic plasticity and function by releasing neurotoxic factors and directly pruning dendritic spines (Miyamoto et al., 2013). In male rats, chronic stress produces a variety of changes in microglial activation and expression of immune factors. For instance, chronic restraint stress increases microglial cell density and heightens microglial activation, as reflected in morphology and expression of immune molecules, in mPFC (Walker et al., 2013). These changes in microglial activation are associated with deficits in spatial working memory (Hinwood et al., 2012). Likewise, chronic unpredictable mild stress increases interleukin (IL)-1β and NLRP3 inflammasome mRNA and protein, tumor necrosis factor (TNF)-α mRNA, and purinergic receptor P2RX7 protein, and decreases anti-inflammatory IL-10 expression in mPFC in male rats (You et al., 2011; Pan et al., 2014).
Recent studies demonstrate dramatic sex differences in microglial density, function, and morphology in several brain regions (Bilbo et al., 2012). For instance, the morphology of microglia in the parietal cortex, hippocampus, and amygdala is consistent with increased activation in adult female rats (Schwarz et al., 2012). Furthermore, levels of various microglia-linked immune factors, such as interleukin (IL)-10 mRNA and IL-1β protein, are higher in female parietal cortex and hippocampus (Schwarz et al., 2012; Hudson et al., 2014). Together, these studies suggest heightened basal microglial activation in females, and therefore the potential for sex differences in the effects of stress on microglial morphology and function. Thus, we assessed microglial cell density, morphological activation state, and immune factor expression in mPFC in male and female rats following acute and chronic restraint stress.
2. Materials and Methods
2.1 Animals
Male and female Sprague-Dawley rats (Male: 75.39 ± 0.65 days old; Female: 72.72 ± 0.89 days old) underwent either acute (1 day; Male: n = 23, Female: n = 20) or chronic (10 consecutive days; Male: n = 24, Female: n = 21) restraint stress, or were left unstressed. Restraint stress was performed by placing rats in a clear plastic semi-cylindrical rodent restrainer in their home cages under bright lights for 3 hours/day. The time of day during which restraint occurred varied over the light phase of the light-dark cycle. This procedure produces significant increases in plasma corticosterone (Cook and Wellman, 2004). Unstressed rats (Male: n = 23, Female: n = 20) were left unhandled except for weighing, with all rats weighed every other day. Rats were group-housed by sex in a temperature- and humidity-controlled vivarium (12 h light/dark cycle, lights on 0800 h), with food and water provided ad libitum. All animals were euthanized between 1100–1630 h, with stressed animals euthanized 1.68 ± 0.11 h post-restraint. There were no systematic differences in time of euthanasia across groups. Brains were then processed for either immunohistochemical visualization of microglia (section 2.4) or qPCR for immune factor expression (section 2.5). All experimental procedures were performed during the light phase, carried out in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals, and approved by the Bloomington Institutional Animal Care and Use Committee.
2.2 Characterization of Estrous Phase
On the final day of restraint, vaginal lavages were performed. All samples were collected between 1100–1500 h. Exfoliate cytology was examined immediately under light microscopy, and estrous phase was determined based on the morphology of the cells present in the smear (Garrett and Wellman, 2009). The majority of females were in diestrus (n = 54; proestrus, n = 6; estrus, n = 1), likely reflecting their relatively young adult status. Proestrous and estrous females were present in all stress conditions (unstressed, n = 1 proestrous and 1 estrous; acute stress, n = 2 proestrous; chronic stress, n = 3 proestrous). Given the limited representation of animals in different estrous phases, we did not analyze our data relative to estrous cycle.
2.3 Stressor Validation
Chronic stress can reduce weight gain and induce adrenal hypertrophy in male rats (Ulrich-Lai et al., 2006). Therefore, adrenals were dissected and weighed to verify stressfulness of the manipulation. Adrenal-weight-to-body-weight ratios and body weight at the end of treatment were compared across groups.
2.4 Effects of Stress on Microglial Cell Morphology in Prelimbic Cortex
On the final day of restraint, a subset of animals from each treatment condition (unstressed: male n = 13, female n = 10; acute stress: male n = 13, female n = 10; chronic stress: male n = 14, female n = 11) were euthanized for immunohistochemical visualization of microglial cells. Density and morphology of microglia were analyzed in the prelimbic region of mPFC.
2.4.1 Iba-1 Immunohistochemistry
Animals were overdosed with urethane and transcardially perfused with 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were removed, postfixed overnight in 4% paraformaldehyde in PBS, and cryoprotected in 30% sucrose. Coronal sections were cut at 44μm using a freezing microtome (American Optical AO860, Buffalo, NY). Microglia were visualized using a protocol adapted from Lenz et al. (2013). Free-floating sections were rinsed in PBS, blocked 1 h in 10% bovine serum albumin (BSA) in PBS + 0.4% Triton X (PBST), and incubated in 0.3% hydrogen peroxide in 50% methanol 1 h at 4°C. Sections were then rinsed and incubated overnight at 4°C with an antibody specific to ionized calcium-binding adaptor protein-1 (Iba-1, 1:1000; Wako Chemicals Inc., Richmond, VA) in 5% BSA + PBST. Iba-1 is constitutively expressed in microglia, involved in cytoskeletal reorganization, and up-regulated in response to microglial cell activation (Imai and Kohsaka, 2002). Sections were then rinsed, incubated 1 h at room temperature with biotinylated secondary antibody (1:500, Vector Laboratories Inc., Burlingame, CA) in 5% BSA + PBST, rinsed, and incubated 1 h with ABC complex (Vector Laboratories Inc.) in PBST. Iba-1-immunopositive cells were visualized using a nickel-intensified DAB reaction. Sections were subsequently rinsed, mounted, counterstained with neutral red to facilitate identification of prelimbic cortex, dehydrated, cleared, and coverslipped.
2.4.2 Stereology
We analyzed microglial cell morphology and density in left and right hemispheres of prelimbic cortex in 4–7 sections per animal, approximately evenly spaced throughout the anterior-posterior axis of prelimbic cortex (Paxinos and Watson, 1998). Cells were counted at a final magnification of 1800× using the optical fractionator method and StereoInvestigator (Microbrightfield Inc., Williston, VT). Microglia were classified as ramified (numerous thin processes, radial branching), primed (thickened processes, increased polarity, and proliferation with reduced secondary branching), reactive (thickened stout processes with highly reduced branching), or amoeboid (rounded soma with no branching; Figure 1A–D) based on standard morphological criteria (Ransohoff and Perry, 2009). The counting frame was 100 μm × 100 μm, with approximately 19 frames counted per section, per hemisphere (mean CE: 0.04, combined hemispheres), for a total of approximately 563 cells counted per animal. Guard zones were set with a centered-probe thickness of 8 μm. Counts were performed blind to sex and experimental condition. Estimated densities and relative proportions were calculated and analyzed for each cell type. Statistical tests consisted of 2-way ANOVA followed by Tukey’s post hoc comparisons.
Figure 1.
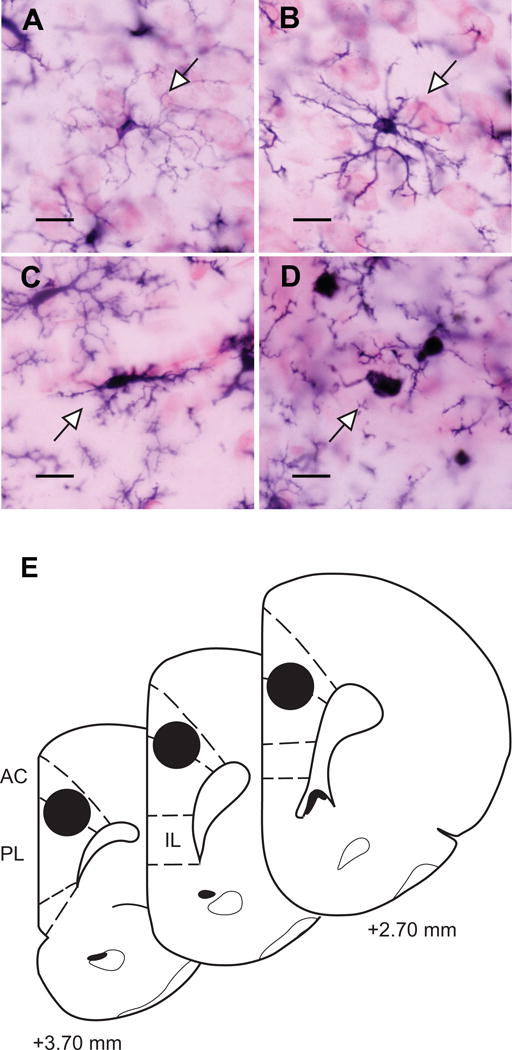
Microglial cell morphology and micropunch sampling in medial prefrontal cortex.
Microglia were classified as ramified (numerous thin processes, radial branching; A), primed (thickened processes, increased polarity, and proliferation with reduced secondary branching; B), reactive (thickened stout processes with highly reduced branching; C), or amoeboid (rounded soma with no branching; D). Scale bars = 10 μm. Arrowheads indicate exemplars of each type. E: Micropunch samples in dorsal mPFC were obtained from both hemispheres using standard gross morphology (Paxinos and Watson, 1998). For simplicity, only one hemisphere is shown.
2.5 Effects of Stress on Immune Factor Expression in Medial Prefrontal Cortex
Potential sex differences and stress-induced changes in immune factor expression in mPFC were analyzed using quantitative real time PCR (qPCR) following the final session of restraint. We examined immune factors expressed by microglia and perivascular and meningeal macrophages, and in neuron-microglia specific interactions. The antigen presentation-associated molecules major histocompatibility complex (MHC) II and cluster of differentiation (CD) 40 were assessed as markers of microglial activation (Lynch, 2009). The proinflammatory cytokine IL-6 was examined as an indicator of microglial cell function. The chemokine, fractalkine (CX3CL1) and its cognate receptor (CX3CR1) were also analyzed, as recent evidence suggests that neuronally expressed CX3CL1 can modulate glutamatergic tone, inhibit microglial cell activation, and regulate the effects of chronic stress on neuronal plasticity and depressive-like behaviors through microglial CX3CR1-mediated actions (Paolicelli et al., 2014; Sheridan et al., 2014; Milior et al., 2015).
2.5.1 Tissue Collection, Processing, and RNA Isolation
Animals (Male: n = 10 per group, Female: n = 10 per group) were overdosed with urethane and transcardially perfused with ice-cold saline for 3 min to clear the brain vasculature of blood and peripheral immune molecules (Frank et al., 2007). Brains were rapidly extracted and the rostral forebrain was snapfrozen on dry ice and stored at −80°C. Slices through prefrontal cortex (1–2 mm) were obtained using a precision brain slicer (Braintree Scientific Inc., Braintree, MA) equilibrated to −20°C. Dorsal mPFC (prelimbic and anterior cingulate cortex) was identified using standard gross neuroanatomical landmarks (Paxinos and Watson, 1998; see Figure 1E) and micropunch samples (1 mm dia., 2–6 mg tissue/animal) from both hemispheres of dorsal mPFC were collected.
Total RNA was isolated using a modified Trizol extraction technique (Invitrogen, Carlsbad, CA). Frozen micropunch samples were rapidly disrupted by pestle grinding in 500 μl Trizol reagent (Invitrogen, Carlsbad, CA). Homogenates were then incubated at room temperature for 5 min before being frozen on dry ice for 1 h. Homogenates were thawed and mixed with 100 μl chloroform, incubated at room temperature for 3 min, and centrifuged (12,000 × g) at 4°C for 15 min. The resulting aqueous phase was then transferred to a new tube and mixed with 1 μl of the RNA carrier GlycoBlue (Invitrogen) followed by 250 μl propanol, incubated at room temperature for 10 min and centrifuged (12,000 × g) at 4°C for 15 min. The nucleic acid precipitant was washed with 500 μl ethanol (75%), resuspended in 11 μl of nuclease-free water, and stored at −80°C. RNA integrity and concentration were subsequently analyzed using gel electrophoresis (Agilent TapeStation 2200, Agilent Technologies Inc., Santa Clara, CA). All samples exhibited an RNA Integrity Number (RIN) greater than 8, indicating quality RNA.
2.5.2 cDNA Synthesis
Total RNA samples were reverse transcribed into first strand cDNA using SuperScript III Reverse Transcriptase (Invitrogen) for RT-PCR. Just prior to reverse transcription, aliquots of total RNA (1 μg, 11 μl) were DNAse treated at 37°C for 30 min followed by 65°C for 10 min. Treated samples were chilled to 4°C before being incubated with oligo-dT primers (1.5 μl, 5 μM, Invitrogen) and dNTPs (0.5 μl, 25 mM, Invitrogen) at 65°C for 10 min, held post-cycle at 4°C. A mixture of SuperScript III Reverse Transcriptase (1 μl) and an RNAse inhibitor (1 μl, RNAseOut, Invitrogen) was then added to the reaction and incubated at 42°C for 50 min, immediately followed by enzyme denaturation and reaction termination at 70°C for 15 min. cDNA samples (20 μl) were stored at −20°C.
2.5.3 Quantitative PCR
Primers were designed to measure expression of each gene of interest, as well as the reference gene glyceraldehyde-6-phosphate dehydrogenase (GAPDH), using either the National Center for Biotechnology Information (NCBI) Primer-BLAST (ncbi.nlm.nih.gov/tools/primer-blast/) or IDT PrimerQuest (idtdna.com/Primerquest/Home/Index) tools (Table 1). Primers were tested for sequence specificity using MFEprimer-2.0 (http://biocompute.bmi.ac.cn/CZlab/MFEprimer-2.0/index.cgi/) and obtained from Eurofins Genomics (Eurofins MWG Operon LLC, Huntsville, AL).
Table 1.
Primer specifications for qPCR
| Gene | Function | Primer sequence (5′ – 3′) | Genbank Accession No. |
|---|---|---|---|
| MHCII | Antigen presentation | F – AATGGCCACACTTGGAGACC R – GTGTGCTCCTCTCTGATAGCC |
NM_001008847.2 |
| CD40 | Antigen presentation | F – GCCACTGAGACTACTGATACTG R – TGACTTGTTCCTTCCCGTAG |
NM_134360.1 |
| IL6 | Pro-inflammatory cytokine | F – GACCAAGACCATCCAACTCATC R – GCTTAGGCATAGCACACTAGG |
NM_012589.2 |
| CX3CL1 | Chemokine | F – GAATTCCTGGCGGGTCAGCACCTCGGCATA R – AAGCTTTTACAGGGCAGCGGTCTGGTGGT |
NM_134455.1 |
| CX3CR1 | Chemokine receptor | F – AGCTGCTCAGGACCTCACCAT R – GTTGTGGAGGCCCTCATGGCTGAT |
NM_133534.1 |
| GAPDH | Glycolysis | F – ACCACAGTCCATGCCATCACTG R – GATGACCTTGCCCACAGCCTT |
NM_017008.4 |
Formation of PCR product was measured in real time using the Roche LightCycler 480 System (Roche Diagnostics, Indianapolis, IN). For each sample, triplicate reactions were performed in 384-well plates at a volume of 10 ul, with each reaction consisting of 3 μl cDNA (12 ng for MHCII, CX3CL1, and CX3CR1, and 3.75 ng for CD40 and IL-6), 5 ul of PerfeCta SYBR Green SuperMix (Quanta BioSciences, Gaithersburg, MD) and 2 ul primer (300 nM). PCR cycling conditions consisted of a pre-incubation phase (10 min, 95°C), followed by 45 cycles of denaturation (15 s, 95°C), annealing (40 s, 60°C), and product extension (10 s, 72°C), with SYBR green I fluorescence captured at 72°C. Melt curves were generated using the machine default setting post-cycling to assess primer specificity and primer dimer formation.
To evaluate the relative abundance of mRNA, mean CT values were computed across reaction triplicates for each sample, with replicate CT standard deviations above 1 removed from analysis. Relative gene expression was then quantified using the 2−ΔΔCT method (ΔΔCT, internal reference gene subtracted from respective gene of interest, normalized to internal reference gene subtracted from respective gene of interest for sample measured across plates; Schmittgen and Livak, 2008) and evaluated using two-way ANOVA followed by Fisher’s LSD post hoc comparisons. Pearson’s correlations were used to assess the relationship between CX3CL1 and CX3CR1 transcript levels.
3. Results
3.1 Body and Adrenal Weight
Chronic stress affected body and adrenal weight in male and female rats (Figure 2). Body weight was significantly higher in male rats, regardless of stress (effect of sex: F(1, 125) = 1196.28, p < .05). There was a main effect of stress on weight gain (F(2, 125) = 28.02, p < .05) that varied between males and females (stress × sex interaction: F(2, 125) = 3.22, p < .05). Post hoc comparisons revealed reduced body weight in chronic stress males, compared to unstressed and acute stress males (p < .05). Consistent with previous research (Konkle et al., 2003), adrenal-weight-to-body-weight ratios were significantly higher in female rats (effect of sex: F(1, 125) = 713.84, p < .05). Chronic stress significantly increased adrenal weights in both male and female rats (effect of stress: F(2, 125) = 43.93, p < .05). This stress-induced increase did not differ between the sexes (stress × sex interaction: F(2, 125) = 0.05, ns).
Figure 2.
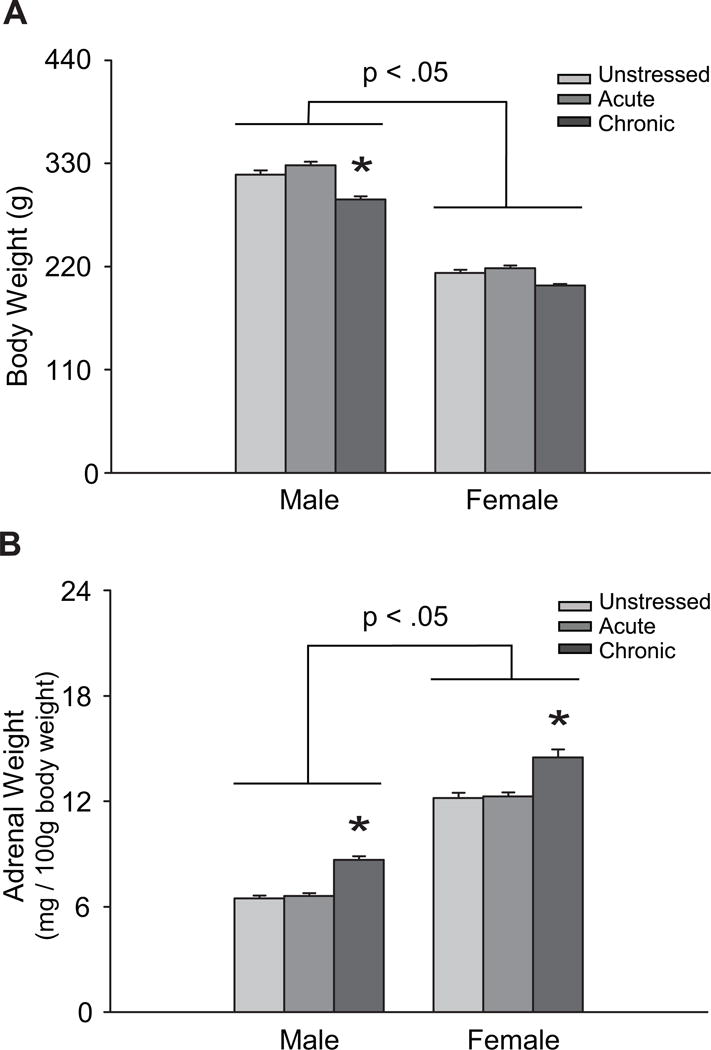
Chronic restraint stress reduces weight gain in male rats and induces adrenal hypertrophy in male and female rats.
Data are displayed as A: mean body weights, and B: mean adrenal-weight-to-body-weight ratios (adrenal weight/100g body weight). *p < .05 compared to same-sex unstressed group. Error bars indicate SEM.
3.2 Microglial Cell Density and Morphology in Prelimbic Cortex
Analyses revealed no significant differences across groups in total density of microglia (main effect of sex: F(1, 63) = 1.12, ns; main effect of stress: F(2, 63) = 0.37, ns; sex × stress interaction: F(2, 63) = 1.13, ns; Figure 3A,C). Very few reactive or amoeboid microglia were present (reactive, < 2% of cells; amoeboid, < 1% of cells). Therefore, subsequent analyses focused on ramified and primed microglia, with subtype proportions expressed as primed cell density over ramified cell density (Torres-Platas et al., 2014). There were no significant main effects of sex (F(1, 63) = 0.68, ns) or stress (F(2, 63) = 1.55, ns) on the proportion of primed to ramified microglia. However, the effect of stress varied between males and females (F(2, 63) = 5.50, p < .05). Post hoc comparisons revealed that the proportion of primed to ramified microglia was significantly higher in unstressed females relative to unstressed males (p < .05; Figure 3B). Although acute stress produced a non-significant tendency toward an increased proportion of primed to ramified microglia in males (p = 0.11), chronic stress did not alter microglial cell morphology (ns). In contrast, both acute and chronic stress markedly decreased the proportion of primed to ramified microglia in females (p < .05; Figure 3D).
Figure 3.
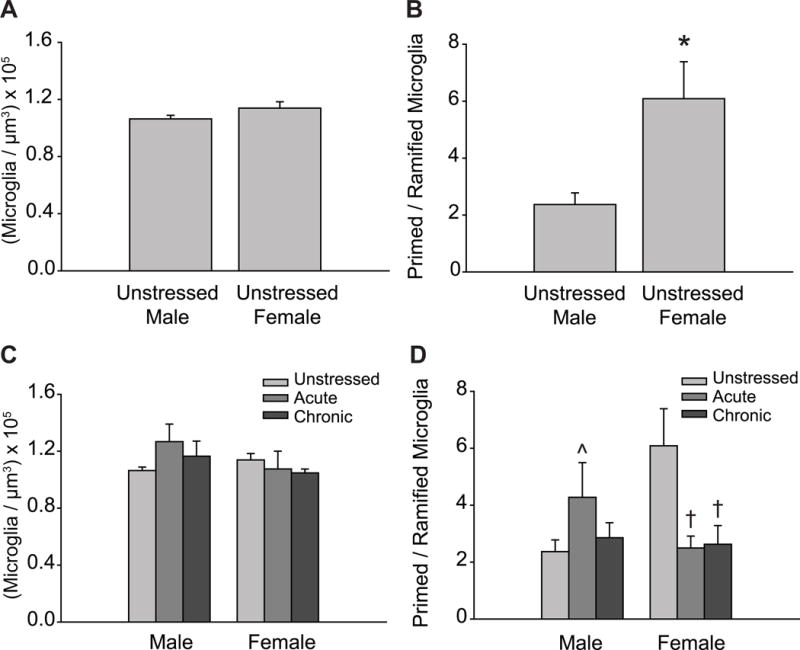
Sex and stress effects on microglial cell morphology in prelimbic cortex.
A: Total microglial density based on estimated volume, collapsed across morphological classifications. Unstressed male and female rats exhibit similar microglial densities. B: The proportion of primed to ramified microglia is significantly higher in unstressed females compared to unstressed males. C: Total microglial cell density based on estimated volume. Neither acute nor chronic stress significantly altered microglial cell density in prelimbic cortex. D: Acute and chronic stress significantly decreased the proportion of primed to ramified microglia in female but not male rats. *p < .05 compared to unstressed males, † p < .05 compared to unstressed females. ˆp = 0.11 compared to unstressed males. Error bars indicate SEM.
3.3 Immune Factor Expression in Dorsomedial Prefrontal Cortex
Gene expression analyses revealed no significant main effects of stress on chemokine CX3CL1 transcript levels (F(2, 48) = 0.72, ns). However, CX3CL1 expression differed significantly between males and females (F(1, 48) = 5.15, p < .05). Post hoc comparisons revealed higher levels of CX3CL1 mRNA in unstressed female rats compared to unstressed males (p < .05; Figure 4A). There were no significant interactions present (F(2, 48) = 1.05, ns).
Figure 4.
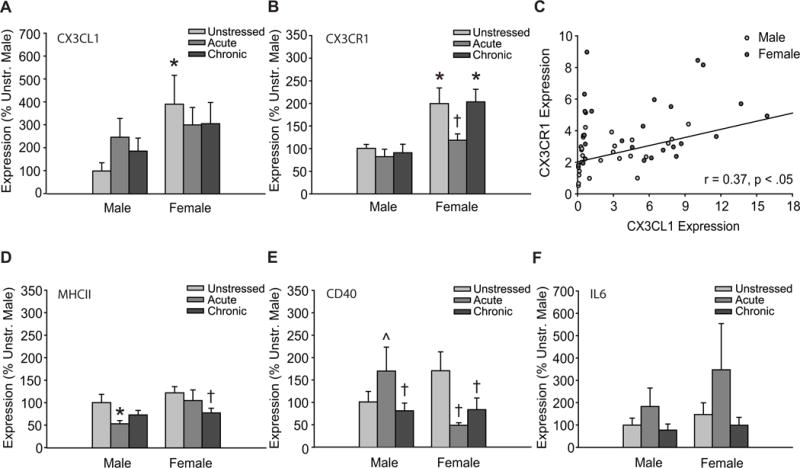
Sex and stress effects on immune factor expression in dorsomedial prefrontal cortex.
A: Expression of CX3CL1 is significantly greater in unstressed female rats compared to unstressed males. B: Transcript levels of CX3CR1 are significantly greater in unstressed and chronic stress female rats compared to unstressed males. Acute stress reduced CX3CR1 expression in female rats. C: Transcript levels of CX3CL1 and its cognate receptor, CX3CR1, are positively correlated in male but not female rats (combined linear relationship indicated on graph). D: Levels of MHCII expression were significantly different between males and females. Acute stress reduced MHCII expression in male rats, whereas chronic stress reduced MHCII transcript in females. E: Both acute (p = 0.06) and chronic stress decreased the expression of CD40 in female but not male rats. Acute stress induced a tendency toward greater levels of CD40 transcript in males. F: Levels of IL-6 mRNA were unaffected by sex or stress. Error bars indicate SEM. *p < .05 compared to unstressed males, † p < .05 compared to unstressed females, ˆp = 0.11 compared to unstressed males. Data in graphs A, B, D–F represent the percent change relative to unstressed males. Data points in graph C are 2−ΔΔCT values.
Chemokine receptor CX3CR1 expression varied with both sex (F(1, 51) = 14.44, p < .05) and stress (F(2, 51) = 5.42, p < .05). The effect of stress on CX3CR1 expression did not differ between males and females (F(2, 51) = 1.40, ns; Figure 4B). Follow up comparisons indicated significantly greater CX3CR1 transcript levels in unstressed and chronic stress females compared to unstressed males (p < .05), and significantly reduced CX3CR1 mRNA in acute stress females compared to unstressed females (p < .05). Furthermore, expression of CX3CL1 and its cognate receptor, CX3CR1, were moderately correlated (collapsed across sexes, r(54) = 0.37, p < .05; Figure 4C). Further analysis indicated a significant positive correlation between CX3CL1 and CX3CR1 expression in male (r(25) = 0.44, p < .05) but not female rats (r(27) = 0.19, ns).
MHCII expression varied with both sex (F(1, 43) = 4.01, p < .05) and stress (F(2, 43) = 3.31, p < .05; sex × stress interaction: F(2, 43) = 1.04, ns; Figure 4D). Post hoc comparisons revealed reduced MCHII mRNA in acute stress males compared to unstressed males (p < .05), and greater MCHII transcript levels in unstressed females compared to acute and chronic stress males (p < .05). Chronic stress reduced MCHII expression in female rats (p < .05).
There were no main effects of sex (F(1, 49) = 0.38, ns) or stress (F(2, 49) = 0.45, ns; Figure 4E) on CD40 transcript levels. However, the effects of stress varied between males and females (F(2, 49) = 4.11, p < .05). Post hoc comparisons indicated significant acute (p < .05) and marginally significant (p = 0.06) chronic stress-induced reductions in the expression of CD40 in female rats. Acute stress produced a non-significant tendency towards increased CD40 expression in male rats (p = 0.13). Expression of CD40 was greater in unstressed female rats compared to chronic stress males (p < .05).
IL6 expression did not significantly vary across sex or stress conditions (Figure 4F; main effect of sex: F(1, 46) = 1.70, ns; main effect of stress: F(2, 46) = 0.61, ns; sex × stress interaction: F(2, 46) = 2.67, ns).
4. Discussion
This study is the first examination of sex differences in microglial cell density and morphology in mPFC and, moreover, the first investigation of sex differences in stress effects on microglial cell activation. Our data demonstrate both a basal sex difference and differential effects of stress on microglia in male and female mPFC.
4.1 Heightened Morphological Activation of Microglia in Female mPFC
Unstressed females had increased proportions of primed to ramified microglia in mPFC compared to unstressed males. Given that morphological alterations in microglia are highly coupled with activation (Ransohoff and Perry, 2009), these findings suggest a heightened basal state of microglial activation in females. This is consistent with the increased microglial cell activation seen in parietal cortex, hippocampus and amygdala of adult female rats (Schwarz et al., 2012). The morphology of pyramidal neurons in prelimbic cortex is sexually dimorphic: apical dendritic arbors of unstressed female rats are smaller than those of unstressed males (Garrett and Wellman, 2009). Given the contribution of microglial cell activation to synaptic plasticity and the regulation of dendritic spine density (Miyamoto et al., 2013), it is interesting to speculate that differential morphological activation of microglia may contribute to this sex difference.
4.2 Stress Decreases Morphological Activation of Microglia in Females but not Males
Chronic stress did not significantly affect microglial morphology in males. In females, both acute and chronic restraint stress greatly reduced the proportion of primed to ramified microglia, reflecting a significant decrease in microglial activation.
Neither acute nor chronic restraint induced a significant change in microglial cell morphology in males. This finding contrasts with prior reports of restraint stress-induced microglial activation in males (Hinwood et al., 2012; Hinwood et al., 2013), but is consistent with a study demonstrating no change in microglial morphology in males following chronic variable stress (Kopp et al., 2013). Consistent with previous work from our lab, the present study varied the time of day at which restraint occurred, thus decreasing the predictability of the stressor and, likely, stressor habituation. This produced adrenal hypertrophy in males consistent with chronic variable stress (Ulrich-Lai et al., 2006). Therefore, as Kopp et al. (2013) posited, varying the time of restraint may have led to hypothalamic-pituitary-adrenal (HPA) axis hypersensitivity, greater cumulative glucocorticoid exposure and, potentially, increased glucocorticoid-mediated anti-inflammatory actions in mPFC. This would further support the hypothesis that chronic stress with low glucocorticoid exposure primes the brain’s immune response, whereas chronic stress with sustained, high glucocorticoid exposure reduces neuroimmune activation (Kopp et al., 2013).
Alternatively, we evaluated microglial cell activation using standard morphological criteria and an unbiased stereological technique, whereas most preceding investigations assessed activation using densitometry-based measurements of Iba-1-positive material (Hinwood et al., 2012; Kopp et al., 2013). Thus, differences across studies may reflect differential sensitivity of each technique to subtle changes in microglial cell morphology.
Regardless, we found robust stress-induced morphological deactivation in females. Though the mechanisms underlying this sex difference are not yet known, several pathways are likely involved. For instance, females exhibit heightened baseline levels and lower stress-induced increases in circulating corticosterone (Galea et al., 1997). Microglia express glucocorticoid receptors and, through glucocorticoid signaling, are capable of altering the immunological milieu of the brain (Bellavance and Rivest, 2014). In males, the immunomodulatory actions of glucocorticoids in the CNS are complex, with context, severity, and chronicity of glucocorticoid exposure shaping either an anti- or pro-inflammatory response (Bellavance and Rivest, 2014). For example, acute exposure to glucocorticoids can reduce inflammation and exert numerous adaptive and neuroprotective effects, while prolonged glucocorticoid exposure, as seen during chronic stress, can promote a pro-inflammatory environment and microglial cell sensitization (Bellavance and Rivest, 2014). Thus, glucocorticoids might affect microglial activation differentially in males and females, at least in part due to sex differences in baseline and stress-linked HPA axis activity.
Our findings in microglia parallel sex differences in the effects of stress on neuronal morphology. For example, chronic restraint stress induces dendritic atrophy in mPFC in male rats, yet dendritic hypertrophy in females (Garrett and Wellman, 2009). This stress-linked increase in dendritic complexity in females is, in part, estradiol-dependent (Garrett and Wellman, 2009). Microglia express both estrogen and progesterone receptors (Sierra et al., 2008). Under pathological conditions, endogenous estrogens and progesterone regulate microglial function, reducing the expression of inflammatory mediators and exerting neuroprotective effects (Sierra et al., 2008). Accordingly, female sex steroids may exert a modulatory effect on stress-induced microglial cell activation. The majority of the females in the present study were in diestrous on the day of euthanasia. Although stress can disrupt the estrous cycle and induce a state of persistent diestrus in rats (Konkle et al., 2003; Antunes et al., 2006), this is unlikely to account for our results, as the rats in proestrus/estrus were essentially evenly distributed across stress conditions. Nonetheless, due to the limited representation of rats across the estrous cycle, we did not examine potential differences in stress effects across the estrous cycle. Future studies should address the effects of sex steroids and stress on microglial morphology and activation in mPFC.
Nonsteroidal mechanisms may also contribute to the differential effects of stress on microglial morphology in mPFC. For instance, dopamine mediates effects of stress on cortical structure and function in males (Rey et al., 2014; Lin et al., 2015) and dopaminergic innervation of mPFC is sexually dimorphic in young adult rats (Kritzer and Creutz, 2008). Notably, microglia express receptors for most neurotransmitters, including dopamine (Domercq et al., 2013), and can respond to and modulate neurotransmitter signaling. Thus, sex differences in the dopaminergic or other neurotransmitter systems could contribute to the differential effects of stress on microglial morphology in males and females.
4.3 Heightened Fractalkine and Fractalkine Receptor Expression in Female mPFC
Paralleling the morphological data, the immune-associated expression profile in mPFC differed in unstressed male and female rats, with greater levels of CX3CL1 and CX3CR1 expression in mPFC in females compared to males. Notably, CX3CL1-CX3CR1 signaling can mediate the release and subsequent actions of IL-1β in the brain (Rogers et al., 2011), and unstressed female rodents exhibit greater levels of IL-1β protein in various cortical regions (Schwarz et al., 2012; Hudson et al., 2014). Therefore, greater CX3CL1-CX3CR1 expression could underlie heightened IL-1β levels in female rats. Future studies investigating the CX3CL1-CX3CR1 signaling pathway and its downstream effects on pro-inflammatory cytokine release in females should test this hypothesis.
Neurons constitutively express high levels of CX3CL1, whereas CX3CR1 is found almost exclusively on microglia (Jung et al., 2000; Cardona et al., 2006). We found greater CX3CR1 expression in unstressed female rats. Heightened CX3CL1 and CX3CR1 expression in females could indicate greater neuron-microglia cross talk and, potentially, a greater need for neuronally expressed CX3CL1 in the regulation of microglial activation state and function in females compared to males (Sheridan, 2013).
Additionally, CX3CL1 and CX3CR1 expression were positively correlated in male but not female rats. This may be due to a disproportionately high level of microglial CX3CR1 expression, relative to CX3CL1 transcript, in females. Heightened CX3CR1 expression in the face of low-level CX3CL1 expression could again indicate greater neuron-microglia crosstalk in female rats, and may implicate microglia-dependent mechanisms in the regulation of this signaling pathway. CX3CL1-CX3CR1 signaling has been associated with the modulation of synaptic plasticity, cytokine release, stress responsivity, cognition, and behavior (Rogers et al., 2011; Paolicelli et al., 2014; Sheridan et al., 2014). Therefore, different levels of CX3CL1-CX3CR1 signaling could contribute to sex differences in stress susceptibility, microglial morphology, and neuronal plasticity.
Acute but not chronic stress reduced CX3CR1 expression in female rats, whereas stress did not alter CX3CL1-CX3CR1 expression in males. Numerous mechanisms that attract microglial processes (i.e. purinergic signaling, glutamatergic neurotransmission) have been associated with the effects of acute stress on brain and behavior (Popoli et al., 2012). These mechanisms could enhance neuron-microglia contact and CX3CL1 signaling, as well as the subsequent compensatory downregulation of CX3CR1 expression in microglia. Given that CX3CL1-CX3CR1 signaling is linked to microglial regulation, this pathway may be involved in the observed acute stress-induced deactivation of microglial morphology in female rats. However, different mechanisms would seem to be active during chronic stress, and in male rats. Identifying these differential mechanisms, as well as downstream pathways involved in synaptic plasticity, immune factor release, and behavior, is an important question for future research.
4.4 Stress Decreases MHCII and CD40 Expression in Females but not Males
In males, acute stress significantly reduced MHCII expression but induced a tendency toward heightened CD40 expression, whereas chronic stress did not affect MHCII, CD40, or IL6 expression. In females, acute restraint stress reduced CD40 expression and chronic stress reduced both MHCII and CD40 expression, while stress did not affect IL-6 expression in females. Thus, acute and chronic stress decreased monocyte and microglial activation, as assessed by a significant shift in immune-associated gene expression, in mPFC in female but not male rats.
Although acute stress induced a tendency toward increased CD40 expression and reduced MHCII transcript levels in males, chronic stress did not affect these immune markers. This finding differs from a prior report of stress-induced increases in MHCII transcript levels in male rats (Frank et al., 2007), but is consistent with other studies showing no change in MHCII expression following various acute and chronic stressors (Blandino et al., 2009; Hinwood et al., 2012; Hinwood et al., 2013; Kreisel et al., 2014). This may be due to differences in stressor type and severity, as acute inescapable shock, perhaps a more physically aversive stressor, induces MHCII expression (Frank et al., 2007) whereas chronic restraint and variable stress do not (Hinwood et al., 2012; Hinwood et al., 2013; Kreisel et al., 2014). These differences may indicate stressor-dependent pathways in the regulation of microglial activation.
Our results, along with those reported by Hinwood et al. (2013), suggest that different signals or pathways may contribute to the regulation of microglial cell morphology versus other measures of activation state. We found a tendency toward acute stress-induced morphological activation along with enhanced CD40 expression, but reduced MHCII expression in males. This again is consistent with the hypothesis that sustained high levels of glucocorticoids may reduce neuroinflammatory state (Kopp et al. (2013), and could indicate that different pathways may be involved in the regulation of acute and chronic stress effects in males. Given that microglial cells are highly responsive to various extracellular perturbations, differing signaling molecules, and biochemical cascades (Salter and Beggs, 2014), variation in stressor parameters might affect microglial activation patterns and the neuroimmunological milieu. These findings underscore the importance of analyzing multiple immune factors and pathways in the assessment of stress effects on microglial activation.
We found no effects of stress on inflammatory cytokine IL-6 expression in mPFC in male or female rats. The literature on stress-induced alterations in inflammatory cytokines in the brain is mixed, with reports of increases in inflammatory cytokines (e.g., IL-1β and TNF-α; Grippo et al., 2005; Cao et al., 2007; Hudson et al., 2014) and reports of no effects (Frank et al., 2007; Kreisel et al., 2014). Moreover, many studies reporting stress-induced increases in the expression of inflammatory cytokines in the brain find only one or a few cytokines to be upregulated, and the specific cytokine(s) induced by stress vary across studies (Grippo et al., 2005; Cao et al., 2007; Hudson et al., 2014). Therefore, it is possible that other cytokines, receptors, or cytokine-associated pathways were differentially altered by stress in males and females.
Nonetheless, robust stress-induced decreases in microglial activation-associated gene expression were present in female rats. This finding is consistent with the stress-induced morphological deactivation we found in females but not males. Given the parallel patterns of CD40 expression and morphological alteration in females, it is interesting to speculate that CD40 may contribute to the effects of acute and chronic stress on microglial activation in females. Moreover, our finding of chronic stress-induced downregulation in MHCII expression suggests an overall reduction in microglial activity in mPFC in female rats. CD40 is involved in the activation of several intracellular pathways (e.g. nuclear factor (NF)-κB signaling), inflammatory cytokine release, and chemotaxis, and has been linked with neuroinflammation-associated cognitive and behavioral dysfunction (Chen et al., 2006; Lynch, 2009; Michels et al., 2015; Muller et al., 2015). Moreover, CD40 can modulate microglial phagocytic capacity (Lynch, 2009). Therefore, differing patterns of CD40 expression could contribute to sex differences in the effects of stress on mPFC structure and function, as well as mPFC-mediated behaviors. In vitro studies indicate that both glucocorticoids and estrogens regulate levels of CD40 expression (Dimayuga et al., 2005; Li et al., 2007). Thus, both sex steroids and glucocorticoids could mediate the differential effects of stress on CD40 expression in mPFC.
5. Conclusion
We have shown a sex difference in the baseline morphological state and immune-associated gene expression profile of microglia and a sex-dependent effect of stress on microglial cell activation in mPFC. In male rats, stress-induced increases in microglial activation in mPFC disrupt prefrontally mediated behaviors (Hinwood et al., 2012). Similarly, stress-linked dendritic retraction in mPFC is associated with deficits in prefrontally mediated behaviors (Mika et al., 2012). In response to the same stressor, females exhibit stress-induced dendritic proliferation in mPFC (Garrett and Wellman, 2009) and enhancements in prefrontally mediated behaviors (Bowman et al., 2003). Given the differential effects of stress on microglial cell morphology and immune-associated gene expression in males and females, and the link between microglial morphology, activation state, and synaptic remodeling (Miyamoto et al., 2013), it is interesting to speculate that microglia may contribute to the sex-specific effects of stress on neuronal morphology and function in mPFC.
Finally, psychological stress and depression are associated with immune activation and increased pro-inflammatory cytokine production (Grippo et al., 2005). Considering the greater prevalence of stress-linked psychological disorders in women, and recent reports showing a correlation between perturbations in microglial morphology in anterior cingulate cortex and suicide risk in patients with depression (Torres-Platas et al., 2014), understanding the complex interactions among sex, stress, immune factors, and microglial activation in mPFC may elucidate important mechanisms underlying the sex-specific expression of stress-linked psychopathology.
Acknowledgments
We thank Dr. Kimberly Rosvall for providing expert advice on qPCR technique, troubleshooting, and primer design, and Indiana University and the Harlan Scholars Program for their generous support of this research.
Footnotes
Conflict of Interest Statement: The authors declare no competing financial interests.
References
- Antunes IB, Andersen ML, Baracat EC, Tufik S. The effects of paradoxical sleep deprivation on estrous cycles of the female rats. Horm Behav. 2006;49:433–440. doi: 10.1016/j.yhbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Bellavance M-A, Rivest S. The HPA – Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Frontiers in immunology. 2014;5:136. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, Schwarz JM. A Lifespan Approach to Neuroinflammatory and Cognitive Disorders: A Critical Role for Glia. J Neuroimmune Pharm. 2012;7:24–41. doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Cao L, Hudson CA, Moynihan JA. Chronic foot shock induces hyperactive behaviors and accompanying pro- and anti-inflammatory responses in mice. J Neuroimmunol. 2007;186:63–74. doi: 10.1016/j.jneuroim.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang DR, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. Journal of Neuroscience. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KQ, Huang J, Gong WH, Zhang LZ, Yu PC, Wang JM. CD40/CD40L Dyad in the Inflammatory and Immune Responses in the Central Nervous System. Cell Mol Immunol. 2006;3:163–169. [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Dimayuga FO, Reed JL, Carnero GA, Wang CM, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, Bruce-Keller AJ. Estrogen and brain inflammation: Effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161:123–136. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Domercq M, Vazquez-Villoldo N, Matute C. Neurotransmitter signaling in the pathophysiology of microglia. Frontiers in Cellular Neuroscience. 2013;7:17. doi: 10.3389/fncel.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: Sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, Walker FR. Evidence that Microglia Mediate the Neurobiological Effects of Chronic Psychological Stress on the Medial Prefrontal Cortex. Cereb Cortex. 2012;22:1442–1454. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic Stress Induced Remodeling of the Prefrontal Cortex: Structural Re-Organization of Microglia and the Inhibitory Effect of Minocycline. Cereb Cortex. 2013;23:1784–1797. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- Hudson SP, Jacobson-Pick S, Anisman H. Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience. 2014;277:239–249. doi: 10.1016/j.neuroscience.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: Role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle ATM, Baker SL, Kentner AC, Barbagallo LSM, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Kopp BL, Wick D, Herman JP. Differential effects of homotypic vs. heterotypic chronic stress regimens on microglial activation in the prefrontal cortex. Physiol Behav. 2013;122:246–252. doi: 10.1016/j.physbeh.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatr. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. Journal of Neuroscience. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia Are Essential to Masculinization of Brain and Behavior. Journal of Neuroscience. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MQ, Wang YY, Guo RW, Bai Y, Yu ZP. Glucocorticoids impair microglia ability to induce T cell proliferation and Th1 polarization. Immunol Lett. 2007;109:129–137. doi: 10.1016/j.imlet.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Lin GL, Borders CB, Lundewall LJ, Wellman CL. D1 receptors regulate dendritic morphology in normal and stressed prelimbic cortex. Psychoneuroendocrinology. 2015;51:101–111. doi: 10.1016/j.psyneuen.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. The Multifaceted Profile of Activated Microglia. Molecular Neurobiology. 2009;40:139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- Michels M, Danieslki LG, Vieira A, Florentino D, Dall’Igna D, Galant L, Sonai B, Vuolo F, Mina F, Pescador B, Dominguini D, Barichello T, Quevedo J, Dal-Pizzol F, Petronilho F. CD40-CD40 Ligand Pathway Is a Major Component of Acute Neuroinflammation and Contributes to Long-term Cognitive Dysfunction after Sepsis. Mol Med. 2015;21:219–226. doi: 10.2119/molmed.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika A, Mazur GJ, Hoffman AN, Talboom JS, Bimonte-Nelson HA, Sanabria F, Conrad CD. Chronic Stress Impairs Prefrontal Cortex-Dependent Response Inhibition and Spatial Working Memory. Behav Neurosci. 2012;126:605–619. doi: 10.1037/a0029642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior G, Lecours C, Samson L, Bisht K, Poggini S, Pagani F, Deflorio C, Lauro C, Alboni S, Limatola C. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain, behavior, and immunity. 2015 doi: 10.1016/j.bbi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Moorhouse AJ, Nabekura J. Microglia and synapse interactions: fine tuning neural circuits and candidate molecules. Frontiers in Cellular Neuroscience. 2013;7:6. doi: 10.3389/fncel.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AF, Strauss L, Greter M, Gast H, Recher M, Becher B, Fontana A. Neutralization of colony-stimulating factor 1 receptor prevents sickness behavior syndrome by reprogramming inflammatory monocytes to produce IL-10. Brain Behav Immun. 2015;48:78–85. doi: 10.1016/j.bbi.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1 beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Frontiers in Cellular Neuroscience. 2014;8:10. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of Mood Disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Annual Review of Immunology. Palo Alto: Annual Reviews; 2009. Microglial Physiology: Unique Stimuli, Specialized Responses; pp. 119–145. [DOI] [PubMed] [Google Scholar]
- Rey CD, Lipps J, Shansky RM. Dopamine D1 Receptor Activation Rescues Extinction Impairments in Low-Estrogen Female Rats and Induces Cortical Layer-Specific Activation Changes in Prefrontal Amygdala Circuits. Neuropsychopharmacology. 2014;39:1282–1289. doi: 10.1038/npp.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 Deficiency Leads to Impairment of Hippocampal Cognitive Function and Synaptic Plasticity. Journal of Neuroscience. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Beggs S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C-T method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan GK. Neuron – glia crosstalk in health and disease: fractalkine and CX(3)CR1 take centre stage. Open Biol. 2013;3:14. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan GK, Wdowicz A, Pickering M, Watters O, Halley P, O’Sullivan NC, Mooney C, O’Connell DJ, O’Connor JJ, Murphy KJ. CX(3)CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity. Frontiers in Cellular Neuroscience. 2014;8:15. doi: 10.3389/fncel.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Herman JP. Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol-Endocrinol Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Walker FR, Nilsson M, Jones K. Acute and Chronic Stress-Induced Disturbances of Microglial Plasticity, Phenotype and Function. Curr Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZL, Luo CM, Zhang WZ, Chen YB, He JJ, Zhao QY, Zuo R, Wu YH. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behav Brain Res. 2011;225:135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]


