Abstract
A new non-invasive and potentially inexpensive frontier in the diagnosis of cancer relies on the detection of volatile organic compounds (VOCs) in exhaled breath samples. Breath can be sampled and analyzed in real-time, leading to fascinating and cost-effective clinical diagnostic procedures. Nevertheless, breath analysis is a very young field of research and faces challenges, mainly because the biochemical mechanisms behind the cancer-related VOCs are largely unknown. In this review, we present a list of 115 validated cancer-related VOCs published in the literature during the past decade, and classify them with respect to their “fat-to-blood” and “blood-to-air” partition coefficients. These partition coefficients provide an estimation of the relative concentrations of VOCs in alveolar breath, in blood and in the fat compartments of the human body. Additionally, we try to clarify controversial issues concerning possible experimental malpractice in the field, and propose ways to translate the basic science results as well as the mechanistic understanding to tools (sensors) that could serve as point-of-care diagnostics of cancer. We end this review with a conclusion and a future perspective.
1. Introduction
According to the WHO statistics for 2008, cancer is a leading cause of mortality with more than 7.5 million deaths worldwide and more than 12 million new cases every year.2 While the incidence of lung cancer, as reflected by occurrence and mortality, is among the highest in the world, other cancers (e.g., stomach, liver, colon and breast cancer) are also responsible for many cancer deaths each year.2,3 Approximately 30% of the cancer deaths are associated with one or a combination of the following risk factors: high body mass index, low fruit and vegetable intake, lack of physical activity, tobacco use, and alcohol use.2 In a few instances, the cause for cancer is hereditary.2 Patterns of cancer incidence and mortality differ strongly from region to region worldwide; more than 50% of cancer incidence and 60% of deaths occur in less-developed countries.2,3
1.1. Available approaches for cancer diagnosis
Evaluation of cancer prognosis involves disease confirmation and disease staging.4 Depending on the cancer type, a variety of techniques for the diagnosis and staging are applied in the clinical setting. These techniques include blood tests, X-ray,5 mammography,6 colonoscopy,7 computed tomography (CT),8 magnetic resonance imaging (MRI),9 positron emission tomography (PET),10 and ultrasonography.11 Although one or a combination of these techniques can show, to a limited extent, the presence, location and size of an abnormal mass, the final determination of cancer is made through a biopsy taken from the specific tissue.12 In this approach, the tissue is generally examined under a microscope by a pathologist to determine the shape and/or concentration of the cells which, in turn, could give indications of the stage(s), sub-type(s) and/or genetic mutations of the disease. Nevertheless, a biopsy is neither convenient for the patient nor free of complications.13 Furthermore, there is a possibility to miss small lesions, because the diseased areas may be patchy.14 In some instances, such as in the lower stages of gastric mucosal atrophy,14,15 there are great inter-observer variations in the identification of pre-malignant lesions. In other instances, such as in the lung or liver biopsy, there is a morbidity and even mortality risk following a biopsy process, mainly due to bleeding.11,13,16
Currently, there is a trend towards personalized medicine in cancer care to optimize clinical response and to minimize toxicity.4,17–19 This trend is based on the search for molecular cancer biomarkers that could complement the conventional diagnostic methods and improve their diagnostic yield.17–31 Gene expression profiling of cancer cells is associated with tumor heterogeneity and treatment outcome, thus allowing a global picture of cellular functioning. Protein expression profiling of cancer cells provides important information to the treating physician, as most targeted therapeutic agents are designed to inhibit the activity of proteins.27–31 Although much progress has been made in these fields, some difficulties must still be overcome towards developing effective biomarkers, including tumor heterogeneity, genetic, epigenetic, and micro environmental effects. Moreover, the related technologies require relatively large amounts of tissue, and are often costly, time consuming, and not available in many medical facilities as described earlier.32–38
1.2. Volatile organic compounds for cancer diagnosis
An evolving approach in cancer diagnostics is based on volatile organic compounds (VOCs), which are organic compounds that have a high vapor pressure under ordinary room-temperature conditions. Cancer VOCs originate from the cell or disease location, enter the surrounding environment39 and can be identified (i) from the headspace of cancer cell lines (i.e., the blend of VOCs confined above the cells in a sealed flask);40–51 (ii) through the urine;52 (iii) through the skin;53,54 (iv) through the blood;55,56 and/or (v) through the exhaled breath.15,40–49,51,55,57–81 The current review focuses on the cancer VOCs examined through exhaled breath.
A typical population of breath samples might contain around 3000 different VOCs in total, mostly at low concentrations that range from pptv to ppbv.82 A major part of the VOC spectrum varies amongst different individuals, while only few VOCs share a common health condition in a given population.83,84 This outcome has been supported by extensive empirical data.4,39,85–88 A number of first-rate reviews on cancer-related VOCs and an outlook on the potential developments in the area of VOC analysis can be found elsewhere.4,39,57,58,80,87–91 Nevertheless, the pathophysiology underlying the alteration of the cancer VOCs has been vague to a large extent. In this review, we shed light on the pathophysiology causing metabolic changes in the VOC levels and compositions in cancer. Towards this end, we have narrowed the wide spectrum of reported cancer-related VOCs (approximately 3000; the significance for most is unknown)92 to some hundred candidates. We have then used specific VOCs and combinations thereof to discuss important issues related to their possible biochemical origin and the underlying pathophysiological causes (Section 2) – a subject that has so far been insufficiently targeted.4 In our discussion, we have tried to clarify controversial issues concerning possible experimental malpractice in the field. Based on this discussion, we propose ways to translate the lab results as well as the mechanistic understanding to tools (sensors) that could serve as point-of-care diagnostics of cancer (Section 3). We end this review with a conclusion and a future perspective (Section 4).
2. Assessing the origin of cancer VOCs
2.1. Emission of VOCs from cancer cells
In normal and abnormal processes in the body, metabolic changes occur all the time. It has been shown, for example, that different liver enzymes affect the construction of cell membranes.93,94 In metabolic illnesses, such abnormal processes can alter the body’s chemistry either by changing the VOCs concentration or by producing new VOCs.
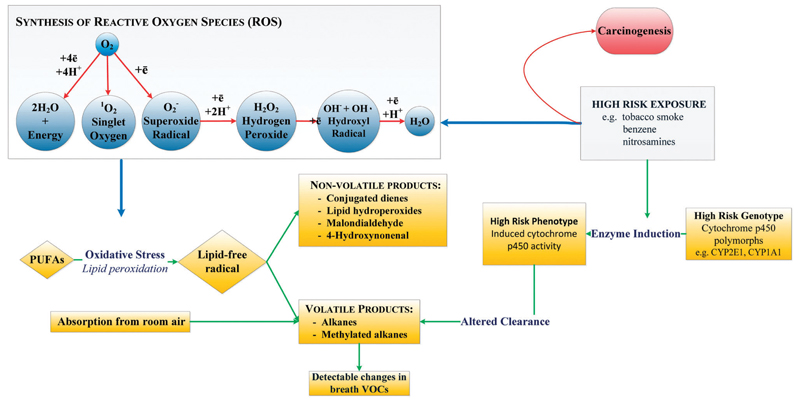
A vital risk factor for cancer development is linked to boosted oxidative stress and induction of cytochrome p-450 enzymes (CYP450, a group of oxidase enzymes).95 Oxidative stress in the body is related to the general equilibrium between formation and deactivation of reactive oxygen species (ROS) and free radicals. As part of the cellular process in the mitochondria, the cell manufactures ROS that have an unpaired electron in the outer shell. Other sources of ROS could be from exogenous origins, for example cigarette smoke, pollution and radiation.4,72 Once accumulated in the tissue, ROS can attack different molecules in the body such as polyunsaturated fatty acids (PUFA) and proteins. During oxidative stress, ROS and free radicals are excreted from the mitochondria in the cell, generating volatile alkanes that are emitted in the breath (see Fig. 1).4 In addition, cytochrome p-450 enzymes that catalyze the oxidation of organic chemicals can be upregulated by ROS molecules in the human tissue.96,97 This enzyme family has been shown to be over-expressed in human breast cancer tissue, for example aromatase which synthesizes estrogens.98 Note that most inflammatory conditions are associated with ROS production, and, hence, ROS products might not be specific for cancer.
Fig. 1.
Hypothetical basis of the breath test for lung cancer: lung cancer could result from the interaction of hereditary and environmental factors. Several cytochrome p450 mixed oxidases are activated by exposure to environmental toxins such as tobacco smoke. The induced phenotype may increase the risk of lung cancer by increased conversion of precursors to carcinogens. An altered pattern of cytochrome p450 mixed oxidase activity could potentially modulate catabolism of endogenous VOC products of oxidative stress and generate an altered pattern of breath VOCs. Reprinted from ref. 4.
A complementary pathophysiologic model suggests that during the early stages of cancer development, some of the normal cells proliferate at prompt rates, reach the oxygen diffusion boundary and become hypoxic (less than 0.1% oxygen in the gaseous phase).99 Because of the increased demand for energy and macromolecular biosynthesis these cells prefer the use of glycolysis over oxidative phosphorylation (Warburg effect). This process is associated with high rate of glycolysis and lactic acid formation,100–103 thus allowing cell survival in the hypoxic micro-environment.104,105 The excessive lactate production causes the tissue to become acidic and eventually causes the breakage of the basement membrane. Moreover, the acidic surroundings defend the tumor from the immune system.106
Tumor growth generally goes along with gene changes and/or protein changes.107,108 As a result, individual alleles can create a unique VOC profile that is further secreted in the body fluids.109 Although most models relate to VOCs that are produced endogenously, exogenous VOCs detected in breath are of great interest as well, mainly because they relate to exposure of an individual to carcinogens. Exogenous VOCs are typically highly reactive, causing peroxidative damage to DNA, proteins, and PUFA. The negative impact of such processes accumulates during the years and is assumed to promote cancer.110 Particularly, very lipophilic chemical compounds are stored in the fat compartments of the body and can be released over a period of weeks and months after exposure.111
2.2. VOC exchange between various body fluids
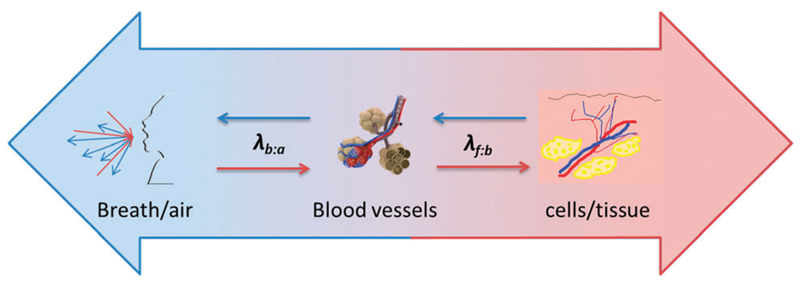
As indicated in the previous section, it has been hypothesized that the abnormal cancer VOCs are produced by tumor cells, from which they are excreted into the endobronchial cavity, from where they are exchanged and excreted via various body fluids. An idealized approach to check this hypothesis would rely on the comparison of VOC profiles from the different organs and body fluids of the same cancer patient and/or the same animal model. In this context, the simplest starting point would be a comparison between the VOC profiles in the headspace of cancer tumor tissue, (headspace of) blood samples, and breath samples. Due to not yet mature technical/experimental methods, no experimental results have been achieved with such an approach. Therefore, and given the unmet need to gain an understanding of the biochemical pathway of the cancer-related VOCs, we have simulated such an experiment with the help of thermodynamics viewpoint. In our simulation, we have targeted the equilibrium concentrations of a given VOC between “breath–blood–fat”, through estimation of the respective thermodynamic partition coefficients (see Fig. 2):
Partition coefficient between fat and blood (λf:b): this coefficient is designed to estimate the equilibrium concentration of VOCs in fat tissue and (lipophilic) cell membranes with respect to blood.
Partition coefficient between blood and air (λb:a): this coefficient is designed to simulate the equilibrium of VOCs between blood and exhaled air. If the respective VOC is systemic, the blood concentration can be estimated using the blood–air partition coefficients, λb:a.1,4,66,112–115 If experimentally determined λb:a are not available, their values can be estimated by either theoretical molecular descriptors or on semi-empirical calculations using experimental physical properties (for example, water–air, rat-λb:a, or olive-oil–air partition coefficients).116–120
Fig. 2.
Simulation scheme of the two main thermodynamic parameters responsible for the diffusion of cancer VOCs between “breath–blood–fat”: λf:b – partition coefficient between fat and blood, which simulates the diffusion of VOC from the (cancer or healthy) tissue to the blood; and λb:a – partition coefficient between blood and air, which simulates the diffusion of VOC from the blood to the exhaled air.
This partition-coefficient simulation is a straightforward and simple approach, which does not need elaborate modelling, as in the papers of King et al.121–129 To implement this approach, we have listed 115 VOCs that were reported in the literature as cancer biomarkers during the past 10 years, together with the constituent λb:a and λf:b (Table 1). The full list of 115 VOCs is divided into the following compound families: hydrocarbons, aromatic compounds, alcohols, ketones, aldehydes, acids, esters, ethers, heterocyclic compounds, nitriles, sulfides, terpenes and others.
Table 1.
Candidates for cancer VOCs published during the past decade.4,15,50,56,63,68,71,72,74–76,78,133,202,248,249 Identification of these compounds was achieved through spectral library match. A number of authors validated their identification by comparison of retention time or retention index, e.g., in ref. 1, 15, 42–45, 59, 65, 66, 131 and 133. For these VOCs the λb:a and λf:b are given in separate columns. If these partition coefficients are not known from the literature, they are estimated by regression from data in ref. 115 for hydrocarbons or estimated by the algorithms of Poulin and Krishnan139 for other compounds based on the partition coefficients for water:air(λw:a) and for octanol:water (λo:w). All partition coefficients are given in dimensionless units [mol L−1/mol L−1]. Based on these physicochemical parameters, the equilibrium concentrations of VOCs in blood and fat can be estimated based on the concentration in alveolar breath
| Chemical family | CAS number | Compound name | λb:a at 37 °C in dimensionless units [(mol L−1)/(mol L−1)] | References for λb:a at 37 °C | log[λfb] | References for λf:b at 37 °C | Tentative origin | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Alkanes, branched-chain alkanes and branched-chain alkenes | 74-84-0 | Ethane | 8.50 × 10−02 | Predicted252 | 1.934 | 120 | Natural or petrol, product of lipid peroxidation | Lung cancer | 4 |
| 109-66-0 | Pentane | 4.16 × 10−01 | Measured115 | 1.998 | 253 | Natural, possibly petrol, product of lipid peroxidation | Lung cancer | 78 | |
| 142-82-5 | Heptane | 2.71 × 10+00 | Measured115 | 2.190 | 253 | Natural or petrol, plastics | Lung cancer | 78 | |
| 111-65-9 | Octane | 5.77 × 10+00 | Measured115 | 1.606 | 120 | Natural or petrol | Lung cancer | 56,78 | |
| 111-84-2 | Nonane | 1.39 × 10+01 | Measured115 | 1.777 | 254 | Natural or petrol | Breast cancer | 74 | |
| 124-18-5 | Decane | 2.48 × 10+01 | Predicted using data from ref. 115 | 1.656 | 254 | Natural or petrol | Lung cancer | 78 | |
| 1120-21-4 | Undecane | 5.46 × 10+01 | Predicted using data from ref. 115 | 2.342 | Natural or petrol | Breast cancer | 76 | ||
| 112-40-3 | Dodecane | 1.20 × 10+02 | Predicted using data from ref. 115 | 1.901 | Fuels | Lung cancer, breast cancer | 76,248 | ||
| 629-50-5 | Tridecane | 2.67 × 10+02 | Predicted using data from ref. 115 | 2.540 | Fuels | Breast cancer | 76 | ||
| 629-59-4 | Tetradecane | 5.99 × 10+02 | Predicted using data from ref. 115 | 2.990 | Breast cancer | 76 | |||
| 629-62-9 | Pentadecane | 1.36 × 10+03 | Predicted using data from ref. 115 | 3.515 | Breast cancer | 76 | |||
| 75-28-5 | 2-Methyl-propane | 7.90 × 10−02 | Measured115 | 1.795 | Refrigerant, contaminant from plastics, tubing, medical equipment | Breast cancer | 74 | ||
| 107-83-5 | 2-Methyl-pentane | 4.73 × 10−01 | Measured115 | 2.295 | 253 | Petrol | Lung cancer | 78 | |
| 78-79-5 | Isoprene | 9.50 × 10−01 | Measured114 | 1.043 | 120 | Mevalonic pathway – biosynthesis of cholesterol | Lung cancer and gastric cancer | 15 | |
| 61141-72-8 | 4,6-Dimethyl-dodecane | 7.38 × 10+02 | Predicted | 2.178 | Kerosene fuel | Head and neck cancer | 63 | ||
| 17302-37-3 | 2,2-Dimethyl-decane | 1.18 × 10+02 | Predicted | 1.316 | Head and neck cancer | 63 | |||
| 473-19-8 | 2,2,3-Trimethyl-exobicyclo[2.2.1]heptane | 1.25 × 10+02 | Predicted | 2.296 | Head and neck cancer | 63 | |||
| 562-49-2 | 3,3-Dimethyl-pentane | 1.20 × 10+00 | Predicted | 1.786 | Breast cancer | 68 | |||
| 62185-53-9 | 5-(2-Methylpropyl)-nonane | 2.86 ×10+02 | Predicted | 1.684 | Breast cancer | 68 | |||
| 62238-15-7 | 2,3,4-Trimethyl-decane | 2.99 × 10+02 | Predicted | 1.755 | Breast cancer | 68 | |||
| 589-34-4 | 3-Methyl-hexane | 1.30 × 10+00 | Measured253 | 2.329 | 253 | Unnatural, environmental contaminant | Head and neck cancer | 63 | |
| 2213-23-2 | 2,4-Dimethylheptane | 7.55 × 10+00 | Predicted | 1.492 | High chance of mis-assignment of isomer, petrol | Head and neck cancer, lung cancer | 4,63 | ||
| 3221-61-2 | 2-Methyl-octane | 3.31 × 10+00 | Measured for rat blood254 | 2.040 | 254 | Breast cancer | 74 | ||
| 2216-34-4 | 4-Methyl-octane | 8.21 × 10+00 | Predicted | 1.284 | Contaminant from plastics, tubing, medical equipment | Head and neck cancer, lung cancer | 4,63 | ||
| 54166-32-4 | 2,6,6-Trimethyl-octane | 4.64 × 10+01 | Predicted | 1.593 | Head and neck cancer | 63 | |||
| 5911-04-6 | 3-Methyl-nonane | 5.76 × 10+00 | Measured for rat blood254 | 2.079 | 254 | Natural or petrol | Head and neck cancer | 63 | |
| 16747-26-5 | 2,2,4-Trimethylhexane | 7.08 × 10+00 | Predicted | 1.426 | Petrol | Lung cancer | 202 | ||
| 25117-31-1 | 5-Methyl-tridecane | 7.67 × 10+02 | Predicted | 2.559 | Breast cancer | 74 | |||
| 1002-43-3 | 3-Methyl-undecane | 1.27 × 10+02 | Predicted | 1.726 | Breast cancer | 74 | |||
| 10105-38-1 | 6-Methyl-pentadecane | 4.86 × 10+03 | Predicted | 3.689 | Breast cancer | 74 | |||
| 6418-45-7 | 3-Methyl-nonadecane | 2.13 × 10+05 | Predicted | 6.830 | Breast cancer | 74 | |||
| 6117-97-1 | 4-Methyl-dodecane | 3.08 × 10+02 | Predicted | 2.176 | Breast cancer | 74 | |||
| 763-29-1 | 2-Methyl-1-pentene | 1.41 × 10+00 | Predicted | 1.783 | Contaminant from plastics, tubing, medical equipment | Lung cancer | 202 | ||
| 2847-72-5 | Decane, 4-methyl- | 5.04 × 10+01 | Predicted | 2.038 | Lung cancer | 71 | |||
| 764-13-6 | 2,4-Hexadiene, 2,5-dimethyl- | 1.64 × 10+00 | Predicted | 2.284 | Lung cancer | 71 | |||
| 1515-79-3 | 5,5-Dimethyl-1,3-hexadiene | 1.13 × 10+00 | Predicted | 2.281 | Lung cancer | 72 | |||
| Primary and secondary alcohols | 64-17-5 | Ethanol | 1.50 × 10+03 | Measured252 | −0.823 | 255 | Natural, diet, disinfectants, intestinal bacterial flora | Liver cancer | 50 |
| 71-23-8 | 1-Propanol | 1.03 × 10+03 | Measured120 | −0.532 | 255 | Natural, disinfectants | Lung cancer | 4,71 | |
| 67-63-0 | 2-Propanol | 8.30 × 10+02 | Measured252 | −0.634 | 255 | Natural, disinfectants | Lung and breast cancer | 72,75 | |
| 71-36-3 | 1-Butanol | 9.33 × 10+02 | Measured252 | −0.095 | 120 | Natural, diet | Lung cancer | 249 | |
| 104-76-7 | 2-Ethyl-1-hexanol | 1.31 × 10+03 | Predicted | 2.156 | Contaminant from tubing material | Lung cancer | 4 | ||
| 3391-86-4 | 1-Octen-3-ol | 1.13 × 10+03 | Predicted | 2.089 | Natural (produced in plants and fungi) | Liver cancer | 56 | ||
| 625-31-0 | 4-Penten-2-ol | 1.39 × 10+03 | Predicted | 1.006 | Lung cancer | 72 | |||
| Aldehydes and branched aldehydes | 123-38-6 | Propanal | 1.77 × 10+02 | Predicted | 0.418 | Natural or industrial waste product | Lung cancer | 4 | |
| 123-72-8 | Butanal | 1.27 × 10+02 | Predicted | 0.894 | Natural or industrial waste product, diet | Lung cancer | 4 | ||
| 110-62-3 | Pentanal | 8.85 × 10+01 | Predicted | 1.361 | Natural, diet | Lung cancer | 4,133 | ||
| 66-25-1 | Hexanal | 8.21 × 10+01 | Predicted | 1.769 | Natural, diet | Lung cancer, liver cancer | 4,56,133 | ||
| 111-71-7 | Heptanal | 8.87 × 10+01 | Predicted | 2.058 | Natural or industrial waste product, diet, | Lung cancer, breast cancer | 4,75 | ||
| 124-13-0 | Octanal | 1.26 × 10+02 | Predicted | 2.209 | Natural or industrial waste product diet | Lung cancer | 4,133 | ||
| 124-19-6 | Nonanal | 1.63 × 10+02 | Predicted | 2.268 | Possibly natural | Lung cancer | 4,133 | ||
| 98-01-1 | Furfural||furaldehyde | 3.05 × 10+03 | Predicted | 0.705 | Natural or industrial waste product | Gastric cancer | 15 | ||
| Carboxylic acids | 75-98-9 | 2,2-Dimethyl-propanoic acid | 3.82 × 10+03 | Predicted | 0.930 | Head and neck cancer | 63 | ||
| 64-19-7 | Acetic acid | 7.96 × 10+04 | Predicted | −0.190 | Natural or industrial waste product | Liver cancer | 50 | ||
| Ketones | 67-64-1 | Acetone | 3.40 × 10+02 | Measured137 | −0.475 | 255 | Fatty acids metabolism | Lung cancer | 4 |
| 78-93-3 | 2-Butanone | 1.64 × 10+02 | Measured120 | −0.004 | 255 | Diet, environmental contaminant | Lung cancer | 4 | |
| 107-87-9 | 2-Pentanone | 1.50 × 10+02 | Measured256 | 0.206 | 120 | Natural, diet | Lung cancer | 4 | |
| 591-78-6 | 2-Hexanone | 1.68 × 10+02 | Measured120 | 0.356 | Industrial waste product | Lung cancer | 202 | ||
| 106-35-4 | 3-Heptanone | 1.87 × 10+02 | Predicted | 1.813 | Natural, drugs | Lung cancer | 202 | ||
| 623-56-3 | 5-Methyl-3-hexanone | 9.15 × 10+01 | Predicted | 1.702 | Head and neck cancer | 63 | |||
| 7379-12-6 | 2-Methyl-3-hexanone | 8.28 × 10+01 | Predicted | 1.702 | Lung cancer | 72 | |||
| 110-93-0 | 6-Methyl-5-hepten-2-one | 1.92 × 10+02 | Predicted | 1.779 | Squalene oxidation | Gastric cancer | 15 | ||
| 513-86-0 | 3-Hydroxy-2-butanone | 1.02 × 10+03 | Predicted | −0.173 | Lung cancer | 249 | |||
| 119-61-9 | Benzophenone | 4.14 × 10+04 | Predicted | 2.247 | Industrial waste product (used as fragrance in soaps, in pharmaceuticals and ultraviolet absorbers – sunscreen) | Lung cancer | 72 | ||
| 3848-24-6 | 2,3-Hexanedione | 3.42 × 10+04 | Predicted | −0.189 | Lung cancer | 72 | |||
| 565-80-0 | 3-Pentanone, 2,4-dimethyl- | 4.27 × 10+01 | Predicted | 1.582 | Lung cancer | 71 | |||
| Aromatic compounds | 71-43-2 | Benzene | 8.80 × 10+00 | Measured257 | 1.598 | 257 | Petrol, smoking | Lung cancer | 4 |
| 108-88-3 | Toluene | 1.39 × 10+01 | Measured257 | 2.180 | 257 | Petrol, smoking | Lung cancer | 4 | |
| 100-42-5 | Styrene | 5.56 × 10+01 | Measured120 | 1.758 | 120 | Natural, smoking | Lung cancer | 4 | |
| 625-86-5 | 2,5-Dimethylfuran | 2.58 × 10+00 | Predicted | 1.695 | Smoking | Lung cancer | 4,71 | ||
| 106-42-3 | p-Xylene | 3.89 × 10+01 | Measured120 | 0.709 | 257 | Petrol, smoking | Head and neck cancer, prostate cancer | 63,68 | |
| 496-16-2 | 2,3-Dihydro-benzofuran | 8.71 × 10+01 | Predicted | 1.976 | Liver cancer | 50 | |||
| 100-41-4 | Ethylbenzene | 2.82 × 10+01 | Measured120 | 1.796 | 120 | Petrol | Lung cancer | 78 | |
| 98-86-2 | 1-Phenyl-ethanone | 1.27 × 10+03 | Predicted | 1.573 | Breast cancer | 75 | |||
| Nitrils | 75-05-8 | Acetonitrile | 6.98 × 10+02 | Predicted | −0.198 | Smoking | Lung cancer | 4 | |
| 107-13-1 | 2-Propenenitrile | 1.41 × 10+02 | Predicted | 0.210 | Smoking and car exhaust | Gastric cancer | 15 | ||
| 93946-48-6 | 2-Amino-5-isopropyl-8-methyl-1-azulenecarbonitrile | 9.65 × 10+06 | Predicted | 2.294 | Breast cancer | 68,76 | |||
| Terpens and terpenoids | 138-86-3 | dl-Limonene | 6.21 × 10+01 | Predicted | 2.296 | Industrial waste (used in food flavorings and cosmetics) | Breast cancer | 76 | |
| 98-55-5 | p-Menth-1-en-8-ol | 2.63 × 10+03 | Predicted | 2.152 | Cosmetics | Lung cancer | 72 | ||
| 21368-68-3 | Camphor | 2.07 × 10+02 | Predicted | 1.873 | Natural | Lung cancer | 72 | ||
| Others | 110-27-0 | Isopropyl myristate | 5.75 × 10+04 | Predicted | 2.298 | Breast cancer | 75 | ||
| 124-63-0 | Methane-sulfonyl chloride | 2.65 × 10+02 | Predicted | 0.021 | Transamination pathways (incomplete metabolism of methionine) | Liver cancer | 50 | ||
| 631-61-8 | Ammonium acetate | Head and neck | 63 | ||||||
| 35242-43-4 | 2,3-Dihydro-1-phenyl-4(1H)-quinazolinone | 3.84 × 10+07 | Predicted | 2.260 | Breast cancer | 68,75 | |||
| 4282-42-2 | 1-Iodo-nonane | 3.72 × 10+02 | Predicted | 2.298 | Breast cancer | 68 | |||
| 24310-22-3 | 2-[(1,1-Dimethylethyl)thio]-acetic acid | 9.66 × 10+05 | Predicted | 1.767 | Colon cancer | 68 | |||
| 82406-83-5 | 4-(4-Propylcyclohexyl)-, 4′-cyano[1,1′-biphenyl]-4-yl ester benzoic acid | 1.77 × 10+11 | Predicted | 2.298 | Colon cancer | 68 | |||
| NIST 282650 | 2-Trifluoromethylbenzoic acid, 6-ethyl-3-octyl ester | 1.18 × 10+05 | Predicted | 2.298 | Breast cancer | 68 | |||
| 21064-19-7 | 1,5,9-Cyclododecatriene, 1,5,9-trimethyl- | 1.05 × 10+03 | Predicted | 2.298 | Lung cancer | 71 | |||
| 6846-50-0 | Pentan-1,3-dioldiisobutyrate, 2,2,4-trimethyl | 8.50 × 10+04 | Predicted | 2.293 | Plasticizer | Lung cancer | 71 | ||
| 23676-09-7 | Benzoic acid, 4-ethoxy-, ethyl ester | 2.07 × 10+04 | Predicted | 2.266 | Lung cancer | 71 | |||
| 74381-40-1 | Propanoic acid, 2-methyl-, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester | 7.68 × 10+04 | Predicted | 2.293 | Lung cancer | 71 | |||
| 494-19-9 | 10,11-Dihydro-5H-dibenz-[b,f]-azepine | 5.37 × 10+05 | Predicted | 2.286 | Lung cancer | 71 | |||
| 719-22-2 | 2,5-Cyclohexadiene-1,4-dione, 2,6-bis(1,1-dimethylethyl)- | 1.69 × 10+07 | Predicted | 2.283 | Lung cancer | 71 | |||
| 101-84-8 | Benzene, 1,1-oxybis- | 2.43 × 10+03 | Predicted | 2.292 | Lung cancer | 71 | |||
| 13049-35-9 | 1,1-Biphenyl, 2,2-diethyl- | 6.68 × 10+04 | Predicted | 2.298 | Lung cancer | 71 | |||
| 87-44-5 | trans-Caryophyllene | 1.81 × 10+02 | Predicted | 2.298 | Lung cancer | 71 | |||
| 3910-35-8 | 1H-Indene, 2,3-dihydro-1,1,3-trimethyl-3-phenyl- | 3.22 × 10+04 | Predicted | 2.298 | Lung cancer | 71 | |||
| 84-66-2 | 1,2-Benzenedicarboxylic acid, diethyl ester | 3.85 × 10+04 | Predicted | 2.154 | Plasticizer | Lung cancer | 71 | ||
| 76-13-1 | Ethane, 1,1,2-trichloro-1,2,2-trifluoro- | 2.40 × 10−01 | Predicted | 2.245 | Lung cancer | 72 | |||
| 1634-04-4 | Propane, 2-methoxy-2-methyl- | 1.59 × 10+01 | Predicted | 0.728 | 258 | Gasoline | Lung cancer | 72 | |
| 42848-06-6 | 1-Propene, 1-(methylthio)-, (E)- | 8.18 × 10+00 | Predicted | 2.043 | Diet (onion, garlic) | Lung cancer | 72 | ||
| 824-22-6 | 1H-Indene, 2,3-dihydro-4-methyl- | 1.32 × 10+02 | Predicted | 2.280 | Lung cancer | 72 | |||
| 915392-37-9 | 5-Isopropenyl-2-methyl-7-oxabicyclo[4.1.0]heptan-2-ol | 5.97 × 10+05 | Predicted | 1.473 | Lung cancer | 72 | |||
| 127-51-5 | Isomethyl ionone | 1.95 × 10+03 | Predicted | 2.291 | Lung cancer | 72 | |||
| 710336-76-8 | 2,2,7,7-Tetramethyltricyclo-[6.2.1.0(1,6)]undec-4-en-3-one | 4.33 × 10+02 | Predicted | 2.274 | Lung cancer | 72 | |||
| 24238-73-1 | Bicyclo[3.2.2]nonane-1,5-dicarboxylic acid, 5-ethyl ester | 3.20 × 10+06 | Predicted | 1.868 | Lung cancer | 72 | |||
| 959016-51-4 | Pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester | 1.27 × 10+04 | Predicted | 2.295 | Lung cancer | 72 | |||
| 16204-36-7 | 1,2,4,5-Tetroxane, 3,3,6,6-tetraphenyl- | 9.14 × 10+08 | Predicted | 2.295 | Lung cancer | 72 | |||
| 6738-27-8 | 2,5-Cyclohexadien-1-one, 2,6-bis(1,1-dimethylethyl)-4-ethylidene- | 3.38 × 10+03 | Predicted | 2.292 | Lung cancer | 72 | |||
| 55162-49-7 | Furan, 2-[(2-ethoxy-3,4-dimethyl-2-cyclohexen-1-ylidene)methyl]- | 3.47 ’ 10+03 | Predicted | 2.297 | Lung cancer | 72 | |||
| 7694-30-6 | Benzene, 1,1-(1,2-cyclobutanediyl)bis, cis- | 2.85 × 10+04 | Predicted | 2.296 | Lung cancer | 72 | |||
| 53699-80-2 | Benzene, 1,1-[1-(ethylthio)propylidene]bis- | 1.09 × 10+05 | Predicted | 2.295 | Lung cancer | 72 | |||
| 101580-33-0 | Anthracene, 1,2,3,4-tetrahydro-9-propyl- | 2.45 × 10+05 | Predicted | 2.298 | Lung cancer | 72 | |||
| 839-73-6 | 9,10-Anthracenediol, 2-ethyl- | 2.79 × 10+11 | Predicted | 2.284 | Lung cancer | 72 | |||
| 10224-91-6 | Benzene, 1,1-ethylidenebis, 4-ethyl- | 6.30 × 10+04 | Predicted | 2.298 | Lung cancer | 72 |
Based on the data presented in Table 1, the equilibrium concentrations in blood and fat are estimated (see Fig. 3 and Section 2.3), while waiving several normalization and standardization gaps that are present among the various published studies. Regarding the rest of these normalization and standardization gaps, we mention:
-
(a)
variances and inconsistencies in the control groups of the clinical trials (healthy smokers, healthy non-smokers, age-matched groups, hospital personnel, relatives of the patients, etc.);
-
(b)
differences in the instrumentations used for the identification of disease-related VOCs (e.g., GC-MS,65,73 PTR-MS46,81);
-
(c)
uncertainties in the identification of the VOCs, even though qualitative analysis by retention time and spectral library match is quite reliable in the case of GC-MS;42,43,45
-
(d)
differences in the sampling procedures (e.g., collection of mixed expiratory breath,81 CO2-controlled sampling of end-tidal breath,65,130,131 sampling with Tedlar or Mylar bags,81,132 portable breath collection apparatus (BCA),73 etc.);
-
(e)
differences in the pre-concentration procedures used in conjugation with the mass spectrometry technique (e.g., solid phase micro-extraction (SPME) fibers,58,59 and thermal desorption units with cryo-focusing77);
-
(f)
differences in the normalization procedures (e.g., data normalization according to a specific VOC concentration in the examined sample59,81 or based on the difference in the examined sample and the inhaled air VOC concentrations);72,73,77
-
(g)
differences in the data analysis procedure (e.g., peak identification and integration in the chromatograms of each sample,42–45 comparison among the quantitative data from different study groups,71,81,133 statistical analysis using regression and pattern recognition algorithms); and
-
(h)
differences in the applied calibration standards between the groups involved in breath cancer studies; indeed, approximately 50% of the published breath-related studies still present qualitative data on potential VOC breath bio-markers for a variety of diseases, but with no quantification of their concentration levels.
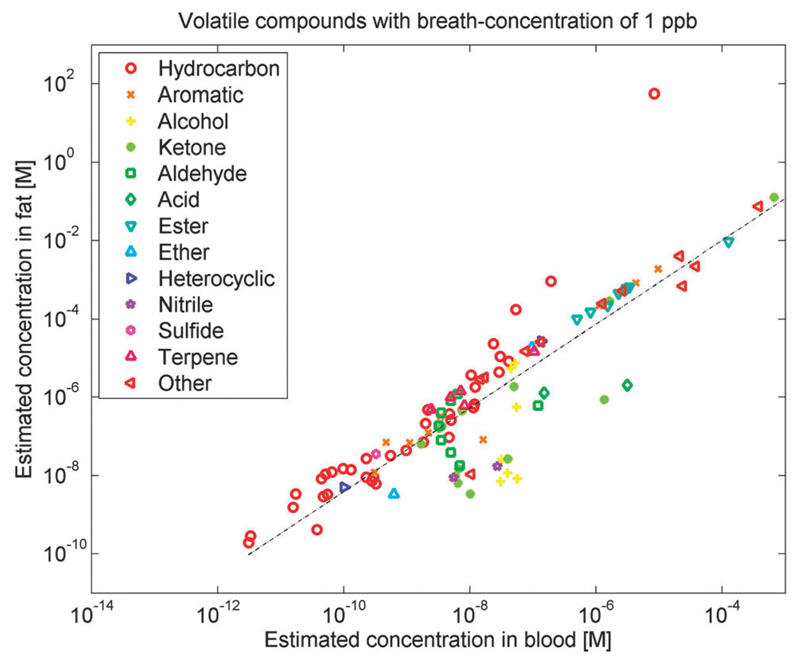
Fig. 3.
Estimated equilibrium concentrations in blood and fat for candidates of volatile cancer biomarkers published during the past decade.4,15,50,56,63,68,71,72,74–76,78,133,202,248,249 These equilibrium concentrations have been estimated under the assumption that the concentration in alveolar breath is 1 part-per-billion (ppb), based on the λb:a (partition coefficient between blood and air) and λf:a (partition coefficient between fat and blood) from Table 1. Hence, for different VOCs showing the same concentration in exhaled breath, the concentration in fat and blood may be very different (up to a factor of 108). Different VOCs, therefore, carry distinctive information on the various compartments of the human body. In the figure, various chemical classes of compounds (such as hydrocarbons or sulfides) are indicated by different symbols and colors.
Considering these variations, the current efforts might not provide precise or definite answers to the puzzling pathophysiological pathways of some cancer VOCs. Nevertheless, this effort would help stimulating constructive discussions and new ideas.
2.3. VOC exchange into the breath
The principle behind breath cancer detection is that cancer-related VOCs in the (fat) tissue are emitted to the blood and that the VOC blood chemistry is reflected in measurable changes in the breath through exchange via the lungs.134 It is found that some gases exchange in the airways, rather than in the alveoli, depending on the λb:a. Theoretical and experimental studies have shown that gases with low solubility in blood, mainly nonpolar VOCs (λb:a < 10; λb:a in dimensionless units [mol Lb−1/mol La−1]), exchange almost exclusively in the alveoli, while VOCs that are well soluble in blood, e.g., polar VOCs (λb:a > 100), tend to exchange also in the airways.113,121,127,128 Further studies predicting the location of the pulmonary gas exchange have shown that VOCs with 10 < λb:a < 100 interact significantly both with the airways and with the alveoli.113 An important conclusion of these studies is that the airways play a more significant role in pulmonary gas exchange than previously assumed.127,128 Hence, the implications of pulmonary tests and breath tests might have to be re-evaluated.113 The VOC profile is also influenced by the retention of VOCs in the lungs, viz. the fraction of the molecules that remains in the respiratory tract at any time, after inhalation and exhalation, because of the λb:a.135 Thus, the final partition and exhalation of the VOCs depends on their physical and chemical properties, and on their interaction with the different alveolar clearance processes.135,136
We illustrate the blood–breath concentration relations using the examples of isoprene and acetone. Isoprene is more volatile and less soluble in blood, compared to acetone. This is expressed in that the λb:a value for isoprene (~0.95114) is smaller than for acetone (~340137). Nevertheless, acetone has been reported to appear in noticeably higher concentrations in the breath, compared to isoprene. This difference is attributed to the fact that the concentration of acetone in the blood is generally more than three orders of magnitude higher than that of isoprene. This result might reflect the absence of direct out-gassing of marker VOCs into the airways, leading to low expression of the high boiling point (BP) VOCs that are not “picked up” in the breath analysis.138
In addition to λb:a, the λf:b is a very important quantity. Together, these two physicochemical partition constants determine the equilibrium concentration of a given compound between breath, blood and fat. Most of the proposed cancer biomarkers are lipophilic, and, hence, can be expected to be stored in the fat compartment. For lipophilic compounds, a low concentration in exhaled breath (like ~1 ppb) can be associated with a relatively high concentration in the fat compartment.
For many VOCs, λb:a and λf:b are unknown.114,115 They may, however, be estimated based on the water:air partition coefficient (λw;a) and the octanol:water partition coefficient (λo:w) using the method of Poulin and Krishnan.139 If the λb:a are not available in the literature, we estimate them by different methods. For alkanes, methylated alkanes and 1-alkenes, we use data from ref. 115 to estimate λb:a by regression based on the number of carbon atoms, the BP and the molecular weights. For other VOCs, we use the estimate by Poulin and Krishnan139 given by the formula
| (1) |
Here, a ≈ 0.0033 is the fraction of neutral lipids in blood, b ≈ 0.0024 is the fraction of phospholipids in blood, and c ≈ 0.82 is the fraction of water in blood. The λo:w values are taken from Scifinder (https://scifinder.cas.org). The λw:a (Henry constants) at 25 °C are taken from the compilation of Sander,140 estimated using the EPI Suite™ software developed by the US Environmental Protection Agency (EPA, http://www.epa.gov/opptintr/exposure/pubs/episuitedl.htm). Alternatively, they are estimated by use of surrogate compounds, for which λw:a is known, with correction by the quotient of the respective vapor pressures (of the compound in question and its surrogate compound). To estimate the Henry constants at 37 °C, we use the derivative dln(λw:a)/d(1/T) as given in the compilation by Sander,140 or the corresponding enthalpy of vaporization (ΔHvap) divided by the gas constant, R. This is the standard procedure recommended by the US Environmental Protection Agency (EPA),141 for compounds whose data on temperature-dependence of the Henry constant are not accessible in the literature.141 The λf:b values are computed from the λb:a and the λf:a using λf:b=λf:a/λb:a. If the λf:a is not available in the literature, we use the estimate by Poulin and Krishnan139 given by the equation
| (2) |
Here, A ≈ 0.798 is the fraction of neutral lipids in adipose tissue (fat), B ≈ 0.002 is the fraction of phospholipids in adipose tissue, and C ≈ 0.15 is the fraction of water in adipose tissue.
Different VOCs carry different information about the various compartments of the human body. In particular, the storage capacity in the human body is quite different for various VOCs. Also, the time necessary to deplete storage for a certain compound is very different. Fig. 3 illustrates that different VOCs with the same concentration in exhaled breath may show very different concentrations in fat and blood (up to a factor of 108). In Fig. 3, the respective estimated concentrations in blood and fat are shown under the assumption that the concentration in breath is 1 ppb. The figure shows that different VOCs in blood or in the fat compartment might have very different concentrations, even though their concentration in exhaled breath is identical. Ethane, for example, with 1 ppb in exhaled breath is estimated to appear in blood at a concentration of 3.3 × 10−12 M and in fat at a concentration of 2.9 × 10−10 M. Tridecane, on the other hand, with 1 ppb in exhaled breath is estimated to appear in blood at a concentration of 1.0 × 10−8 M and in fat at a concentration of 3.3 × 10−6 M. Therefore, ethane and tridecane behave very differently in blood and fat, even when their concentrations in exhaled breath are identical (1 ppb).
The concentration information in Fig. 3 is most interesting, but rather limited. Much more detailed information than just partition coefficients and estimates of concentrations in blood and fat are available for some compounds like isoprene, which is the hydrocarbon displaying the highest concentration in exhaled breath. This more detailed information has been elucidated through real-time measurements of exhaled breath during exertion of an effort, and contains, in particular, the concentrations or amounts of isoprene in the periphery of the human body (e.g., the muscles of the limbs). The isoprene stores in the body can be depleted by exertion of an effort, e.g., on a stationary bicycle.121,124 After about 45 min of cycling, a large part of the stored isoprene is exhaled and it takes about 1–2 h to re-synthesize isoprene in the body and fill up the stores. Similar information is available for acetone and, in part, for 2-pentanone. We expect similarly interesting effects for the other compounds presented in Table 1, with the λb:a and λf:b playing a central role.
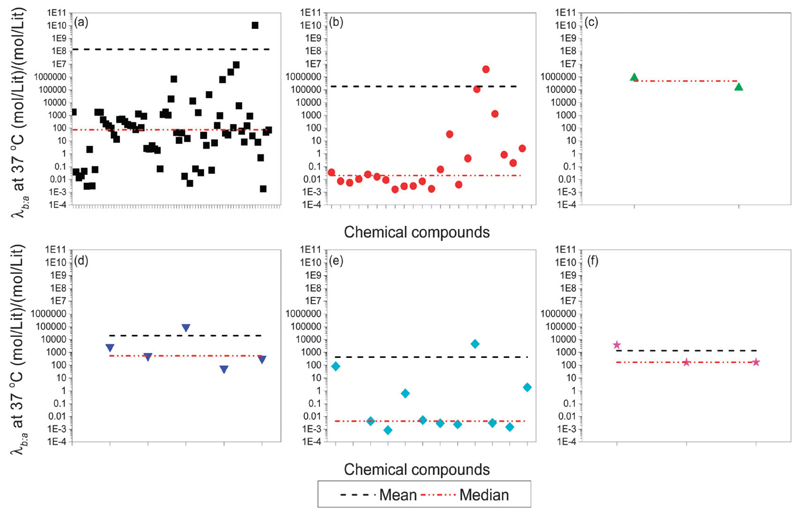
When examining the variation of the VOCs’ λb:a according to a specific cancer type, some connections are revealed (Fig. 4). The results show that lung cancer, gastric cancer and liver cancer have rather similar values as seen from the median line, while breast cancer and head and neck cancer are similar. Colon cancer is different from the rest (see Fig. 4). While no obvious reason currently explains this difference, we can hypothesize that metabolic processes in cancer development and compound storage in tissue might be similar in these cancer types. In some of the cancers, a few VOCs are “outliers” with respect to the λb:a within the general trend of the group. In breast cancer, three VOCs present a high λb:a opposed to the rest: 2-amino-5-isopropyl-8-methyl-1-azulenecarbonitrile, which can be found in fragrances, 2,3-dihydro-1-phenyl-4(1H)-quinazolinone, which has been suggested as a cholecystokinin (CCK) antagonist,142 and 1-phenyl-ethanone (acetophenon), which can be found in fragrances, chewing gums, cigarettes and as an excipient. In head and neck cancer, two VOCs presented a high λb:a: 5-methyl-3-hexanone – a VOC that is found in human body fluids and feces143 – and 2,2-dimethyl-propanoic acid, which is an odiferous compound that exists in liquid phase at body temperature. Such “outliers”, if confirmed and validated for a particular disease, could be particularly interesting due to very different concentration levels in blood, fat and breath, in comparison to other biomarkers of the disease.
Fig. 4.
λb:a as a function of the VOCs from different types of cancer: (a) lung cancer; (b) breast cancer; (c) colon cance ; (d) liver cancer; (e) head and neck cancer; (f) gastric cancer. Data show that lung cancer, gastric cancer and liver cancer have rather similar values as can be seen from the median line. On the other hand, breast cancer and head and neck cancer are similar and colon cancer is different from the rest.
2.4. The biochemical pathway of cancer VOCs
Different VOCs carry different information about the various compartments of the human body. In particular, the storage capacity of the human body is quite distinctive for different volatile compounds. Also the time necessary to deplete stores for a certain compound varies. With this in mind, we utilize the data and discussion presented in the previous sections to provide further insight into the biochemical pathways of the various chemical families of cancer VOCs, viz., hydrocarbons (alkanes, branched-chain alkanes and branched-chain alkenes), primary and secondary alcohols, aldehydes and branched aldehydes, ketones, esters, nitriles, and aromatic compounds.
2.4.1. Hydrocarbons
The key mechanism that relates to hydrocarbon production in the body is oxidative stress (see Section 2.1). Alkanes are mainly produced by peroxidation of PUFA, found mainly in cellular and subcellular membranes (lipid peroxidation). Lipid peroxidation is responsible for damage of tissues in vivo. It may be a cause of cancer, inflammatory diseases, atherosclerosis, and aging. The human body tries to control and reduce lipid peroxidation by the use of anti-oxidants.4 Saturated hydrocarbons such as ethane and pentane are the end products of lipid peroxidation. Pentane and ethane in the breath have been extensively used as non-invasive in vivo indicators of lipid peroxidation.144 Although the occurrence of other saturated hydrocarbons (e.g., C3–C11) can be related to the lipid peroxidation process, in the case of branched hydrocarbons, this mechanism seems to be irrelevant. Due to their low solubility in the blood, hydrocarbons that are not metabolized in the body are emitted into the breath within minutes.145,146
2.4.2. Alcohols
Alcohols can be absorbed from all parts of the gastrointestinal tract mainly by diffusion into the blood. Alcohols can as well be a product of hydrocarbon metabolism. Short-chain alcohols are absorbed rapidly in the blood due to their high affinity to water. Alcohol metabolism is prone to be affected by confounding factors in the body, mostly the changes in water and fat content among different people and genders.4 Possibly, enzymes such as alcohol dehydrogenase (ADH) and cytochrome p450 (CYP2E1, which predominantly works in the liver) are responsible for alcohol metabolism in the body. ADH can catalyze the oxidation of several different alcohols in humans; remaining VOCs are removed through the excretion of alcohol in breath, urine, sweat, feces, breast milk and saliva.4
2.4.3. Aldehydes
Aldehydes are produced in the body as part of common physiological processes. Some of the aldehydes are essential for functional processes. Others are thought to be cytotoxic intermediates with several functions, such as signal transduction, gene regulation and cellular proliferation.147,148 There are a number of sources of aldehydes in the body. The first source relates to metabolized alcohols. The second source of aldehydes in the body relates to the reduction of hydroperoxide by cytochrome p450 as a secondary product of lipid peroxidation.149 The third source for the aldehydes in the body relates to smoking. Saturated and unsaturated aldehydes such as formaldehyde, acetaldehyde, and acrolein are found in tobacco smoke.150 The fourth source for the aldehydes in the body is the detoxification process by cytochrome p450 as a result of the by-product of tobacco metabolism.151,152 Finally, aldehydes can also originate from dietary sources.153,154
2.4.4. Ketones
During cancer progression, an increase in the rate of fatty acid oxidation due to changes in metabolic conditions results in the formation of ketone bodies including acetone. Such compounds are also related to weight loss, which is, in turn, one of the symptoms of cancer.155 Acetoacetate and b-hydroxybutyrate are synthesized in the liver in significant quantities, followed by spontaneous decarboxylation of acetoacetate to yield acetone. Of the ketone bodies, acetone is produced in smaller quantities, and due to its high vapor pressure it can be secreted through the breath, urine and skin. Protein metabolism can result as well in ketone bodies. In the state of cachexia, typical in diseased conditions such as cancer, protein metabolism increases resulting in higher levels of ketone bodies.155 However, acetone is not suitable to be used as a cancer biomarker as its concentration levels in the breath are altered due to exercising, fasting and/or food consumption.156,157 Finally, other exogenous sources like food or chemical industries can result in ketone production that could eventually be absorbed in the body.15,34
2.4.5. Esters
This group of compounds can be found in natural fats and fatty oils, natural wax and fruit essential oils in large amounts. In humans, esterases hydrolyze esters into alcohol and acid at temperatures below 40 °C.158 One example of such an enzyme is lipase which catalyzes lipid hydrolysis as part of the natural metabolism in the body.
2.4.6. Nitriles and aromatic compounds
Nitriles and aromatic VOCs are usually considered to be pollutants of exogenous sources. Such sources include exposure to cigarette smoke, alcohol, pollution and radiation. While such compounds are most likely to be of exogenous origin, they could be of interest for cancer patient follow-up, since some are known to be carcinogens.4 These molecules are highly reactive, resulting in peroxidative damage to PUFA, proteins, and DNA. Such damage accumulates during a lifetime, while the natural fixing mechanisms in the body become less efficient, leading to age-dependent diseases such as cancer.110 These compounds are stored in the fatty tissues of the body; thus it is likely that cancer patients, previously exposed to continuous occupational pollutants or excessive smoking, could slowly release them in high concentrations through the exhaled breath. In addition, mechanical, cellular, and enzymatic defense mechanisms act to eliminate hazardous chemicals and xenobiotics by a two phase process, resulting in a more soluble and excretable form of molecule.4,159 One such compound is acetonitrile which is found in smokers. The pathway suggested for acetonitrile is the bio-transformation to cyanohydrin by cytochrome P450 monooxygenase, which spontaneously breaks down to hydrogen cyanide and formaldehyde. Because of the rather slow metabolism of acetonitrile in the body, substantial amounts of acetonitrile can be emitted as-is through exhaled breath and/or urine.4,160
2.5. Challenges and future directions for better understanding of cancer VOC biochemical pathways
In the following, we present a few proposals to improve the understanding of the metabolic pathways that generate potential cancer VOCs as well as to VOC production/consumption in the body:
Many metabolic pathways such as glycolysis, apoptosis, loss of tumor suppressor genes, and angiogenesis are activated or over-activated in the case of cancer.161 These pathways may alter the production of VOCs in the body. To identify the exact change in the VOC pattern, we propose blocking such metabolic processes in various cell lines, each in a separate assay. This could be achieved by deactivating the specific enzyme (e.g., hexokinase, pyruvate kinase dehydrogenase or matrix metallo-proteases) that initiates or is crucial to the process, in order to compare between the measured VOC profiles before and after the blocking. According to the specific blocking, the cancer VOCs can be associated with the different mechanisms occurring in the same cancer cell.
The hypothesis that certain VOCs are associated with the cell metabolism per se, rather than with the microenvironment of the cancer or other indirect metabolic pathways in the body (human or animal), needs to be confirmed through direct observation. This hypothesis could be resolved by using cell lines from well-documented sources,42–45 so that they can be directly correlated to metabolic pathways without any confounding factors. In this context, using a variety of different cell lines, rather than replicas of the same cell line, could be helpful to simulate the natural diversity of cancer while eliminating potential confounding effects that are associated with clinical samples.
Many cancer VOCs are related to non-cancer sources, such as environmental and tobacco compounds. Following inhalation, these molecules might affect the respiratory system, and later on, also the blood composition. In this case, the lipophilic species are stored in the fat compartment, with subsequent comparatively slow release through exhalation. Therefore, it is important to examine the effect on the blood and the fat compartment of inhaling these molecules, as well as examining the breath VOC profile. Using an animal model, such compounds could be introduced via inhalation or they could be directly introduced into the bloodstream to monitor the resulting breath VOC profile of the treated animals. In addition, oxidative stress could be determined through measuring the amount of glucose and the activity of G-6 PD. A comparison between the animal model and the introduction of the same molecules in vitro to cancer cells would allow gaining a detailed understanding of how these VOCs affect the body both at a cellular level and as a whole.
It is hypothesized that a malignant tumor is a “free organ” having its own cancer stem cells (CSC). These cells present a chemotherapy-resistant population capable of self-renewal. Stem cells have high levels of ALDH activity, yet ALDH activity varies among different cells. A focused study on CSC, both in vitro and in vivo, might reveal variances in the patterns of VOCs that are released as a response to ALDH activity. This study could serve as a launching platform for developing a CSC (and/or ALDH activity) biomarker, namely a single VOC or a VOC pattern that could be indicative of recurring tumor initiation or metastasis initiation, thus aiding the prediction of patient prognosis, and tailoring personalized treatments.
These proposals can be implemented by means of mass-spectrometry techniques. A very promising approach in this endeavor is real-time analysis of exhaled breath by direct mass-spectrometric methods, such as proton-transfer-reaction mass spectrometry (PTR-MS),121–129,162,163 proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF-MS),164–171 or selected ion flow tube mass spectrometry (SIFT-MS).88,172–176 With these real-time techniques, exhaled breath is directly analyzed by mass spectrometry, without any need for sample storage or preconcentration. These techniques can even be carried out with breath-to-breath resolution. The mere possibility of real-time analysis (e.g., when exerting an effort on a stationary bicycle or during sleep123,162) is a decisive advantage compared to investigations of blood samples. It allows detecting very fast processes, such as a quick release of isoprene during physical effort.124–126,163
Undoubtedly, conventional and real-time mass-spectrometry techniques are powerful tools that can provide qualitative and quantitative information on the cancer VOCs and, subsequently, enable extracting important information on the biochemical pathways of the release of cancer VOCs. However, to date, the use of these techniques has been impeded by the need for expensive equipment, high levels of expertise required to operate such instruments, low speed required for sampling and analysis, and the need for preconcentration techniques. For cancer VOC testing to become a clinical reality, the advances in the knowledge of spectrometry-based specific cancer VOCs have to be translated to sensor development.
3. Sensors for testing cancer VOCs
Sensor matrices are likely to become a clinical and laboratory diagnostic tool, because they are significantly smaller, easier to use, and less expensive. An ideal chemical sensor for VOC analysis should be sensitive at very low VOC concentrations in the presence of water vapour, because the headspace of clinical samples is fully humidified. Furthermore, it should respond rapidly and differently to small changes in concentration, and provide a consistent output that is specific to a given exposure. When not in contact with the VOC, the sensor should return to its baseline state rapidly, or be simple and inexpensive enough to enable manufacturing large numbers of disposable units.
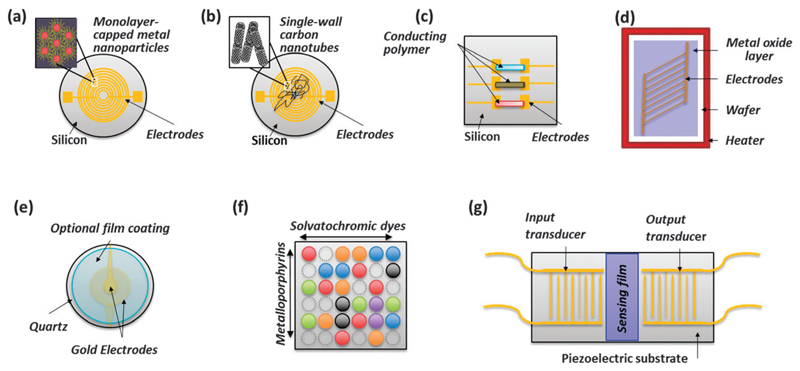
Sorption-based sensors are a candidate for low-power, compact chemical vapor detection for breath analysis. Such sensors combine a (semi-)selective transducer with chemo-selective materials that serve as a vapor concentrator, resulting in a highly sensitive detector that responds selectively to a particular class of chemical vapors. Among the choice of transducers are chemiresistors that monitor the resistance of polymers laced with conductive nanomaterials (see Fig. 6a and b) or conducting polymers (see Fig. 6c); chemiresistors or chemicapacitors based on metal oxide films that monitor changes of either resistance or dielectric properties (see Fig. 6d); mechanical oscillators and surface acoustic wave devices that respond to changes in mass (see Fig. 6e and g); and colorimetric sensors that monitor changes in optics (see Fig. 6f). Among these transducers, chemicapacitors and chemiresistors are best suited for low-power sensor arrays. Chemiresistors are simple to implement, but instability of the conductive particle/polymer interface can be a disadvantage. Chemicapacitors are more stable, but can take minutes to respond and recover. This slow response is limited by the time required to load and then remove the VOC from the relatively thick layers of chemo-selective dielectric (~1 µm) that are typically used.
Fig. 6.
Schematic illustration of different nanomaterial-based sensors: (a) chemiresistor based on monolayer-capped metal nanoparticles; (b) chemiresistor based on single-walled carbon nanotubes; (c) chemiresistor based on conducting polymers; (d) chemiresistor or chemicapacitor based on metal-oxide films; (e) quartz microbalance (QMB) with selective coating; (f) colorimetric sensor; and (g) surface acoustic wave (SAW) sensor. Reconstructed from ref. 39.
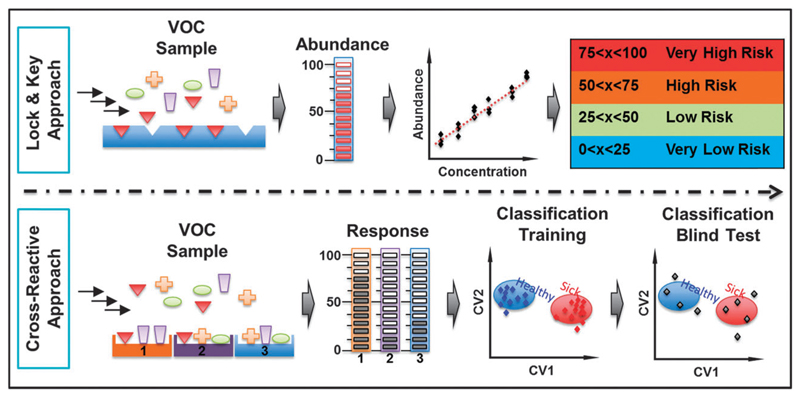
In this review, we consider two complementary approaches to profile cancer-related VOCs by sensor matrices. The first approach relies on sensors with selective recognition characteristics, which aim to detect one or a few specific VOCs. The second approach uses cross-reactive (i.e., semi-selective) sensors, which have a broad-spectrum of sensitivity to volatiles and gain their selectivity through pattern recognition.
3.1. Selective sensors for cancer VOCs
In the selective sensing concept, a highly selective receptor/detector is designed to specifically bind or detect the cancer VOC of interest.39 Sensor selectivity is defined here as higher sensitivity to a specific or mixture of gases/vapors in the presence of interfering gaseous species. This approach is suitable for detecting a well-defined target cancer VOC in the presence of interfering species and/or background (see Fig. 5, upper panel). In light of the difficulties to find distinctive cancer VOC(s), in the presence of controlled backgrounds and interferences, the development of selective sensors for cancer VOCs is still lagging. Additional limitation stems from the need to synthesize separate, highly selective nanomaterials for each VOC to be detected.177 Nevertheless, most currently available selective sensing techniques have aimed for non-volatile compounds.
Fig. 5.
Schematic illustration of the selective sensing approach versus the cross-reactive sensing approach. Reconstructed from ref. 39.
3.2. Cross-reactive sensors for cancer VOCs
An emerging strategy that is complementary to the selective sensing approach is the cross-reactive sensor array.39 Bio-inspired, this approach performs detection through use of an array of broadly cross-reactive sensors in conjunction with pattern recognition methods.39 In contrast to the selective sensing approach, each sensor in this architecture produces a distinct fingerprint from the array of broadly cross-reactive sensors. This allows considerably widening the variety of compounds to which a given matrix is sensitive, to increase the degree of component identification and, in specific cases, to perform an analysis of individual components in complex multi-component (bio)-chemical media.89 Pattern recognition algorithms can then be used to obtain information on the identity, properties and concentration of the vapor exposed to the sensor array (see Fig. 5, lower panel).39,178 Although such sensor arrays are mostly qualitative or semi-quantitative in nature, such methodologies are ideal for rapid disease screening as the results can be obtained in minutes.39,179 Fig. 6 illustrates the schematic representation of different sensors used. An overview of some of them, in the context of detection of cancer VOCs, is presented.
3.2.1. Nanomaterial-based sensors
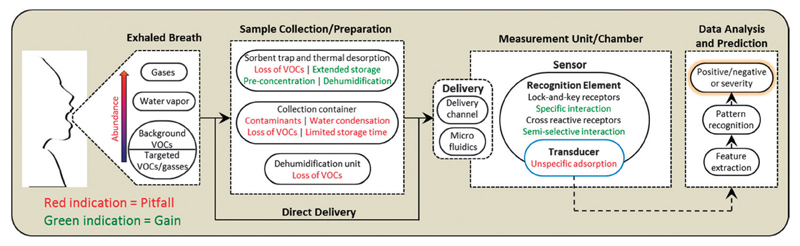
Distinct attention has been given in the past few years to approaches incorporating nanomaterial-based VOC/gas sensors (NMVSs). This is because NMVSs can lead to the development of sensitive, fast, and responsive diagnostic tools, though in relatively inexpensive manner.89 These advantages are the result of the nanoscale dimensions of the nanomaterials used, dimensions which provide them with superior physical, chemical, and optical properties, together with their high surface-to-volume ratio and low-priced fabrication. Thus, NMVSs allow high plasticity when fabricating sensors for breath analysis with the option to tailor them for specific disease related VOCs achieving high-level detection accuracy. Still, the choice of the breath analysis setup must take into consideration the potential restrictions of the applied sensor system, mainly because of potential gains and pitfalls in the NMVSs’ breath analysis methodology (see Fig. 7). Nanoparticles, nanowires and carbon nanotubes are examples for nanomaterials that have been exploited for VOC sensing.
Fig. 7.
Overview of the processes involved in breath testing: exhaled breath is a complex mixture of gases, water vapor, and thousands of VOCs in which only a small number of specific VOCs and gases comprise the clinically significant breath print. In order to perform the breath test, a sample is prepared from the complex mixture of exhaled breath by “trapping” the breath components on a sorbent material (followed by thermal desorption for their release), within a collection container (for example, a bag, vial, or canister), a dehumidification unit, or a channeling unit for direct delivery. The sample is then delivered to a measurement chamber through a simple delivery channel or a microfluidic system. In the measurement chamber, the breath components interact with the recognition element of the NMVS, inducing a measurable change (that is, electrical or optical) in the transducer that is translated into an output signal. Data analysis is then performed on the output signals in order to make the clinical prediction of the breath test. Reconstructed from ref. 138.
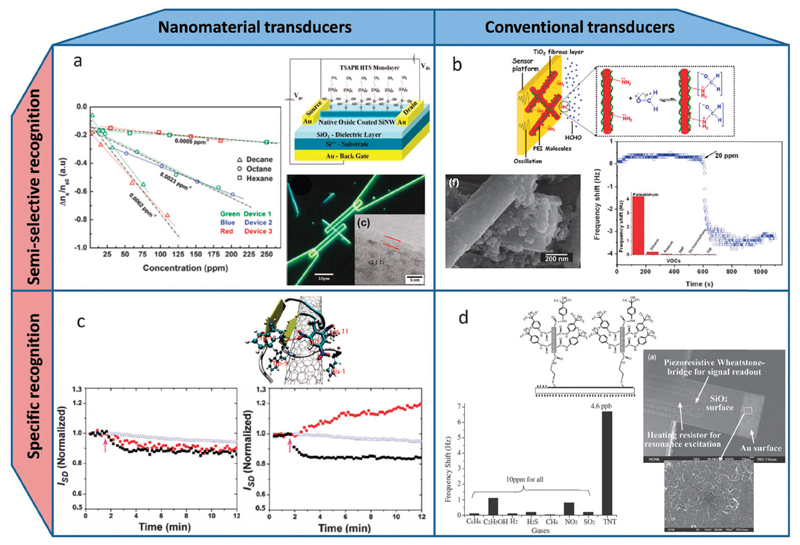
Nanomaterials combined with different molecular-sized organic functionalities has been used as sensitive transduction elements (see Fig. 8a and c).180 Examples of nanomaterial based transducers include field effect transistors (FETs) based on single-walled carbon nanotubes (CNTs)181,182 (see Fig. 8c) or nanowires (NWs) of various materials (see Fig. 8a),183–186 nanoelectromechanical oscillators,187–190 nanoporous chemi-optical materials,191,192 coaxial-chemicapacitors based on CNTs coated with nanoporous alumina193 and chemiresistors based on monolayer capped metal nanoparticle (MCNP) films,80,194,195 porous metal–oxide (cf. Fig. 6d),196 and random networks of single-walled CNTs183,197 or silicon NWs.198
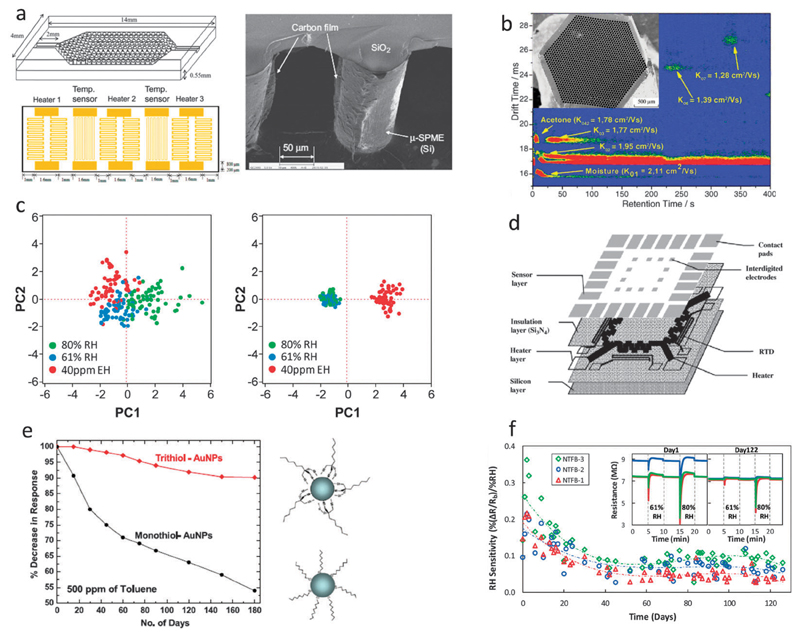
Fig. 8.
Nanomaterial-based VOC sensors can be divided into sensors based on nanomaterial transducers (left column, a and c) or conventional transducers (right column, b and d), with the recognition elements being either semi-selective (upper row, a and b) or specific (lower row, c and d), with the latter types typically more sensitive than the former. (a) Top right: schematic of a Si-NW FET configuration functionalized and passivated with an organic self-assembled monolayer of hexyltrichlorosilane. Bottom right: optical micrograph of a Si-NW FET with an inset showing a TEM image of a representative Si-NW. Left: semi-selectivity of the device shown by the relative surface-state density change (Δns/ns0) as extracted from three different devices exposed to three different nonpolar VOCs (hexane, octane, and decane) at increasing concentrations.185 (b) Top: schematic of a QCM oscillator coated with a sensing layer of polyethyleneimine functionalized TiO2 (PEI–TiO2) nanoporous fibers. Bottom left: SEM image of a representative PEI–TiO2 nanoporous fiber. Right: responses of QCM-based PEI–TiO2 sensors on exposure to 20 ppmv formaldehyde. The inset shows the frequency shift of the sensor versus 20 ppmv of various VOCs demonstrating the increased selectivity (semi-selectivity) of the sensor towards formaldehyde.250 (c) Top: a computational modeling predicting the specific binding of TNT to a peptide–CNT hybrid through a H-bond with Trp17 of the peptide and π–π interaction with the CNT surface (as part of a SWCNT-FET sensor for TNT vapor). Bottom left and right: response of a bare and peptide-coated (respectively) CNT-FET sensor to vapors of TNT (red circles), RDX (blue triangles), and HPT (black squares) showing the specific response to TNT. The arrow indicates when the vapor is introduced into the device.181 (d) Top: schematic of the surface modification of a gold-coated cantilever end with multi-walled CNTs functionalized with TNT-specific AHFP molecules. Right: SEM image of amicro-cantilever sensor immobilized with multi-walled CNTs. The inset shows a magnification of the random network of immobilized CNTs. Bottom left: response of a surface modified cantilever sensor, with HFIP functionalized multi-walled CNTs, to various interfering gases (all in about 10 ppmv concentration) in comparison with its response to 4.6 ppbv TNT vapor and demonstrating the specific response to TNT.251 Reconstructed from ref. 138.
The most common nanomaterial-based sensors are usually based on conductive inorganic nanomaterials (e.g., metal nanoparticles, single walled carbon nanotubes, carbon black) that are capped with or in organic functionality.39,69,70,179,197 In these films, the inorganic nanomaterials provide the electric conductivity and the organic film component provides sites for the sorption of VOCs (see Fig. 6a and b).89,199 On exposure, VOCs reach the sensing surface or diffuse into the sensing film and react with the capping ligands or the functional groups that cap the inorganic nanomaterials. As a result of the latter, a volume expansion/shrinkage in the nanomaterial film occurs.39,89 The connection between the inorganic nanomaterial blocks becomes lower/higher, and the conductivity decreases–increases.39,89 In a few instances, exposure of the nanomaterial film to VOCs causes charge transfer from/to the inorganic nanomaterial, thus causing changes in the measured conductivity, even in the absence of any steric changes within the sensing film.39,69,194 The chemical diversity of the functional group(s) that cap the inorganic nanomaterial can be tailored for each sensor type, with the aim that each sensor responds to a particular fingerprint of VOCs in a different way. Consequently, a pattern of resistance changes is obtained from the sensor array to a given vapor.200
Clinical studies on breath samples with a cross-reactive array of MCNPs have shown the capability to distinguish lung cancer breath samples from control groups68,69,201 as well as distinguish between various types (lung cancer, colon cancer, breast cancer, prostate cancer, and head and neck cancer),63,68 in the presence of confounding factors. Peled et al.39,40 have shown that a cross-reactive MCNP array and a molecule-terminated single-walled carbon nanotube (SWCNT) array of chemiresistors discriminated between malignant and benign pulmonary nodules and between adeno- and squamous-cell carcinomas with 85–91% accuracy; additionally, it could also discriminate between early-stage and advanced-stage lung cancer with 86–90% accuracy.201 Similar results were achieved on cancer cell lines in an in vitro study.39–41 Another study included exhaled breath of 14 individuals with bronchogenic carcinoma and 45 control subjects without cancer using an array of chemiresistive films of polymer and carbon black.200 The sensor arrays detected lung cancer with 71.4% sensitivity and 91.9% specificity; positive and negative predictive values were 66.6% and 93.4%, respectively.200 Broza et al.202 included early-stage lung cancer (stages Ia, Ib and IIa) before and three weeks after tumor resection in their study. A modified array of MCNP-based sensors discriminated between pre-surgery and post-surgery lung cancer samples (80% accuracy), as well as between pre-surgery benign and lung cancer conditions (94% accuracy). In contrast, the same sensor array could not discriminate between pre-surgery and post-surgery benign states, nor between lung cancer and benign states post-surgery.202 These results point to the use of such MCNP-based chemiresistors array for short-term follow-up after lung cancer resection.202
Based on the effective classification of lung cancer, researchers studied malignant mesothelioma against an asbestos-related disease group and a control group. Breath analysis was carried out with an array of carbon black/polymer sensors enabling the discrimination of malignant mesothelioma from all other groups with 88% accuracy.203 The sensors could discriminate with 80.8% accuracy the malignant mesothelioma group from people with asbestos exposure and discriminate with 84.6% accuracy the malignant mesothelioma group from healthy controls.204 Haick, Liu and co-workers used an array of MCNP and SWCNT sensors and showed excellent ability to differentiate between (i) gastric cancer and benign gastric conditions (90% accuracy); (ii) early stage gastric cancer (I–II) and late stage (III–IV) (92% accuracy); and (iii) ulcer and less severe cancer conditions (86% accuracy).15 The common effect between gastric disorders and respiratory disorders was recently studied using an array of polymers and carbon black chemiresistors.205 Study results presented an ability to differentiate between breath prints of obstructive lung disease patients without gastro-oesophageal reflux disease (GORD) and obstructive lung disease patients with GORD (with 67.6% accuracy), and asthmatic patients with reflux from asthmatics without GORD (85% accuracy). But in the case of patients with COPD and COPD with GORD, only 64% accuracy was achieved by the array.205 A larger prospective interventional study is needed as the results described were influenced by several different confounders.39,205
3.2.2. Colorimetric sensors
Colorimetric sensors are composed of a diverse range of chemically responsive dyes, whose colors depend on their chemical environment (see Fig. 6f).206,207 Since the measurable responses of the sensors are the color changes in each of the dyes, a colorimetric sensor array can easily be read out with the naked eye.206,207 Alternatively, auxiliary equipment such as a spectrometer is needed. Another advantage of colorimetric sensor arrays is their ease of fabrication: they can simply be printed on a variety of substrates using a disposable cartridge printer.
Colorimetric sensors array was applied successfully to lung cancer breath testing, using different classes of chemically responsive dyes.208 The colorimetric sensors are made from dye-containing metal ions (e.g., metalloporphyrins) that respond to Lewis basicity, pH indicators that respond to Bronsted acidity/basicity, and dyes with large permanent dipoles that respond to polar breath VOCs. The sensitivity of the system is in the low ppmv range for many relevant VOCs, but it is not established for humid gas mixtures. An array of 24 colorimetric sensors was used in a clinical trial on 229 subjects (92 LC with different histology, 137 healthy controls).208 Results showed that better accuracies are achieved in the comparison of individual histologies and the control group (e.g., squamous cell carcinoma, adenocarcinoma) than in the case of non-small cell lung cancer compared with the control group, which gave a sensitivity and specificity of 70% and 86%, respectively.39
3.2.3. Electro-acoustic sensors
Electro-acoustic sensors measure the electrical response to applied mechanical stress: mechanical stress generates a voltage in piezoelectric materials, and vice versa. An oscillating potential near the material’s resonant frequency induces a variety of wave modes.209,210 Covering piezoelectric substrates with organic films provides the moderate chemical selectivity that is required for sensor array elements. The electro-acoustic sensors use either bulk acoustic waves (BAKs) or surface acoustic waves (SAWs).
3.2.3.1. Quartz microbalance (QMB) sensors
Quartz crystal microbalance (QCM) sensors constitute the simplest implementations of BAK sensors (see Fig. 6e).211–213 In a QCM, the acoustic wave propagates through the bulk of the crystal in a direction perpendicular to the surface.211–213 QCMs with chemo-active coatings have been widely used in gas and vapor sensing. Adsorption and desorption of the breath VOCs from the coated membrane cause changes in its mass, which, in turn, gives rise to shifts in the resonator’s frequency. The resonant frequency is also affected by variation in temperature and humidity, which could be important confounding factors during direct breath sampling. These two parameters should be controlled when using QCM sensor arrays for cancer breath testing, in order to minimize their effect during the exposure to the samples.
Lung cancer VOCs have been successfully demonstrated in a small-scale pilot study, using QCM sensor arrays with metalloporphyrin coatings.187,188 These sensors presented decent sensitivity towards aromatic compounds, amines, alcohols, and ketones. Additionally, they have been shown to correctly classify breath prints of three groups of volunteers: (i) lung cancer patients before surgical treatment; (ii) control group including hospital staff; and (iii) lung cancer patients after the surgery. The accuracy of the array of QMB sensors was 90.3% with 100% correct classification of the lung cancer patients.39,213
3.2.3.2. Surface Acoustic Wave (SAW) sensors
In a SAW device, wave motion occurs only at the surface, penetrating to a depth of approximately one acoustic wavelength into the crystal (see Fig. 6g).214 The direction of propagation is parallel to the surface, which can be covered with different chemiselective films. Adsorption and desorption of the breath VOCs from the coated membrane cause changes in its mass, resulting in a change in the mass (acoustic field of the SAW) and in the electrical conductivity (electric field of the SAW, associated with the acoustic field) of the chemical interface, influencing the SAW amplitude and phase velocity.214 SAW sensors have a higher sensitivity than QMB sensors to most VOCs and the devices offer better possibilities for surface modifications. Preliminary results showed promise for deriving a breath print marker for LC malignancy, using a pair of chemically modified (polyisobutylene) SAW sensors, but the study population was too small to draw far-reaching conclusions.
In a study on lung cancer, a pair of SAW sensors was used as detectors in breath analysis. The first sensor was coated using a poly(isobutylene) film and the other sensor was used as the reference.215 The study outline included a few steps: preconcentration of the breath samples with a solid-phase microextraction (SPME) fiber followed by their injection into a gas chromatography capillary column. The eluted VOCs were then introduced to the polymer-coated SAW sensor, one by one and measured as frequency change steps. The responses were evaluated by the back-propagation artificial neural network (ANN) algorithm. Results of 10 breath prints presented a diagnostic ability for lung cancer states with 80% sensitivity and specificity.39,215
3.3. Challenges and future directions for detection of cancer VOCs
3.3.1. Tailoring advanced materials for improved detection of VOCs
Disease detection by breath analysis, particularly cancer, requires the capability to detect disease-related irregularities in the levels of breath VOCs regardless of characteristic variations in the levels of confounding VOCs.138 This requires comprehensive knowledge of breath composition and of possible factors that influence VOC breath levels. Standard exhaled breath samples contain nitrogen, oxygen, carbon dioxide, water vapor, argon, and a selection of thousands of VOCs, mostly at parts per billion levels.89,138 Most VOC spectrum varies in abundance amongst different individuals in most breath samples of a given population. In rare cases, a specific VOC could be uniquely found in the breath of diseased subjects as opposed to non-diseased subjects. Therefore, VOCs that indicate a clinical state generally display distinct levels and concentrations associated with the disease. The number of shared VOCs potentially indicative of a definite clinical state ranges from only a few to tens of VOCs.4,216 Thus, constructing suitable sensors for the detection of a certain disease is challenging and should take into account these aspects: (i) the sensor’s detection range based on the predicted VOC concentrations in breath; (ii) increasing specificity to relevant VOCs while reducing sensitivity to background noise;87 (iii) knowledge of the chemical identity of the target VOCs and their breath concentrations.
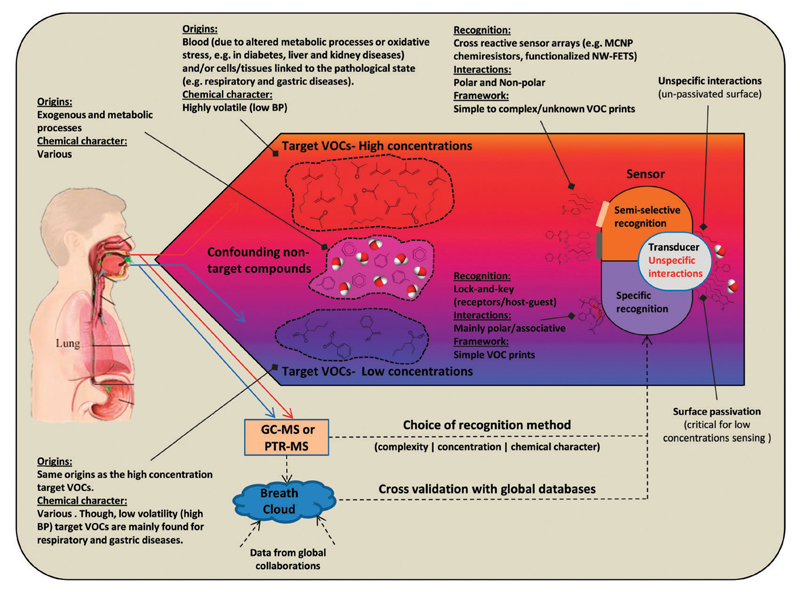
If initial VOC profiling for a given cancer reveals that a few specific marker VOCs are expected to appear at elevated concentrations (e.g., methanol, acetone, and methane, up to a few ppmv)59,112,217 in breath, then a sensing platform of semi-selective or highly selective sensors based on specific recognition would be suitable (see Fig. 9). Yet, when a varied composition of VOCs must be identified or when a doubt exists regarding the target VOCs’ exact nature, a less specific sensing approach would be better (see Fig. 9). Sensor arrays based on chemiresistive layers of MCNPs or RN-CNTs are very attractive for such uses.138
Fig. 9.
Illustration showing the two main sensing approaches (specific vs. cross-reactive approaches) and how they can be coupled to the different types of VOC prints originating from different types of clinical states. When the detection of a single or a few target breath markers is required, maximal selectivity is required from the NMVSs, so a lock-and-key approach is most suitable. This approach is especially important for compounds that tend to appear in breath at low concentrations, such as unvolatile (high boiling point) compounds. If the targeted breath print is composed of many compounds or their identity is unknown, an array of more semi-selective NMVSs should be used. Such a setup is especially suitable for volatile (low boiling point) compounds that tend to appear at more elevated levels. Reconstructed from ref. 138.
High BP VOCs should be found in breath at low concentrations of single ppbv (for example, propofol)66 and even lower concentrations, especially the water soluble compounds (for example, indole218), due to a high λb:a. To enable sufficient limit of detections (LODs) for such compounds, their detection requires highly sensitive nanomaterial transducers, such as nanowire- or nanotube-based FETs as well as specific recognition features. If not, background VOCs “noise” from non-specific interactions would probably affect the signals of the target VOCs which can eventually result in positive false detection for the determination outlined in Fig. 9.180
When focusing efforts on fine-tuning an applied sensing technology for a specific clinical state, rough estimates are inadequate; an accurate picture of the indicative VOC print should be obtained. Therefore, analytical evaluations of the variances among the characteristic VOCs must be performed to distinguish breath composition patterns of healthy people against people suffering from a disease. The analytical evaluations should be done using standardized techniques, such as gas-chromatography mass spectrometry (GC-MS) or proton transfer reaction mass spectrometry (PTR-MS).59,164
The physical and chemical characteristics of the target VOCs are very important for creating a suitable sensing platform to a given condition. Polarity is the main physical characteristic related to VOC sensing, while polar VOCs are generally easier to identify by sensors.80,89 This easier detection is because polar VOCs are either directly detected through charge transfer between the sensing material and the molecule, or indirectly detected through a molecular layer as in the case of sensors based on functionalized single Si NW FETs or SWCNT FETs.184,185 Additionally, highly specific recognition elements are more available for polar VOCs because they offer a wider range of possible molecular interactions. For nonpolar VOCs, a sensing mechanism relies on indirect recognition through dielectric changes and steric interactions.138 Thus the size and shape of VOCs are vital factors for developing novel selective recognition for these chemicals. For instance, molecular imprinted gold MCNP composites can serve as artificial biomimetic receptors (host–guest lock-and-key architecture) in conjugation with surface plasmon resonance (SPR) transduction to detect low (nM) concentrations of RDX in a selective manner.219 The current architecture of this approach is probably limited to sensing only large-sized compounds that can be accommodated through host–guest interactions within the interlinked MCNPs’ matrix. An additional example in chemiresistive films would be the use of cube shaped MCNPs, as opposed to spherical MCNPs, that was shown to discriminate among VOCs based only on size.194,220 By applying this strategy, sensor selectivity can be tuned towards compound polarity characteristics based on the organic layer coating the MCNPs. Furthermore, the use of self-assembled polycyclic aromatic hydrocarbon (PAH) layers covering RN-CNT chemiresistive films was shown to provide the sensors with a selective response to polar and nonpolar VOCs in a changing humidity background.197 FETs based on single Si-NWs were successfully passivated to block silicon oxidation and functionalized with alkane-backbone silanes and alkenes, which enabled sensing straight alkanes by an “indirect” steric molecular gating mechanism.138,184,185
3.3.2. Overcoming confounding factors
To develop a detection system for real-world analysis, the system must be able to deal with various confounding factors. In particular, breath analysis sensors for trace-amount VOC detection must cope with chemical or physical factors such as ambient temperature and humidity or the instability of breath samples and sensing elements.216 From the first step of the breath analysis process, sampling, storage and transport of the exhaled breath to and into the sensor apparatus might result in VOC loss and/or involve considerable amounts of contaminants.221 These difficulties can be minimized by integrating appropriate sampling and preparation techniques for delivery of the sensors. Currently, a common technique used for sample storage involves the use of containers such as collection bags, vials, or canisters. These containers often introduce contamination and cause VOC loss during storage.222–224 A promising alternative option would be “trapping” the VOCs on a sorbent material (for example, Tenax® and/or Carbopack X and/or Carboxen) followed by thermal desorption (TD).42,43,50,178,225–230 The latter can be accomplished by thermal desorption tubes or by needle trap devices.131,231,232 This technique allows using a semi-selective sorbent material that can trap a range of VOCs (see Fig. 10a). By performing pre-concentration of the breath sample, one can gain both a reduction of the sample volume (increased VOC concentration) and a decrease in its complexity. Because the various target VOCs are adsorbed/absorbed differently, an appropriate assessment should be performed on the choice of sorbent material.178,225 The use of solid phase extraction can provide a breath sample storage solution up to a number of months, depending on the storage system. In addition, the solid phase extraction could allow integration of sensor systems with low volume delivery methods such as microfluidics. In this respect, microfluidics – the science and technology implementing microscale fluidic channels to manipulate microliter to nanoliter volumes – should be integrated with the TD system to optimize sample handling and delivery. Another important advantage of using sorbent material, especially ones with low breakthrough volumes for water (e.g., Tenax), is the ability to trap high moisture content samples such as breath. The dehumidification of the sample improves the performance of the VOC sensor in most cases. By using a multi-capillary column (MCC), research studies could effectively separate moisture from other breath components, by simply enabling higher chromatographic flow rates of up to 250 ml min−1 (ref. 233) allowing isothermal separation of VOCs at ambient temperature (see Fig. 10b).234–236 Besides the various dehumidification techniques that might cause the loss of VOCs,237 other approaches such as enhancing recognition element surface coverage238 and humidity calibration algorithms of the sensors can be applied to reduce the effects of humidity among samples (see Fig. 10c).216 If the sensor responses to VOCs and water molecules are not independent due to competitive binding, this approach alone is limited and requires new recognition elements that are selective to the VOCs and to water vapors in the matrix.198,219 Thus, practical sensing should always account for the VOC/humidity sensitivity ratios,216 which should be tested at humidity levels typical for the breath samples.
Fig. 10.
Means for tackling the implications of real-world confounding factors. (a) Top left: schematic diagram of a µ-preconcentrator chip that utilizes an array of solid-phase microextraction (SPME) needles coated with an in situ-grown carbon adsorbent film (as the sorbent material). Right: cross-section SEM image of an array of µ-SPME needles coated with the carbon film. Bottom left: schematic diagram of the heater and temperature sensors of the thermal desorption (TD) unit of the µ-preconcentrator chip.225 (b) A topographic plot of an ion mobility spectrometer (IMS) coupled to a multi-capillary column (MCC) from the breath of a patient suffering from lung infection. The plot shows on the bottom left hand side that the moisture of the breath sample is separated from the signals of the other breath components.234 The inset shows a micrograph of a transverse section of a MCC with ~1400 capillaries having a diameter of ~40 µm.244 (c) A comparison between the response patterns of an array of four gold-nanoparticle (Au-NP) based chemiresistors to clean moist air samples (blue and green closed circles) and air samples contaminated by ~40 ppm of 2-ethylhexanol before humidity compensation (left) and after humidity compensation (right). The plot shows the major improvement in the performance of the sensor array resulting from the humidity compensation procedure.216 (d) A schematic view of the different layers composing a CNT-FET sensor integrated with an embedded heating layer situated between the substrate and the dielectric layer, which is useful for reducing the recovery time of the sensor by desorbing the bound molecules more rapidly.182 (e) Left: a plot showing the major improvement in the stability of the sensitivity to toluene of Au-NP based sensors capped by trithiols instead of monothiols, which is explained to be a result of slower oxidation of the thiolate groups in the case of the trithiol capping layer. Right: a schematic drawing showing the differences between the trithiol capped Au-NPs (top) and the monothiol capped Au-NPs (bottom).242 (f) A plot of the sensitivity of three identically fabricated Au-NP based chemiresistors towards water vapor over a period of ~124 days, which shows that their sensitivity drastically drifts down over the first few weeks and stabilizes after an aging period of ~40 days. The inset shows the resistance response profiles of the three sensors that become almost identical towards the end of the experiment.216
Another important aspect of breath analysis with sensing matrices is the working temperature. Breath samples, as well as most sensors, should be handled in a restricted range of operating temperatures.222 In the case of breath samples, the working temperature should not be too high, to protect VOCs from oxidation or thermo-degradation at high temperatures. Additionally, the short thermal desorption process of volatiles could lead to degradation of some compounds.239 Conversely, at low temperatures water condensation occurs in the storage containers causing polar VOCs to dissolve in the condensed humidity. To avoid condensation effects, breath samples in containers should be warmed to a temperature approximately ~40 °C before analysis. Unless the VOCs are extracted and transferred into an inert carrier gas (for example, nitrogen or argon), this approach limits using sensors based on metal oxide nanostructures that operate at high temperatures (for example, 260 °C),240 especially in the case of easily oxidizing compounds.80,241 Maintaining a stable temperature throughout the measurement process is important and can be achieved by incorporating an on-chip embedded heating layer (see Fig. 10d).
Yet another aspect of breath analysis is the exposure of sensors to continuous thermal cycles as a result of multiple exposures of breath samples, which might enhance drift effects of the sensors. The drift effects can be overcome by doing sensitivity calibrations216 or by achieving stable sensing layers by inhibition of oxidation processes (see Fig. 10e).242 For stable sensor operation over time, an alternative option could be a long aging process (see Fig. 10f).216 Future breath testing technologies are expected to incorporate multidisciplinary approaches for minimizing the various limiting factors linked to breath analysis together with nanomaterials tailored specifically for target VOCs.
4. Conclusion and future perspective
In this review, we have discussed the possible cellular and biochemical origin of the cancer-related VOCs as well as the relation between the VOCs in the blood and in the exhaled breath. The data presented might not yet provide precise or definite answers to the puzzling pathophysiological pathways of cancer VOCs, but the data will help stimulating constructive discussions and new ideas. We have also discussed the important milestones that have been reached and those that still need to be achieved on the way to detecting a wide range of diseases by breath testing. The outcome of the comparative study we presented is based on cell biology, by means of one or a combination of the following biochemical pathways: oxidative stress and cytochrome P450, liver enzymes, carbohydrate metabolism (glycolysis/gluconeogenesis pathways), and/or lipid metabolism.
Although the biological mechanisms discussed affect the concentration of the VOCs in both blood and breath, we believe that there is an enormous advantage of breath sampling compared to blood sampling. First, the blood and breath concentrations are related through the respective λb:a of each VOC, so that in certain cases the breath concentration could be higher than the concentration of the same VOC in blood. Another aspect considers the reliability of the sampling technique. In the common process of blood sampling, VOCs are quickly released into the surrounding air. Hence, the sampling of VOCs from blood131 needs very careful preparation and processing of the sample to avoid degassing (and resultant loss) of the VOCs of interest and avoid contamination by VOCs present in the surrounding environment.
A third aspect relates to the analytical techniques. Measuring VOCs in gaseous samples is well developed and comparatively simple, because all the other (non-volatile) compounds do not interfere. However, measuring VOCs in blood samples (where they are surrounded by a much more complicated matrix) needs sampling of blood headspace (after equilibration).131
The last aspect concerns medical applications. Breath sampling is non-invasive and breath can be sampled as often as deemed necessary. Exhaled breath can even be sampled continuously during an ergometer challenge or during sleep,123,125 as opposed to blood, which cannot be sampled continuously.
With respect to current and future technologies for VOC analysis in general, and breath analysis in particular, comprehensive work has yet to be carried out. The exploration of new technologies and new biomarkers for basic and advanced disease detection is constantly gaining momentum. While highly sophisticated analytical methods and molecular methods are currently used in well-equipped clinical and professional laboratories, the future goal is to achieve fast and inexpensive personalized medicine that could be introduced to all parts of the globe including the developing world. As new communication technologies are invented every day, integration of nanoscale medical technologies into this framework would be highly desirable and would allow high-speed global diagnostics. Highly selective sensors could guarantee high sensitivity. Using arrays of cross-reactive sensors may limit the sensitivity, but on the other hand, would relax the stress constraints on the sensor design. The result could be a multi-purpose device with low to medium levels of sensitivity towards the VOCs of interest. In practice, most sensors suffer from some interference by responding to chemical species that are structurally or chemically similar to the desired VOC. This interference in the sensors could be overcome by using different (inorganic) material types and organic functionalities. The responses of the sensors to VOCs can be obtained from equilibrium or kinetic responses, with the latter often providing additional discriminating power. Both binding and solubility properties can be interrogated with advanced functional materials. For example, broadly responsive nanomaterials can be employed to allow a range of structurally similar molecules to bind, membranes could be used as size-selective sensors, and materials with highly selective functional groups could be employed to make selections on the basis of polarity. Often, all of these recognition mechanisms, along with others described in this review, exist simultaneously in these systems but with different domination ratios. An array of sensors combining all these recognition approaches naturally is needed to yield a unique signal for complex but distinctive VOCs without requiring the mixture to be broken down into its individual components. This condition is a disadvantage when precise VOC composition of a complex mixture is required, but is advantageous when the only required information is the composition of the VOCs of interest.
Improved breath testing systems should combine various technologies that are highly sensitive to cancer-related VOCs and barely sensitive or not at all sensitive to parasitic responses that originate from different confounding factors. This could be achieved, for example, by pre-concentrating and dehumidifying the cancer-related VOCs, by means of the micro-adsorption process followed by TD,225 MEMS-based µ-preconcentrator,243 MCCs,165,233,234,236,244 and micro-column gas chromatography (MCGC).245–247 The processed cancer-related VOCs are then delivered through a microfluidic system to highly sensitive and selective on-chip sensors that are integrated with a temperature control unit. Following the trend of miniaturization in the world of technology, a breath testing system should eventually be able to fit into a casing as small as a smart phone.
Acknowledgements
Hossam Haick gratefully acknowledges the funding from the FP7-Health Program under the LCAOS (grant agreement no. 258868) and the FP7’s ERC grant under DIAG-CANCER (grant agreement no. 256639). Additionally, Hossam Haick acknowledges Gady Konvalina and Nisreen Shachada (Technion) for assistance and helpful discussions and the FP’7 Lung Cancer Artificial Olfactory System (LCAOS) partners for support. Anton Amann gratefully appreciates funding from the Austrian Federal Ministry for Transport, Innovation and Technology (BMVIT/BMWA, project 836308, KIRAS) and support from the Oncotyrol-project 2.1.1. The Competence Centre Oncotyrol is funded within the scope of the COMET – Competence Centers for Excellent Technologies through BMVIT, BMWFJ, through the province of Salzburg and the Tiroler Zukunftsstiftung/Standortagentur Tirol. The COMET Program is conducted by the Austrian Research Promotion Agency (FFG). Pawel Mochalski acknowledges support from the Austrian Science Fund (FWF) under Grant no. P24736-B23. Vera Ruzsanyi gratefully acknowledges a Lise-Meitner fellowship from the Austrian Science Fund (FWF) under Grant no. M1213-B18. A.A., P.M. and V.R. thank the government of Vorarlberg (Austria) for its generous support.
Nomenclature
- λb:a
Partition coefficient of ‘blood:air’
- λf:b
Partition coefficient of ‘fat:blood’
- λo:w
Partition coefficient of ‘octanol:water’
- λw:a
Partition coefficient of ‘water:air’
- ΔHvap
Enthalpy of vaporization
- ADH
Alcohol dehydrogenase
- AHFP
2-(4-Aminophenyl)-1,1,13,3,3-hexafluoro-2-propanol
- ALDH
Aldehyde dehydrogenase
- ANN
Artificial neural network
- BAK
Bulk acoustic waves
- BCA
Breath collection apparatus
- BP
Boiling point
- CCK
Cholecystokinin
- CNT
Carbon nanotubes
- COPD
Chronic obstructive pulmonary disease
- CSC
Cancer stem cells
- CT
Computed tomography
- CYP
Cytochrome p450
- DNA
Deoxyribonucleic acid
- EPA
Environmental Protection Agency
- FET
Field effect transistor
- GC-MS
Gas chromatography – mass spectrometry
- GORD
Gastro-oesophageal reflux disease
- HPT
2-Heptanone
- LC
Lung cancer
- LOD
Limit of detection
- MCC
Multi-capillary column
- MCGC
Micro-column gas chromatography
- MCNP
Monolayer capped metal nanoparticle
- MEMS
Microelectromechanical system
- MRI
Magnetic resonance imaging
- NMVS
Nanomaterial based VOC sensor
- NP
Nanoparticle
- NW
Nanowire
- PAH
Polycyclic aromatic hydrocarbon
- PEI
Polyethyleneimine
- PET
Positron emission tomography
- ppbv
Parts per billion by volume
- ppmv
Parts per million by volume
- PTR-MS
Proton-transfer-reaction – mass spectrometry
- PTR-TOF-MS
Proton-transfer-reaction time-of-flight mass spectrometry
- PUFA
Polyunsaturated fatty acid
- QCM
Quartz crystal microbalance
- QMB
Quartz microbalance
- RDX
Cyclotrimethylenetrinitramine
- ROS
Reactive oxygen species
- SAW
Surface acoustic wave
- SEM
Scanning electron microscope
- SIFT-MS
Selected ion flow tube mass spectrometry
- SPME
Solid phase microextraction
- SPR
Surface Plasmon resonance
- SWCNT
Single-walled carbon nanotube
- TD
Thermal desorption
- TNT
Trinitrotoluene
- VOC
Volatile organic compound
Biographies
 Hossam Haick is a Professor in the Technion – Israel Institute of Technology and an expert in the field of nanotechnology and non-invasive disease diagnosis. Hossam Haick is the recipient of more than 42 international honors and prizes for his achievements. Hossam Haick is the founder and the leader of a European consortium of eight universities and companies for the development of advanced generation of nanosensors for cancer diagnosis. His research interests include nano-array devices for screening, diagnosis and monitoring of disease, nanomaterial-based chemical (flexible) sensors, electronic skin, breath analysis, volatile biomarkers, and molecule-based electronic devices.
Hossam Haick is a Professor in the Technion – Israel Institute of Technology and an expert in the field of nanotechnology and non-invasive disease diagnosis. Hossam Haick is the recipient of more than 42 international honors and prizes for his achievements. Hossam Haick is the founder and the leader of a European consortium of eight universities and companies for the development of advanced generation of nanosensors for cancer diagnosis. His research interests include nano-array devices for screening, diagnosis and monitoring of disease, nanomaterial-based chemical (flexible) sensors, electronic skin, breath analysis, volatile biomarkers, and molecule-based electronic devices.
 Yoav Y. Broza received his PhD in Biotechnology & Food Engineering in 2009 from the Technion – Israel Institute of Technology. He has been a researcher in the Laboratory for Nanomaterial-Based Devices, headed by Prof. Hossam Haick, since 2009, and a researcher at the Alfred Mann Institute for biomedical devices between the years 2009 and 2012. Additionally Yoav holds an external lecturer position in the Technion. His current research interests include the identification of disease and infectious agents’ biomarkers through volatile gas analysis by analytical methods and nano-arrays, and the development of novel technologies for gas sampling for clinical and non-clinical applications.
Yoav Y. Broza received his PhD in Biotechnology & Food Engineering in 2009 from the Technion – Israel Institute of Technology. He has been a researcher in the Laboratory for Nanomaterial-Based Devices, headed by Prof. Hossam Haick, since 2009, and a researcher at the Alfred Mann Institute for biomedical devices between the years 2009 and 2012. Additionally Yoav holds an external lecturer position in the Technion. His current research interests include the identification of disease and infectious agents’ biomarkers through volatile gas analysis by analytical methods and nano-arrays, and the development of novel technologies for gas sampling for clinical and non-clinical applications.
 Pawel Mochalski is a senior postdoc at the Breath Research Institute of the Austrian Academy of Sciences led by Prof. Anton Amann. He received his PhD in Physical Sciences in 2004 from the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN). His scientific interests embrace breath analysis, human scent analysis, volatile disease biomarkers, application of chemical analysis for the early detection of entrapped humans, and the development of analytical methods for the detection and identification of new volatile biomarkers (GC-MS, PTR-TOF-MS, IMS).
Pawel Mochalski is a senior postdoc at the Breath Research Institute of the Austrian Academy of Sciences led by Prof. Anton Amann. He received his PhD in Physical Sciences in 2004 from the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN). His scientific interests embrace breath analysis, human scent analysis, volatile disease biomarkers, application of chemical analysis for the early detection of entrapped humans, and the development of analytical methods for the detection and identification of new volatile biomarkers (GC-MS, PTR-TOF-MS, IMS).
 Vera Ruzsanyi is a post doc researcher at the Breath Research Institute of the Austrian Academy of Sciences and working in different projects at Innsbruck Medical University. She received her PhD in Bioengineering in 2006 from ISAS – Institute for Analytical Sciences. Since 2010 she has been an industrial researcher at the company G.A.S. mbH developing sensor systems based on ion mobility spectrometry. She received the Lise-Meitner fellowship from the Austrian Science Foundation in 2010. Her current scientific interest covers volatile metabolite analysis using various techniques and development of novel breath tests.
Vera Ruzsanyi is a post doc researcher at the Breath Research Institute of the Austrian Academy of Sciences and working in different projects at Innsbruck Medical University. She received her PhD in Bioengineering in 2006 from ISAS – Institute for Analytical Sciences. Since 2010 she has been an industrial researcher at the company G.A.S. mbH developing sensor systems based on ion mobility spectrometry. She received the Lise-Meitner fellowship from the Austrian Science Foundation in 2010. Her current scientific interest covers volatile metabolite analysis using various techniques and development of novel breath tests.
 Anton Amann is director of the Breath Research Institute of the Austrian Academy of Sciences and professor at Innsbruck Medical University. He received his PhD degree in Natural Sciences from the Swiss Federal Institute of Technology (ETH-Zürich). Amann’s current research interests include analysis of volatiles in exhaled breath and in the headspace of cells and bacteria. He is the recipient of the Marie Sklodowska Curie medal of the Polish Chemical Society.
Anton Amann is director of the Breath Research Institute of the Austrian Academy of Sciences and professor at Innsbruck Medical University. He received his PhD degree in Natural Sciences from the Swiss Federal Institute of Technology (ETH-Zürich). Amann’s current research interests include analysis of volatiles in exhaled breath and in the headspace of cells and bacteria. He is the recipient of the Marie Sklodowska Curie medal of the Polish Chemical Society.
References
- 1.Tangerman A, Winkel EG. J Breath Res. 2010;4:017003. doi: 10.1088/1752-7155/4/1/017003. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Levin B. World Cancer Report. 2008:1–260. [Google Scholar]
- 3.Culter DM. J Econ Perspect. 2008;22:3–26. doi: 10.1257/jep.22.4.3. [DOI] [PubMed] [Google Scholar]
- 4.Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A, Haick H. Chem Rev. 2012;112:5949–5966. doi: 10.1021/cr300174a. [DOI] [PubMed] [Google Scholar]
- 5.Todd J, McGrath EE. QJM. 2011;104:903–904. doi: 10.1093/qjmed/hcq159. [DOI] [PubMed] [Google Scholar]
- 6.Apantaku LM. Am Fam Physician. 2000;62:596–602. [PubMed] [Google Scholar]
- 7.Singeap AM, Trifan A, Cojocariu C, Stanciu C. Rev Med –Chir Soc Med Nat Iasi. 2012;116:145–149. [PubMed] [Google Scholar]
- 8.Brandman S, Ko JP. J Thorac Imaging. 2011;26:90–105. doi: 10.1097/RTI.0b013e31821639a9. [DOI] [PubMed] [Google Scholar]
- 9.Hochhegger B, Marchiori E, Sedlaczek O, Irion K, Heussel CP, Ley S, Ley-Zaporozhan J, Soares SAJ, Kauczor HU. Br J Radiol. 2011;84:661–668. doi: 10.1259/bjr/24661484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong MT, Viswanathan C, Erasmus JJ. J Thorac Imaging. 2011;26:132–146. doi: 10.1097/RTI.0b013e3182128704. [DOI] [PubMed] [Google Scholar]
- 11.Hunt KK, Newman LA, Copeland EM, Bland KI. Schwart’z Principles of Surgery. 9th. McGraw-Hill; New York: 2010. [Google Scholar]
- 12.Andrews TD, Wallace WA. Clin Oncol. 2009;21:451–463. doi: 10.1016/j.clon.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Wu CC, Maher MM, Shepard JA. AJR Am J Roentgenol. 2011;196:W511–514. doi: 10.2214/AJR.10.4657. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Gotoda T, Sasako M, Saito D. Gastric Cancer. 2006;9:315–319. doi: 10.1007/s10120-006-0399-y. [DOI] [PubMed] [Google Scholar]
- 15.Xu ZQ, Broza YY, Ionsecu R, Tisch U, Ding L, Liu H, Song Q, Pan YY, Xiong FX, Gu KS, Sun GP, et al. Br J Cancer. 2013;108:941–950. doi: 10.1038/bjc.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi ED, Mulè A, Maggiore C, Miraglia A, Lauriola L, Vecchio FM, Fadda G. Rays. 2004;29:357–361. [PubMed] [Google Scholar]
- 17.Potti A, Dressman HK, Bild A, Riedel RF, Chan G, Sayer R, Cragun J, Cottrill H, Kelley MJ, Petersen R, Harpole D, et al. Nat Med. 2006;12:1294–1300. doi: 10.1038/nm1491. [DOI] [PubMed] [Google Scholar]
- 18.Yu S-L, Chen H-Y, Chang G-C, Chen C-Y, Chen H-W, Singh S, Cheng C-L, Yu C-J, Lee Y-C, Chen H-S, Su T-J, et al. Cancer Cell. 2008;13:48–57. doi: 10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Shedden K, Taylor JMG, Enkemann SA, Tsao M-S, Yeatman TJ, Gerald WL, Eschrich S, Jurisica I, Giordano TJ, Misek DE, Chang AC, et al. Nat Med. 2008;14:822–827. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss GJ, Bemis LT, Nakajima E, Sugita M, Birks DK, Robinson WA, Varella-Garcia M, Bunn PAJ, Haney J, Helfrich BA, Kato H, et al. Ann Oncol. 2008;19:1053–1059. doi: 10.1093/annonc/mdn006. [DOI] [PubMed] [Google Scholar]
- 21.Gautschi O, Bigosch C, Huegli B, Jermann M, Marx A, Chasse E, Ratschiller D, Weder W, Joerger M, Betticher DC, Stahel RA, et al. J Clin Oncol. 2004;22:4157–4164. doi: 10.1200/JCO.2004.11.123. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez JL, Rosell R, Taron M, Sanchez-Ronco M, Alberola V, de las Penas R, Sanchez JM, Moran T, Camps C, Massuti B, Sanchez JJ, et al. J Clin Oncol. 2005;23:9105–9112. doi: 10.1200/JCO.2005.02.2905. [DOI] [PubMed] [Google Scholar]
- 23.Chen XQ, Stroun M, Magnenat J-L, Nicod LP, Kurt A-M, Lyautey J, Lederrey C, Anker P. Nat Med. 1996;2:1033–1035. doi: 10.1038/nm0996-1033. [DOI] [PubMed] [Google Scholar]
- 24.Ludovini V, Pistola L, Gregorc V, Floriani I, Rulli E, Piattoni S, Di Carlo L, Semeraro A, Darwish S, Tofanetti FR, Stocchi L, et al. J Thorac Oncol. 2008;3:365–373. doi: 10.1097/JTO.0b013e318168c7d0. [DOI] [PubMed] [Google Scholar]
- 25.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW, Digumarthy S, et al. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H-Y, Yu S-L, Chen C-H, Chang G-C, Chen C-Y, Yuan A, Cheng C-L, Wang C-H, Terng H-J, Kao S-F, Chan W-K, et al. N Engl J Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]
- 27.Yildiz PB, Shyr Y, Rahman JSM, Wardwell NR, Zimmerman LJ, Shakhtour B, Gray WH, Chen S, Li M, Roder H, Liebler DC, et al. J Thorac Oncol. 2007;2:893–901. doi: 10.1097/JTO.0b013e31814b8be7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, et al. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 29.Guo A, Villen J, Kornhauser J, Lee KA, Stokes MP, Rikova K, Possemato A, Nardone J, Innocenti G, Wetzel R, Wang Y, et al. Proc Natl Acad Sci U S A. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanagisawa K, Tomida S, Shimada Y, Yatabe Y, Mitsudomi T, Takahashi T. J Natl Cancer Inst. 2007;99:858–867. doi: 10.1093/jnci/djk197. [DOI] [PubMed] [Google Scholar]
- 31.Taguchi F, Solomon B, Gregorc V, Roder H, Gray R, Kasahara K, Nishio M, Brahmer J, Spreafico A, Ludovini V, Massion PP, et al. J Natl Cancer Inst. 2007;99:838–846. doi: 10.1093/jnci/djk195. [DOI] [PubMed] [Google Scholar]
- 32.Risch A, Plass C. Int J Cancer. 2008;123:1–7. doi: 10.1002/ijc.23605. [DOI] [PubMed] [Google Scholar]
- 33.Yang IV, Schwartz DA. Am J Respir Crit Care Med. 2011;183:1295–1301. doi: 10.1164/rccm.201010-1579PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Mohan A, Guleria R. Biomarkers. 2006;11:385–405. doi: 10.1080/13547500600775011. [DOI] [PubMed] [Google Scholar]
- 35.Herbst RS, Heymach JV, Lippman SM. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coate LE, John T, Tsao M-S, Shepherd FA. Lancet Oncol. 2009;10:1001–1010. doi: 10.1016/S1470-2045(09)70155-X. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal C, Somaiah N, Simon GR. J Natl Compr Cancer Network. 2010;8:822–832. doi: 10.6004/jnccn.2010.0059. [DOI] [PubMed] [Google Scholar]
- 38.Socinski MA. Clin Lung Cancer. 2010;11:149–159. doi: 10.3816/CLC.2010.n.019. [DOI] [PubMed] [Google Scholar]
- 39.Broza YY, Haick H. Nanomedicine. 2013;8:785–806. doi: 10.2217/nnm.13.64. [DOI] [PubMed] [Google Scholar]
- 40.Barash O, Peled N, Tisch U, Bunn PA, Hirsch FR, Haick H. Nanomedicine. 2012;8:580–589. doi: 10.1016/j.nano.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barash O, Peled N, Hirsch FR, Haick H. Small. 2009;5:2618–2624. doi: 10.1002/smll.200900937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filipiak W, Sponring A, Filipiak A, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Cancer Epidemiol Biomarkers Prev. 2010;19:182–195. doi: 10.1158/1055-9965.EPI-09-0162. [DOI] [PubMed] [Google Scholar]
- 43.Filipiak W, Sponring A, Mikoviny T, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Cancer Cell Int. 2008;8:17. doi: 10.1186/1475-2867-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sponring A, Filipiak W, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Cancer Biomarkers. 2010;7:153–161. doi: 10.3233/CBM-2010-0182. [DOI] [PubMed] [Google Scholar]
- 45.Sponring A, Filipiak W, Mikoviny T, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Anticancer Res. 2009;29:419–426. [PubMed] [Google Scholar]
- 46.Brunner C, Szymczak W, Höllriegl V, Mörtl S, OelmeZ H, Bergner A, Huber RM, Hoeschen C, Oeh U. Anal Bioanal Chem. 2010;397:2315–2324. doi: 10.1007/s00216-010-3838-x. [DOI] [PubMed] [Google Scholar]
- 47.Sulé-Suso J, Pysanenko A, Špan l P, Smith D. Analyst. 2009;134:2419–2425. doi: 10.1039/b916158a. [DOI] [PubMed] [Google Scholar]
- 48.Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. Lung Cancer. 2008;59:340–349. doi: 10.1016/j.lungcan.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 49.Smith D, Wang T, Sulè-Suso J, Spanel P, El Haj A. Rapid Commun Mass Spectrom. 2003;17:845–850. doi: 10.1002/rcm.984. [DOI] [PubMed] [Google Scholar]
- 50.Amal H, Ding L, Liu BB, Tisch U, Xu ZQ, Shi DY, Zhao Y, Chen J, Sun RX, Liu H, Ye SL, et al. Int J Nanomed. 2012;7:4135–4146. doi: 10.2147/IJN.S32680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mochalski P, Sponring A, King J, Unterkofler K, Troppmair J, Amann A. Cancer Cell Int. 2013;13:72. doi: 10.1186/1475-2867-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanai Y, Shimono K, Matsumura K, Vachani A, Albelda S, Yamazaki K, Beauchamp GK, Oka H. Biosci Biotechnol Biochem. 2012;76:679–684. doi: 10.1271/bbb.110760. [DOI] [PubMed] [Google Scholar]
- 53.D’Amico A, Bono R, Pennazza G, Santonico M, Mantini G, Bernabei M, Zarlenga M, Roscioni C, Martinelli E, Paolesse R, Di Natale C. Skin Res Technol. 2008;14:226–236. doi: 10.1111/j.1600-0846.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 54.Pennazza G, Santonico M, Martinelli E, Paolesse R, Tamburrelli V, Cristina S, D’Amico A, Di Natale C, Bartolazzi A. Sens Actuators, B. 2011;154:288–294. [Google Scholar]
- 55.Deng C, Zhang X, Li N. J Chromatogr B: Biomed Appl. 2004;808:269–277. doi: 10.1016/j.jchromb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Xue R, Dong L, Zhang S, Deng C, Liu T, Wang J, Shen X. Rapid Commun Mass Spectrom. 2008;22:1181–1186. doi: 10.1002/rcm.3466. [DOI] [PubMed] [Google Scholar]
- 57.Amann A, Corradi M, Mazzone P, Mutti A. Expert Rev Mol Diagn. 2011;11:207–217. doi: 10.1586/erm.10.112. [DOI] [PubMed] [Google Scholar]
- 58.Amann A, Ligor M, Ligor T, Bajtarevic A, Ager C, Pienz M, Denz H, Fiegl M, Hilbe W, Weiss W, Lukas P, et al. Memo. 2010;3:106–112. [Google Scholar]
- 59.Bajtarevic A, Ager C, Pienz M, Klieber M, Schwarz K, Ligor M, Ligor T, Filipiak W, Denz H, Fiegl M, Hilbe W, et al. BMC Cancer. 2009;9:348. doi: 10.1186/1471-2407-9-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buszewski B, Kesy M, Ligor T, Amann A. Biomed Chromatogr. 2007;21:553–566. doi: 10.1002/bmc.835. [DOI] [PubMed] [Google Scholar]
- 61.Cao W, Duan Y. CRC Crit Rev Anal Chem. 2007;37:3–13. [Google Scholar]
- 62.Gordon SM, Szidon JP, Krotoszynski BK, Gibbons RD, Oneill HJ. Clin Chem. 1985;31:1278–1282. [PubMed] [Google Scholar]
- 63.Hakim M, Billan S, Tisch U, Peng G, Dvrokind I, Marom O, Abdah-Bortnyak R, Kuten A, Haick H. Br J Cancer. 2011;104:1649–1655. doi: 10.1038/bjc.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horvath L, Lazar Z, Gyulai N, Kollai M, Losonczy G. Eur Respir J. 2009;34:261–275. doi: 10.1183/09031936.00142508. [DOI] [PubMed] [Google Scholar]
- 65.Kischkel S, Miekisch W, Sawacki A, Straker EM, Trefz P, Amann A, Schubert JK. Clin Chim Acta. 2010;411:1637–1644. doi: 10.1016/j.cca.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Miekisch W, Fuchs P, Kamysek S, Neumann C, Schubert JK. Clin Chim Acta. 2008;395:32–37. doi: 10.1016/j.cca.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 67.O’Neill HJ, Gordon SM, O’Neill MH, Gibbons RD, Szidon JP. Clin Chem. 1988;34:1613–1618. [PubMed] [Google Scholar]
- 68.Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Tisch U, Haick H. Br J Cancer. 2010;103:542–551. doi: 10.1038/sj.bjc.6605810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Haick H. Nat Nanotechnol. 2009;4:669–673. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 70.Peng G, Tisch U, Haick H. Nano Lett. 2009;9:1362–1368. doi: 10.1021/nl8030218. [DOI] [PubMed] [Google Scholar]
- 71.Phillips M, Altorki N, Austin JHM, Cameron RB, Cataneo RN, Greenberg J, Kloss R, Maxfield RA, Munawar MI, Pass HI. Cancer Biomarkers. 2007;3:95–109. doi: 10.3233/cbm-2007-3204. [DOI] [PubMed] [Google Scholar]
- 72.Phillips M, Altorki N, Austin JHM, Cameron RB, Cataneo RN, Kloss R, Maxfield RA, Munawar MI, Pass HI, Rashid A. Clin Chim Acta. 2008;393:76–84. doi: 10.1016/j.cca.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phillips M, Cataneo RN, Cummin ARC, Gagliardi AJ, Gleeson K, Greenberg J, Maxfield RA, Rom WN. Chest. 2003;123:2115–2123. doi: 10.1378/chest.123.6.2115. [DOI] [PubMed] [Google Scholar]
- 74.Phillips M, Cataneo RN, Ditkoff BA, Fisher P, Greenberg J, Gunawardena R, Kwon CS, Rahbari-Oskoui F, Wong C. Breast J. 2003;9:184–191. doi: 10.1046/j.1524-4741.2003.09309.x. [DOI] [PubMed] [Google Scholar]
- 75.Phillips M, Cataneo RN, Ditkoff BA, Fisher P, Greenberg J, Gunawardena R, Kwon SC, Tietje O, Wong C. Breast Cancer Res Treat. 2006;99:19–21. doi: 10.1007/s10549-006-9176-1. [DOI] [PubMed] [Google Scholar]
- 76.Phillips M, Cataneo RN, Saunders C, Hope P, Schmitt P, Wai J. J Breath Res. 2010;4:026003. doi: 10.1088/1752-7155/4/2/026003. [DOI] [PubMed] [Google Scholar]
- 77.Phillips M, Gleeson K, Hughes JMB, Greenberg J, Cataneo RN, Baker L, McVay WP. Lancet. 1999;353:1930–1933. doi: 10.1016/S0140-6736(98)07552-7. [DOI] [PubMed] [Google Scholar]
- 78.Poli D, Carbognani P, Corradi M, Goldoni M, Acampa O, Balbi B, Bianchi L, Rusca M, Mutti A. Respir Res. 2005;6:71. doi: 10.1186/1465-9921-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poli D, Goldoni M, Corradi M, Acampa O, Carbognani P, Internullo E, Casalini A, Mutti A. J Chromatogr, B: Biomed Appl. 2010;878:2643–2651. doi: 10.1016/j.jchromb.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 80.Tisch U, Haick H. Rev Chem Eng. 2010;26:171–179. [Google Scholar]
- 81.Wehinger A, Schmid A, Mechtcheriakov S, Ledochowski M, Grabmer C, Gastl GA, Amann A. Int J Mass spectrom. 2007;265:49–59. [Google Scholar]
- 82.Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. J Chromatogr B: Biomed Appl. 1999;729:75–88. doi: 10.1016/s0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 83.Phillips M. Sci Am. 1992;267:74–79. doi: 10.1038/scientificamerican0792-74. [DOI] [PubMed] [Google Scholar]
- 84.Risby TH. In: Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring. Amann A, Smith D, editors. World Scientific; Singapore: 2005. pp. 251–265. [Google Scholar]
- 85.Thorn RM, Greenman J. J Breath Res. 2012;6:024001. doi: 10.1088/1752-7155/6/2/024001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montuschi P, Mores N, Trove A, Mondino C, Barnes PJ. Respiration. 2013;85:72–84. doi: 10.1159/000340044. [DOI] [PubMed] [Google Scholar]
- 87.Boots AW, van Berkel JJ, Dallinga JW, Smolinska A, Wouters EF, van Schooten FJ. J Breath Res. 2012;6:027108. doi: 10.1088/1752-7155/6/2/027108. [DOI] [PubMed] [Google Scholar]
- 88.Spanel P, Smith D. Curr Opin Clin Nutr Metab Care. 2011;14:455–460. doi: 10.1097/MCO.0b013e3283490280. [DOI] [PubMed] [Google Scholar]
- 89.Tisch U, Haick H. MRS Bull. 2010;35:797–803. [Google Scholar]
- 90.Tisch U, Billan S, Ilouze M, Phillips M, Peled N, Haick H. CML – Lung Cancer. 2012;5:107–117. [Google Scholar]
- 91.Shirasu M, Touhara K. J Biochem. 2011;150:257–266. doi: 10.1093/jb/mvr090. [DOI] [PubMed] [Google Scholar]
- 92.Phillips M, Cataneo RN, Chaturvedi A, Kaplan PD, Libardoni M, Mundada M, Patel U, Zhang X. PLoS One. 2013;8:e75274. doi: 10.1371/journal.pone.0075274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alberts B, Johnson A, Lewis J. Molecular Biology of the Cell. 4th. Garland Publishing; New York: 2002. [Google Scholar]
- 94.Singer SJ, Nicolson GL. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 95.Ambrosone CB. Antioxid Redox Signaling. 2000;2:903–917. doi: 10.1089/ars.2000.2.4-903. [DOI] [PubMed] [Google Scholar]
- 96.Watanabe M. Toxicol Lett. 1998:102–103. 167–171. doi: 10.1016/s0378-4274(98)00303-8. [DOI] [PubMed] [Google Scholar]
- 97.Murray GI. J Pathol. 2000;192:419–426. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH750>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 98.Chen S. Front Biosci. 1998;3:d922–d933. doi: 10.2741/a333. [DOI] [PubMed] [Google Scholar]
- 99.Weinberg RA. The Biology of Cancer. Garland Publishing Inc.; New York: 2006. [Google Scholar]
- 100.Tayek JA. J Am Coll Nutr. 1992;11:445–456. doi: 10.1080/07315724.1992.10718249. [DOI] [PubMed] [Google Scholar]
- 101.Bayley J-P, Devilee P. Curr Opin Genet Dev. 2010;20:324–329. doi: 10.1016/j.gde.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 102.Kim HH, Joo H, Kim T, Kim E, Park S-J, Park JK, Kim HJ. Interdiscip Bio Cent. 2009;1:1–6. [Google Scholar]
- 103.Kroemer G. Oncogene. 2006;25:4630–4632. doi: 10.1038/sj.onc.1209589. [DOI] [PubMed] [Google Scholar]
- 104.Levine AJ, Puzio-Kuter AM. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 105.Sattler UG, Hirschhaeuser F, Mueller-Klieser WF. Curr Med Chem. 2010;17:96–108. doi: 10.2174/092986710790112657. [DOI] [PubMed] [Google Scholar]
- 106.Gatenby RA, Gillies RJ. Nat Rev. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 107.Vousden KH, Ryan KM. Nat Rev Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 108.Okunieff P, Fenton B, Chen Y. Adv Exp Med Biol. 2005;566:213–222. doi: 10.1007/0-387-26206-7_29. [DOI] [PubMed] [Google Scholar]
- 109.Kneepkens CMF, Lepage G, Roy CC. Free Radicals Biol Med. 1994;17:127–160. doi: 10.1016/0891-5849(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 110.Halliwel B. J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 111.Barr DB, Wang RY, Needham LL. Environ Health Perspect. 2005;113:1083–1091. doi: 10.1289/ehp.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Hara ME, Clutton-Brock TH, Green S, Mayhew CA. J Breath Res. 2009;3:027005. doi: 10.1088/1752-7155/3/2/027005. [DOI] [PubMed] [Google Scholar]
- 113.Anderson JC, Babb AL, Hlastala MP. Ann Biomed Eng. 2003;31:1402–1422. doi: 10.1114/1.1630600. [DOI] [PubMed] [Google Scholar]
- 114.Mochalski P, King J, Kupferthaler A, Unterkofler K, Hinterhuber H, Amann A. J Breath Res. 2011;5:046010. doi: 10.1088/1752-7155/5/4/046010. [DOI] [PubMed] [Google Scholar]
- 115.Mochalski P, King J, Kupferthaler A, Unterkofler K, Hinterhuber H, Amann A. Internet J Toxicol. 2012;31:267–275. doi: 10.1177/1091581812442689. [DOI] [PubMed] [Google Scholar]
- 116.Paterson S, Mackay D. Br J Ind Med. 1989;46:321–328. doi: 10.1136/oem.46.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martínez-Lozano P, Zingaro L, Finiguerra A, Cristoni S. J Breath Res. 2011;5:016002. doi: 10.1088/1752-7155/5/1/016002. [DOI] [PubMed] [Google Scholar]
- 118.Basak SC, Mills D, El-Masri HA, Mumtaz MM, Hawkins DM. Environ Toxicol Pharmacol. 2004;16:45–55. doi: 10.1016/j.etap.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Peyret T, Poulin P, Krishnan K. Toxicol Appl Pharmacol. 2010;249:197–207. doi: 10.1016/j.taap.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 120.Meulenberg CJ, Vijverberg HP. Toxicol Appl Pharmacol. 2000;165:206–216. doi: 10.1006/taap.2000.8929. [DOI] [PubMed] [Google Scholar]
- 121.King J, Koc H, Unterkofler K, Mochalski P, Kupferthaler A, Teschl G, Teschl S, Hinterhuber H, Amann A. J Theor Biol. 2010;267:626–637. doi: 10.1016/j.jtbi.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 122.King J, Koc H, Unterkofler K, Teschl G, Teschl S, Mochalski P, Hinterhuber H, Amann A. In: Volatile Biomarkers: Non-invasive Diagnosis in Physiology and Medicine. Amann A, Smith D, editors. Elsevier; Amsterdam: 2013. pp. 27–46. [Google Scholar]
- 123.King J, Kupferthaler A, Frauscher B, Hackner H, Unterkofler K, Teschl G, Hinterhuber H, Amann A, Högl B. Physiol Meas. 2012;33:413–428. doi: 10.1088/0967-3334/33/3/413. [DOI] [PubMed] [Google Scholar]
- 124.King J, Kupferthaler A, Unterkofler K, Koç H, Teschl S, Teschl G, Miekisch W, Schubert J, Hinterhuber H, Amann A. J Breath Res. 2009;3:027006. doi: 10.1088/1752-7155/3/2/027006. [DOI] [PubMed] [Google Scholar]
- 125.King J, Mochalski P, Kupferthaler A, Unterkofler K, Filipiak W, Teschl S, Teschl G, Hinterhuber H, Amann A. Physiol Meas. 2010;31:1169–1184. doi: 10.1088/0967-3334/31/9/008. [DOI] [PubMed] [Google Scholar]
- 126.King J, Mochalski P, Unterkofler K, Teschl G, Klieber M, Stein M, Amann A, Baumann M. Biochem Biophys Res Commun. 2012;423:526–530. doi: 10.1016/j.bbrc.2012.05.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.King J, Unterkofler K, Teschl G, Teschl S, Koc H, Hinterhuber H, Amann A. J Math Biol. 2011;63:959–999. doi: 10.1007/s00285-010-0398-9. [DOI] [PubMed] [Google Scholar]
- 128.King J, Unterkofler K, Teschl G, Teschl S, Mochalski P, Koc H, Hinterhuber H, Amann A. J Breath Res. 2012;6:016005. doi: 10.1088/1752-7155/6/1/016005. [DOI] [PubMed] [Google Scholar]
- 129.King J, Unterkofler K, Teschl S, Amann A, Teschl G. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1001–1004. doi: 10.1109/IEMBS.2011.6090232. [DOI] [PubMed] [Google Scholar]
- 130.Grabowska-Polanowska B, Faber J, Skowron M, Miarka P, Pietrzycka A, Śliwka I, Amann A. J Chromatogr A. 2013;1301:179–189. doi: 10.1016/j.chroma.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 131.Mochalski P, King J, Klieber M, Unterkofler K, Hinterhuber H, Baumann M, Amann A. Analyst. 2013;138:2134–2145. doi: 10.1039/c3an36756h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Musa-Veloso K, Likhodii SS, Cunnane SC. Am J Clin Nutr. 2002;76:65–70. doi: 10.1093/ajcn/76.1.65. [DOI] [PubMed] [Google Scholar]
- 133.Fuchs P, Loeseken C, Schubert JK, Miekisch W. Int J Cancer. 2010;126:2663–2670. doi: 10.1002/ijc.24970. [DOI] [PubMed] [Google Scholar]
- 134.Preti G, Labows JN, Kostelc JG, Aldinger S, Daniele R. J Chromatogr. 1988;432:1–11. doi: 10.1016/s0378-4347(00)80627-1. [DOI] [PubMed] [Google Scholar]
- 135.Kalliomäki P-L, Korhonen O, Vaaranen V, Kalliomäki K, Koponen M. Int Arch Occup Environ Health. 1978;42:83–90. doi: 10.1007/BF01297547. [DOI] [PubMed] [Google Scholar]
- 136.Jakubowski M, Czerczak S. Environ Toxicol Pharmacol. 2009;28:311–315. doi: 10.1016/j.etap.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 137.Anderson JC, Lamm WJ, Hlastala MP. J Appl Physiol. 2006;100:880–889. doi: 10.1152/japplphysiol.00868.2005. [DOI] [PubMed] [Google Scholar]
- 138.Konvalina G, Haick H. Acc Chem Res. 2013 doi: 10.1021/ar400070m. [DOI] [PubMed] [Google Scholar]
- 139.Poulin P, Krishnan K. Toxicol Methods. 1996;6:117–137. [Google Scholar]
- 140.Sander R. 1999 http://www.mpch-mainz.mpg.de/%7Esander/res/henry.html.
- 141.Staudinger J, Roberts PV. Chemosphere. 2001;44:561–576. doi: 10.1016/s0045-6535(00)00505-1. [DOI] [PubMed] [Google Scholar]
- 142.Pentassuglia G, Bertani B, Donati D, Ursini A. J Heterocycl Chem. 1996;33:1163–1170. [Google Scholar]
- 143.Garner CE, Smith S, de Lacy Costello B, White P, Spencer R, Probert CS, Ratcliffe NM. FASEB J. 2007;21:1675–1688. doi: 10.1096/fj.06-6927com. [DOI] [PubMed] [Google Scholar]
- 144.Terelius Y, Ingekman SM. Eur J Biochem. 1986;161:303–308. doi: 10.1111/j.1432-1033.1986.tb10447.x. [DOI] [PubMed] [Google Scholar]
- 145.Kohlmuller D. Anal Biochem. 1993;210:268–276. doi: 10.1006/abio.1993.1195. [DOI] [PubMed] [Google Scholar]
- 146.Risby T. Free Radicals Biol Med. 1999;27:1182–1192. doi: 10.1016/s0891-5849(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 147.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. Am J Respir Crit Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 149.Vaz A, Coon MJ. Proc Natl Acad Sci U S A. 1987;84:1172–1176. doi: 10.1073/pnas.84.5.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Branton PJ, McAdam KG, Winter DB, Liu C, Duke MG, Proctor CJ. Chem Cent J. 2011;5:15. doi: 10.1186/1752-153X-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ahotupa M, Bussacchini-Griot V, Bereziat JC, Camus AM, Bartsch H. Biochem Biophys Res Commun. 1987;146:1047–1054. doi: 10.1016/0006-291x(87)90753-4. [DOI] [PubMed] [Google Scholar]
- 152.Kang JO, Slater G, Aufses AHJ, Cohen G. Biochem Pharmacol. 1988;37:2967–2971. doi: 10.1016/0006-2952(88)90283-3. [DOI] [PubMed] [Google Scholar]
- 153.Burdock GA, Fenaroli G. Fenaroli’s handbook of flavor ingredients. 6th. CRC Press/Taylor & Francis Group; Boca Raton: 2010. [Google Scholar]
- 154.Yannai S. Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients. Chapman & Hall/CRC; Boca Raton: 2004. [Google Scholar]
- 155.Murray R, Granner D, Mayes P, Rodwell V. Harper’s Illustrated Biochemistry. 27. McGraw-Hill Medical; New York: 2006. [Google Scholar]
- 156.Smith D, Wang T, Spanel P. Physiol Meas. 2002;23:477. doi: 10.1088/0967-3334/23/3/301. [DOI] [PubMed] [Google Scholar]
- 157.Lagg A, Taucher J, Hansel A, Lindinger W. Int J Mass Spectrom Ion Processes. 1994;134:55–66. [Google Scholar]
- 158.Riemenschneider W, Bolt HM. Esters, Organic. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2005. [Google Scholar]
- 159.Guengerich FP, Shimada T. Chem Res Toxicol. 1991;4:391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- 160.Greenberg M. Toxicological Review of Acetonitrile. U.S. Environmental Protection Agency; Washington, DC: 1999. [Google Scholar]
- 161.Jones RG, Thompson CB. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Amann A, Telser S, Hofer L, Schmid A, Hinterhuber H. Breath Analysis: for Clinical Diagnosis and Therapeutic Monitoring. 2005:305–316. [Google Scholar]
- 163.Koc H, King J, Teschl G, Unterkofler K, Teschl S, Mochalski P, Hinterhuber H, Amann A. J Breath Res. 2011;5:037102. doi: 10.1088/1752-7155/5/3/037102. [DOI] [PubMed] [Google Scholar]
- 164.Herbig J, Müller M, Schallhart S, Titzmann T, Graus M, Hansel A. J Breath Res. 2009;3:027004. doi: 10.1088/1752-7155/3/2/027004. [DOI] [PubMed] [Google Scholar]
- 165.Ruzsanyi V, Fischer L, Herbig J, Ager C, Amann A. J Chromatogr A. 2013;1316:112–118. doi: 10.1016/j.chroma.2013.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Agapiou A, Mochalski P, Schmid A, Amann A. In: Volatile Biomarkers: Non-invasive Diagnosis in Physiology and Medicine. Amann A, Smith D, editors. Elsevier; Amsterdam: 2013. pp. 515–558. [Google Scholar]
- 167.Kohl I, Herbig J, Dunkl J, Hansel A, Daniaux M, Hubalek M. In: Volatile Biomarkers: Non-invasive Diagnosis in Physiology and Medicine. Amann A, Smith D, editors. Elsevier; Amsterdam: 2013. [Google Scholar]
- 168.Righettoni M, Tricoli A, Gass S, Schmid A, Amann A, Pratsinis SE. Anal Chim Acta. 2012;738:69–75. doi: 10.1016/j.aca.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kushch I, Arendacka B, Stolc S, Mochalski P, Filipiak W, Schwarz K, Schwentner L, Schmid A, Dzien A, Lechleitner M, Witkovsky V, et al. Clin Chem Lab Med. 2008;46:1011–1018. doi: 10.1515/CCLM.2008.181. [DOI] [PubMed] [Google Scholar]
- 170.Kushch I, Schwarz K, Schwentner L, Baumann B, Dzien A, Schmid A, Unterkofler K, Gastl G, Spanel P, Smith D, Amann A. J Breath Res. 2008;2:026002. doi: 10.1088/1752-7155/2/2/026002. [DOI] [PubMed] [Google Scholar]
- 171.Schwarz K, Filipiak W, Amann A. J Breath Res. 2009;3:027002. doi: 10.1088/1752-7155/3/2/027002. [DOI] [PubMed] [Google Scholar]
- 172.Spanel P, Dryahina K, Smith D. J Breath Res. 2013;7:017106. doi: 10.1088/1752-7155/7/1/017106. [DOI] [PubMed] [Google Scholar]
- 173.Spanel P, Smith D. Mass Spectrom Rev. 2011;30:236–267. doi: 10.1002/mas.20303. [DOI] [PubMed] [Google Scholar]
- 174.Spanel P, Dryahina K, Rejskova A, Chippendale TW, Smith D. Physiol Meas. 2011;32:N23–N31. doi: 10.1088/0967-3334/32/8/N01. [DOI] [PubMed] [Google Scholar]
- 175.Spanel P, Smith D. In: Volatile Biomarkers: Non-invasive Diagnosis in Physiology and Medicine. Amann A, Smith D, editors. Elsevier; Amsterdam: 2013. [Google Scholar]
- 176.Ross BM, Esarik A. In: Volatile Biomarkers: Non-invasive Diagnosis in Physiology and Medicine. Amann A, Smith D, editors. Elsevier; Amsterdam: 2013. [Google Scholar]
- 177.Goepel W. Sens Actuators B. 1991;B4:7–21. [Google Scholar]
- 178.Rock F, Barsan N, Weimar U. Chem Rev. 2008;108:705–725. doi: 10.1021/cr068121q. [DOI] [PubMed] [Google Scholar]
- 179.Turner AP, Magan N. Nat Rev Microbiol. 2004;2:161–166. doi: 10.1038/nrmicro823. [DOI] [PubMed] [Google Scholar]
- 180.Farahi RH, Passian A, Tetard L, Thundat T. ACS Nano. 2012;6:4548–4556. doi: 10.1021/nn204999j. [DOI] [PubMed] [Google Scholar]
- 181.Kuang Z, Kim SN, Crookes-Goodson WJ, Farmer BL, Naik RR. ACS Nano. 2010;4:452–458. doi: 10.1021/nn901365g. [DOI] [PubMed] [Google Scholar]
- 182.Bondavalli P, Legagneux P, Pribat D. Sens Actuators B. 2009;140:304–318. [Google Scholar]
- 183.Zhang T, Mubeen S, Myung NV, Deshusses MA. Nanotechnology. 2008;19:332001. doi: 10.1088/0957-4484/19/33/332001. [DOI] [PubMed] [Google Scholar]
- 184.Paska Y, Haick H. ACS Appl Mater Interface. 2012;4:2604–2617. doi: 10.1021/am300288z. [DOI] [PubMed] [Google Scholar]
- 185.Paska Y, Stelzner T, Christiansen S, Haick H. ACS Nano. 2011;5:5620–5626. doi: 10.1021/nn201184c. [DOI] [PubMed] [Google Scholar]
- 186.Dattoli EN, Davydov AV, Benkstein KD. Nanoscale. 2012;4:1760–1769. doi: 10.1039/c2nr11885h. [DOI] [PubMed] [Google Scholar]
- 187.Ekinci KL, Huang XMH, Roukes ML. Appl Phys Lett. 2004;84:4469–4471. [Google Scholar]
- 188.Ekinci KL, Roukes ML. Rev Sci Instrum. 2005;76:061101. [Google Scholar]
- 189.Hwang Y, Gao F, Hong AJ, Candler RN. J Microelectromech Syst. 2012;21:235–242. [Google Scholar]
- 190.Li X, Lee DW. Meas Sci Technol. 2012;23:022001. [Google Scholar]
- 191.Che Y, Gross DE, Huang H, Yang D, Yang X, Discekici E, Zang L. J Am Chem Soc. 2012;134:4978–4982. doi: 10.1021/ja300306e. [DOI] [PubMed] [Google Scholar]
- 192.Liang FX, Kelly TL, Luo LB, Li H, Sailor MJ, Li YY. ACS Appl Mater Interfaces. 2012;4:4177–4183. doi: 10.1021/am300896p. [DOI] [PubMed] [Google Scholar]
- 193.Zhao H, Rizal B, McMahon G, Wang H, Dhakal P, Kirkpatrick T, Ren Z, Chiles TC, Naughton MJ, Cai D. ACS Nano. 2012;6:3171–3178. doi: 10.1021/nn205036e. [DOI] [PubMed] [Google Scholar]
- 194.Dovgolevsky E, Konvalina G, Tisch U, Haick H. J Phys Chem C. 2010;114:14042–14049. [Google Scholar]
- 195.Joseph Y, Guse B, Vossmeyer T, Yasudaa A. J Phys Chem C. 2008;112:12507–12514. [Google Scholar]
- 196.Liu J, Luo T, Meng F, Qian K, Wan Y, Liu J. J Phys Chem C. 2010;114:4887–4894. [Google Scholar]
- 197.Zilberman Y, Ionescu R, Feng X, Mullen K, Haick H. ACS Nano. 2011;5:6743–6753. doi: 10.1021/nn202314k. [DOI] [PubMed] [Google Scholar]
- 198.McAlpine MC, Agnew HD, Rohde RD, Blanco M, Ahmad H, Stuparu AD, Goddard WA, Heath JR. J Am Chem Soc. 2008;130:9583–9589. doi: 10.1021/ja802506d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lewis NS. Acc Chem Res. 2004;37:663–672. doi: 10.1021/ar030120m. [DOI] [PubMed] [Google Scholar]
- 200.Machado RF, Laskowski D, Deffenderfer O, Burch T, Zheng S, Mazzone PJ, Mekhail T, Jennings C, Stoller JK, Pyle J. Am J Respir Crit Care Med. 2005;171:1286–1291. doi: 10.1164/rccm.200409-1184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Peled N, Hakim M, Bunn PA, Jr, Miller YE, Kennedy TC, Mattei J, Mitchell JD, Hirsch FR, Haick H. J Thorac Oncol. 2012;7:1528–1533. doi: 10.1097/JTO.0b013e3182637d5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Broza YY, Kremer R, Tisch U, Gevorkyan A, Shiban A, Best LA, Haick H. Nanomedicine. 2013;9:15–21. doi: 10.1016/j.nano.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 203.Chapman EA, Thomas PS, Stone E, Lewis C, Yates DH. Eur Respir J. 2012;40:448–454. doi: 10.1183/09031936.00040911. [DOI] [PubMed] [Google Scholar]
- 204.Dragonieri S, van der Schee MP, Massaro T, Schiavulli N, Brinkman P, Pinca A, Carratu P, Spanevello A, Resta O, Musti M, Sterk PJ. Lung Cancer. 2012;75:326–331. doi: 10.1016/j.lungcan.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 205.Timms C, Thomas PS, Yates DH. J Breath Res. 2012;6:016003. doi: 10.1088/1752-7155/6/1/016003. [DOI] [PubMed] [Google Scholar]
- 206.Janzen MC, Ponder JB, Bailey DP, Ingison CK, Suslick KS. Anal Chem. 2006;78:3591–3600. doi: 10.1021/ac052111s. [DOI] [PubMed] [Google Scholar]
- 207.Suslick KS. MRS Bull. 2004;29:720–725. doi: 10.1557/mrs2004.209. [DOI] [PubMed] [Google Scholar]
- 208.Mazzone PJ, Wang XF, Xu Y, Mekhail T, Beukemann MC, Na J, Kemling JW, Suslick KS, Sasidhar M. J Thorac Oncol. 2011;7:137–142. doi: 10.1097/JTO.0b013e318233d80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Grate JW, Martin SJ, White RM. Anal Chem. 1993;65:A987–A996. [Google Scholar]
- 210.Grate JW, Martin SJ, White RM. Anal Chem. 1993;65:A940–A948. [Google Scholar]
- 211.Matsuguchi M, Uno T. Sens Actuators B. 2006;113:94–99. [Google Scholar]
- 212.D’Amico A, Penazza G, Santonico M, Martinelli E, Roscioni C, Galluccio G, Paolesse R, Di Natale C. Lung Cancer. 2010;68:170–176. doi: 10.1016/j.lungcan.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 213.Di Natale C, Macagnano A, Martinelli E, Paolesse R, D’Arcangelo G, Roscioni C, Finazzi-Agro A, D’Amico A. Biosens Bioelectron. 2003;18:1209–1218. doi: 10.1016/s0956-5663(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 214.Jakubik WP. Thin Solid Films. 2011;520:986–993. [Google Scholar]
- 215.Chen X, Cao M, Hu W, Wang P, Ying K, Pan H. Meas Sci Technol. 2005;16:1535–1546. [Google Scholar]
- 216.Konvalina G, Haick H. ACS Appl Mater Interface. 2012;4:317–325. doi: 10.1021/am2013695. [DOI] [PubMed] [Google Scholar]
- 217.Szulejko JE, McCulloch M, Jackson J, McKee DL, Walker JC, Solouki T. IEEE Sens J. 2010;10:185–210. [Google Scholar]
- 218.Van den Velde S, Nevens F, Van hee P, van Steenberghe D, Quirynen M. J Chromatogr B: Biomed Appl. 2008;875:344–348. doi: 10.1016/j.jchromb.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 219.Riskin M, Tel-Vered R, Willner I. Adv Mater. 2010;22:1387–1391. doi: 10.1002/adma.200903007. [DOI] [PubMed] [Google Scholar]
- 220.Dovgolevsky E, Tisch U, Haick H. Small. 2009;5:1158–1161. doi: 10.1002/smll.200801831. [DOI] [PubMed] [Google Scholar]
- 221.Amann A, Miekisch W, Pleil J, Risby T, Schubert W. In: European Respiratory Society Monograph 49. Horvath I, de Jongste JC, editors. European Respiratory Society; Lausanne: 2010. pp. 96–114. [Google Scholar]
- 222.Beauchamp J, Herbig J, Gutmann R, Hansel A. J Breath Res. 2008;2:046001. doi: 10.1088/1752-7155/2/4/046001. [DOI] [PubMed] [Google Scholar]
- 223.Barker M, Hengst M, Schmid J, Buers HJ, Mittermaier B, Klemp D, Koppmann R. Eur Respir J. 2006;27:929–936. doi: 10.1183/09031936.06.00085105. [DOI] [PubMed] [Google Scholar]
- 224.Mochalski P, King J, Unterkofler K, Amann A. Analyst. 2013;138:1405–1418. doi: 10.1039/c2an36193k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wong MY, Cheng WR, Liu MH, Tain WC, Lu CJ. Talanta. 2012;101:307–313. doi: 10.1016/j.talanta.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 226.Groves WA, Zellers ET. Ann Occup Hyg. 2001;45:609–623. [PubMed] [Google Scholar]
- 227.Filipiak W, Ruzsanyi V, Mochalski P, Filipiak A, Bajtarevic A, Ager C, Denz H, Hilbe W, Jamnig H, Hackl M, Dzien A, et al. J Breath Res. 2012;6:036008. doi: 10.1088/1752-7155/6/3/036008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Filipiak W, Sponring A, Baur MM, Filipiak A, Ager C, Wiesenhofer H, Nagl M, Troppmair J, Amann A. BMC Microbiol. 2012;12:113. doi: 10.1186/1471-2180-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Filipiak W, Sponring A, Baur MM, Ager C, Filipiak A, Wiesenhofer H, Nagl M, Troppmair J, Amann A. Microbiology. 2012;158:3044–3053. doi: 10.1099/mic.0.062687-0. [DOI] [PubMed] [Google Scholar]
- 230.Filipiak W, Sponring A, Filipiak A, Baur MM, Ager C, Wiesenhofer H, Margesin R, Nagl M, Troppmair J, Amann A. In: Volatile Biomarkers: Non-invasive Diagnosis in Physiology and Medicine. Amann A, Smith D, editors. Elsevier; Amsterdam: 2013. pp. 463–512. [Google Scholar]
- 231.Filipiak W, Filipiak A, Ager C, Wiesenhofer H, Amann A. J Breath Res. 2012;6:027107. doi: 10.1088/1752-7155/6/2/027107. [DOI] [PubMed] [Google Scholar]
- 232.Trefz P, Rosner L, Hein D, Schubert JK, Miekisch W. Anal Bioanal Chem. 2013;405:3105–3115. doi: 10.1007/s00216-013-6781-9. [DOI] [PubMed] [Google Scholar]
- 233.Perl T, Bödeker B, Jünger M, Nolte J, Vautz W. Anal Bioanal Chem. 2010;397:2385–2394. doi: 10.1007/s00216-010-3798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ruzsanyi V, Baumbach JI, Sielemann S, Litterst P, Westhoff M, Freitag L. J Chromatogr A. 2005;1084:145–151. doi: 10.1016/j.chroma.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 235.Rudnicka J, Mochalski P, Agapiou A, Statheropoulos M, Amann A, Buszewski B. Anal Bioanal Chem. 2010;398:2031–2038. doi: 10.1007/s00216-010-4147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ruzsanyi V, Mochalski P, Schmid A, Wiesenhofer H, Klieber M, Hinterhuber H, Amann A. J Chromatogr B: Biomed Appl. 2012;911:84–92. doi: 10.1016/j.jchromb.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Prabhakar A, Iglesias Ra, Shan X, Xian X, Zhang L, Tsow F, Forzani ES, Tao N. Anal Chem. 2012;84:7172–7178. doi: 10.1021/ac301542j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Segev-Bar M, Shuster G, Haick H. J Phys Chem C. 2012;116:15361–15368. [Google Scholar]
- 239.Mochalski P, Wzorek B, Sliwka I, Amann A. J Chromatogr B: Biomed Appl. 2009;877:1856–1866. doi: 10.1016/j.jchromb.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 240.Gu C, Xu X, Huang J, Wang W, Sun Y, Liu J. Sens Actuators B. 2012;174:31–38. [Google Scholar]
- 241.Rogers PH, Benkstein KD, Semancik S. Anal Chem. 2012;84:9774–9781. doi: 10.1021/ac301687j. [DOI] [PubMed] [Google Scholar]
- 242.Garg N, Mohanty A, Lazarus N, Schultz L, Rozzi TR, Santhanam S, Weiss L, Snyder JL, Fedder GK, Jin R. Nanotechnology. 2010;21:405501. doi: 10.1088/0957-4484/21/40/405501. [DOI] [PubMed] [Google Scholar]
- 243.Alfeeli B, Agah M. IEEE Sens J. 2009;9:1068–1075. [Google Scholar]
- 244.Sidelnikov VN, Patrushev YV, Nikolaeva OA. Catal Ind. 2010;2:206–216. [Google Scholar]
- 245.Stadermann M, McBrady AD, Dick B, Reid VR, Noy A, Synovec RE, Bakajin O. Anal Chem. 2006;78:5639–5644. doi: 10.1021/ac060266+. [DOI] [PubMed] [Google Scholar]
- 246.Lambertus G, Elstro A, Sensenig K, Potkay J, Agah M, Scheuering S, Wise K, Dorman F, Sacks R. Anal Chem. 2004;76:2629–2637. doi: 10.1021/ac030367x. [DOI] [PubMed] [Google Scholar]
- 247.Reidy S, Lambertus G, Reece J, Sacks R. Anal Chem. 2006;78:2623–2630. doi: 10.1021/ac051846u. [DOI] [PubMed] [Google Scholar]
- 248.Gaspar EM, Lucena AF, Duro da Costa J, Chaves das Neves H. J Chromatogr A. 2009;1216:2749–2756. doi: 10.1016/j.chroma.2008.10.125. [DOI] [PubMed] [Google Scholar]
- 249.Song G, Qin T, Liu H, Xu GB, Pan YY, Xiong FX, Gu KS, Sun GP, Chen ZD. Lung Cancer. 2010;67:227–231. doi: 10.1016/j.lungcan.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 250.Wang X, Cui F, Lin J, Ding B, Yu J, Al-Deyab SS. Sens Actuators B. 2012;171-172:658–665. [Google Scholar]
- 251.Xu P, Li X, Yu H, Liu M, Li J. J Micromech Microeng. 2010;20:115003. [Google Scholar]
- 252.Luan F, Liu HT, Ma WP, Fan BT. Ecotoxicol Environ Saf. 2008;71:731–739. doi: 10.1016/j.ecoenv.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 253.Perbellini L, Brugnone F, Caretta D, Maranelli G. Br J Ind Med. 1985;42:162–167. doi: 10.1136/oem.42.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zahlsen K, Eide I, Nilsen AM, Nilsen OG. Pharmacol Toxicol. 1993;73:163–168. doi: 10.1111/j.1600-0773.1993.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 255.Fiserova-Bergerova V, Diaz ML. Int Arch Occup Environ Health. 1986;58:75–87. doi: 10.1007/BF00378543. [DOI] [PubMed] [Google Scholar]
- 256.Sato A, Nakajima T. Br J Ind Med. 1979;36:231–234. doi: 10.1136/oem.36.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Thrall KD, Gies RA, Muniz J, Woodstock AD, Higgins G. J Toxicol Environ Health Part A. 2002;65:2075–2086. doi: 10.1080/00984100290071838. [DOI] [PubMed] [Google Scholar]
- 258.Licata AC, Dekant W, Smith CE, Borghoff SJ. Toxicol Sci. 2001;62:191–204. doi: 10.1093/toxsci/62.2.191. [DOI] [PubMed] [Google Scholar]