Abstract
Despite continuous movements of the head, humans maintain a stable representation of the visual world, which seems to remain always upright. The mechanisms behind this stability are largely unknown. To gain some insight on how head tilt affects visual perception, we investigate whether a well-known orientation-dependent visual phenomenon, the oblique effect—superior performance for stimuli at cardinal orientations (0° and 90°) compared with oblique orientations (45°)—is anchored in egocentric or allocentric coordinates. To this aim, we measured orientation discrimination thresholds at various orientations for different head positions both in body upright and in supine positions. We report that, in the body upright position, the oblique effect remains anchored in allocentric coordinates irrespective of head position. When lying supine, gravitational effects in the plane orthogonal to gravity are discounted. Under these conditions, the oblique effect was less marked than when upright, and anchored in egocentric coordinates. The results are well explained by a simple “compulsory fusion” model in which the head-based and the gravity-based signals are combined with different weightings (30% and 70%, respectively), even when this leads to reduced sensitivity in orientation discrimination.
Keywords: oblique effect, visual cortex, gravity, head tilt, supine
Introduction
It is remarkable how, despite continuous movements of the eyes, head, and body, humans maintain a stable representation of their visual world. Much research has centered on how the brain compensates for movements of the eyes (Burr & Morrone, 2012; Wurtz, 2008), but very little research has addressed the problem caused by movements of the head. Horizontal and vertical rotations are largely compensated for by the vestibular ocular reflex (Weber et al., 2012), but tilting the head is potentially more problematic. Head tilt activates the ocular counter-roll reflex (slight rotation of both eyes in the opposite direction of head tilt), but this corrects only about 10% of the total amount of tilt for head tilts up to 45° (Bockisch & Halswanter, 2001). Yet, we are unaware of the consequences of tilting our heads, as the world seems to remain always upright. The mechanisms behind this stability are largely unknown. To gain some insight on how head tilt affects perception, we investigate whether the well-known orientation-dependent visual phenomenon—the “oblique effect” (Appelle, 1972)—is anchored in egocentric or allocentric coordinates.
The oblique effect refers to superior performance for stimuli at cardinal orientations (0° and 90°) compared with oblique orientations (45°). This has led to the idea that sampling resolution along the oblique axis is inferior to sampling along the cardinal axis (Heeley, Buchanan-Smith, Cromwell, & Wright, 1997). Orientation discrimination thresholds, contrast detection thresholds, and visual acuity all reveal oblique effects. Interestingly, the oblique effect has also been observed under conditions of increased uncertainty and was found to correspond very well with estimates of the local orientation distribution in photographs, as shown by a strong prevalence for both vertical and horizontal orientations (Girshick, Landy, & Simoncelli, 2011). However, it is still unclear whether this performance is a product of neural or environmental factors.
Since Hubel and Wiesel (1959) demonstrated that the cat primary visual cortex (V1) comprises orientation-selective cells with a columnar architecture, V1 has been considered to be a likely candidate driving the oblique effect. More recently, functional magnetic resonance imaging (fMRI) has shown that the human primary visual cortex is selective to orientation, showing a global preference for cardinal rather than oblique stimuli (Furmanski & Engel, 2000). This result remains somewhat controversial as other fMRI studies have revealed a radial bias instead (Mannion, McDonald, & Clifford, 2009; Sasaki et al., 2006), which is tightly colocalized with the angular-position component of the retinotopic map in V1 (Freeman, Brouwer, Heeger, & Merriam, 2011).
Importantly, the neural differences at varying orientations reflect differences in psychophysical performance. Banks and Stolarz (1975) reported that head tilt had no effect on the oblique effect, as measured by contrast sensitivity thresholds. Perhaps this is to be expected, as contrast sensitivity is thought to be limited by basic neural mechanisms, probably as early as V1 (Sclar, Maunsell, & Lennie, 1990). However, in visual reproduction of orientation the superior performance for retinal cardinal stimuli did not shift along with the head when the subjects were tilted ±22.5° (Lipshits & McIntyre, 1999) or ±45° (Luyat & Gentaz, 2002). Similarly, early reports on orientation discrimination precision have shown a superior performance around gravity-defined cardinal axes at head tilts of 45° using sine wave gratings (Buchanan-Smith & Heeley, 1993) and at body tilts of 20° using short lines (0.5; Orban, Vandenbussche, & Vogels, 1984). These results are in line with investigations of the subjective visual vertical (SVV) showing an important role of gravity on our perception of “up” (Dyde, Jenkin, & Harris, 2006; Mittelstaedt, 1986). Interestingly, the haptic oblique effect (preference for cardinal axes in discrimination orientation by touch) also depends on the subjective gravitational reference frame (Luyat, Gentaz, Corte, & Guerraz, 2001).
The aim of the present study is to establish whether the asymmetries in orientation discrimination depend on the neural properties of the human visual system, or whether the gravitational system and body orientation play a fundamental role. We disentangled these two alternatives by measuring orientation discrimination precision at different angles of head tilt, both while seated upright and lying supine to discount gravitational effects.
Methods
Upright position
We measured sensitivity for orientation discrimination as a function of the base orientation, with the head vertical and tilted. Two groups of subjects were tested: five (three males, all naïve) in Pisa, with head tilts of 0°, 15°, and 30°; and six (one male, four naïve) in York with head tilts of 0° and 45°. In Pisa, 12 different base orientations were used, varying from 0° (vertical) to ±90° (horizontal) in steps of 15°. In York, the range was spanned by eight orientations in 22.5° steps. The experiment was run using PsychoPy (Peirce, 2007, 2008), in a block design such that the orientation discrimination task for all base orientations was completed at a specific head tilt before moving on to a subsequent head position. The order of head position was counterbalanced across subjects.
We ensured an accurate head tilt position throughout the course of the experiment using a Wii remote controller (Nintendo, Kyoto, Japan), which gave a discrete but noticeable vibrating signal when participants deviated from the desired head tilt position. The experimental protocol was approved by the Ethics Committee of the Scientific Institute Stella Maris in Pisa and was performed in accordance with the Declaration of Helsinki. In York, the study was approved by the University of York Psychology Ethics committee. All participants gave informed written consent.
We used the method of constant stimuli, in a two-alternative forced-choice paradigm. Subjects were required to discriminate which of two successively displayed gratings appeared more clockwise, the base orientation (randomly presented first or second) or a probe stimulus whose orientation deviated from the base by ±1°, ±3°, or ±5° in Pisa, and ±1.5°, ±4.5°, or ±7.5°in York. Six stimulus conditions were randomly interleaved. All stimuli were gratings of 2 c/°, 90% contrast, 200 ms duration, and curtailed within a two-dimensional Gaussian window with full width half maximum of 2° and mean luminance 10 cd/m2. A white noise mask curtailed within a two-dimensional Gaussian window with full width half maximum of 2° was presented for 500 ms after each grating stimulus (see Figure 1A). Following participant’s response for each stimulus there was a 500 ms intertrial interval. Stimuli were presented on a Barco monitor in the Pisa upright setup and on an LCD screen for York upright setups (Figure 2A). We eliminated all visual references by running the experiment in total darkness and displaying the grating patches within a circular screen (diameter = 23° of visual angle). The distance to the screen was 57 cm.
Figure 1.
(A) Timeline and stimuli used to test the oblique effect under different conditions. (B) Psychometric functions from a typical subject in an upright position for three base orientations.
Figure 2.
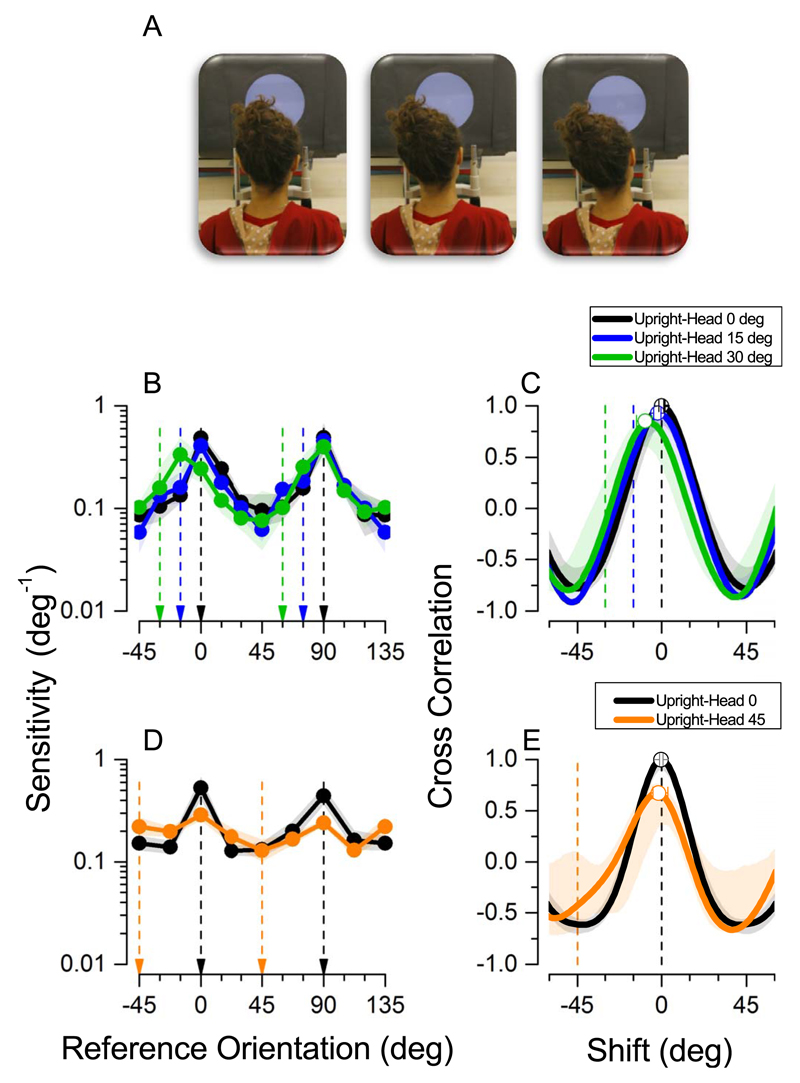
Upright experiment. (A) Schematic of the three head positions. Please note that the experiment was run in total darkness—the lights were kept on only for the purpose of taking the photographs. (B) Sensitivity measurements for 12 base orientations for three different positions (N = 5). Reference Orientation refers to screen coordinates with 0° indicating vertical and 90° horizontal stimuli, vertical lines indicate retinal vertical and horizontal stimuli. (C) Cross correlations of the three conditions against Head at 0°. (D) Sensitivity measurements for eight base orientations for two different head positions (N = 6). (E) Cross correlations of the two head conditions against Head at 0°. In B and D, shading represents ±1 standard error of the mean; in C and E, shading represents the confidence interval 5%–95%. Arrows indicate vertical (0,−15,−30,−45) and horizontal (90, +75, +60, +45) stimuli on the retina at each specific head tilt.
Psychometric error functions like those of Figure 1B were fitted to the data (15 points per condition), with the space constant σ yielding estimates of threshold (inverse of sensitivity). All observers (age range 18–30 years) had normal or corrected-to-normal vision.
Supine position
We repeated the measurement of orientation discrimination thresholds with the Pisa subjects in supine position, observing the stimuli on an LCD screen positioned above them (Figure 3A). There were three conditions: head and the body aligned at 0° or 30° with respect to the screen, or the head at 30° and the body at 0°. We ran this experiment in Pisa with the same subjects who completed the upright condition. All other experimental details were identical to the first experiment.
Figure 3.
Supine experiment. (A) Representation of a control condition upright Head 0° (from Figure 2) and three conditions while lying supine: Head and Body 0°, Head 30° and Body 0°, Head and Body 30°. Please note that the experiment was run in total darkness. (B) Sensitivity measurements for 12 base orientations for a control position (from Figure 2B) and three different supine positions (N = 5). Reference Orientation refers to screen coordinates with 0° indicating vertical and 90° horizontal stimuli, color-coded vertical lines indicate vertical and horizontal stimuli on the retina for each condition. (C) Cross correlations of the three supine conditions against Body and Head at 0° obtained in the previous upright experiment. In B, shading represents ±1 standard error of the mean; in C, shading represents the confidence interval 5%–95%. Arrows indicate vertical (0,−30) and horizontal (90, +60) stimuli on the retina at each specific head tilt.
Results
Upright position
Figure 1B illustrates psychometric functions from a typical subject for three base orientations in upright position. It is evident from the steepness of psychometric functions that performance is quite precise for grating patches at cardinal orientations (0°, 90°), but compromised for the oblique orientation (45°), as is commonly reported.
We define sensitivity as the inverse of standard deviation (1/σ), of the best-fitting cumulative Gaussians for each subject at each of twelve base orientations. Figure 2B shows how sensitivity, averaged over five subjects, varied with base orientation at three different head positions (0°, 15°, 30°). The pattern of results at 0° and 15° head-tilts is very similar, with sensitivity for orientation discrimination reaching its highest point for vertical (0°) and horizontal (90°) grating patches, and that for oblique stimuli (around 45°) severely compromised (troughs in the two functions). At head tilts of 30°, the pattern is similar to the other two conditions, except for a minor shift in peak towards the direction of the head tilt. This small shift seems to occur only for vertical gratings, and is much less than the magnitude of head tilt.
To determine the effect of head tilt on peak sensitivity we cross correlated the sensitivity functions in the head tilted positions against those with the head vertical. Cross correlation is a measure of similarity of the shape of two curves as they are slid over each other. If for a given shift the two normalized functions overlay perfectly, the cross correlation for that shift will be 1. The higher the cross correlation, the higher is the match between the two curves; hence, the peak of the cross correlation function indicates the best shift needed for the two functions to be as similar as possible. The black function in Figure 2C is an autocorrelation of the canonical position with itself, providing a baseline for comparison, and the blue and green functions for 15° and 30° head tilt, respectively. The 15° cross-correlation function is very similar to the autocorrelation with a peak at −1.8°, only slightly but significantly shifted from zero (bootstrap t test, p = 0.01). The cross correlation for 30° head tilt shows a slight shift in the peak of sensitivity for orientation discrimination of 8.4° towards the direction of head tilt (bootstrap t test, p < 0.001).
In a separate group of subjects in York, we measured thresholds with the head tilted 45°: this is of special interest as gravitational vertical and horizontal stimuli will be imaged obliquely on the retina, and oblique stimuli will be imaged at cardinal orientations on the retina.
Figure 2D shows average sensitivity for each of the eight different base orientations at the two head positions (0°, 45°). As before, orientation discrimination remains superior for stimuli at cardinal orientations, with the head vertical. When the head is tilted at 45° the general shape is maintained although the difference between peaks and troughs is less pronounced. Figure 2E shows cross correlations between measurements in the canonical position, Head 0°, for this group of subjects, and the Head 45° position. The cross correlation with the head at 45° shows a clear peak at −0.9°, which is not significantly different from zero shift (autocorrelation: p = 0.34).
In summary, when subjects were sitting upright, sensitivity was generally finest at gravitational 0° or 90°, irrespective of head tilt, with very little difference between the four head positions (0°, 15°, 30°, 45°). We only observed a minor shift of 8.4° in sensitivity towards the direction of head tilt when the head was tilted 30°.
Supine position
In the previous section we showed that the oblique effect follows the gravitational frame of reference, as orientation discrimination abilities were optimum at gravitational cardinal rather than retinal cardinal orientations, suggesting that the effect of gravity is fundamental for orientation discrimination. To better understand the role of gravity, we discount gravitational effects by having the subjects perform the orientation discrimination task lying on their backs. We also included an experimental condition in which the orientations of the head and the body were dissociated.
Figure 3B shows how average sensitivity varied as a function of base orientation for the three different supine positions: Head and Body at 0°, Head at 30° and Body at 0°, Head and Body at 30°. For comparison purposes, the thin black lines reproduce data when participants were sitting upright with head at 0° (from Figure 2B). Despite the loss in sensitivity at the cardinal axes, the pattern of results when lying supine with the head straight is similar to that in the canonical position from the previous section. The pattern of results for the other two conditions was similar to this condition, except both shifted by 30° in the same direction of the head tilt, irrespective of the position of the body.
Figure 3C shows cross correlations between the canonical position from the previous section (sitting upright, Head 0°) and the three supine positions. The main result is that when the head is tilted 30° irrespective of body position, the peaks shift in the same direction, both by 26°. As the pattern of results does not change with body position, we can safely deduce that body orientation does not contribute to orientation discrimination.
These results show that when gravitational effects are discounted, orientation discrimination depends primarily on the orientation of the head, which also governs retinal orientation. At this stage we cannot determine which is more important.
Model
Taken together, the results suggest that at least two distinct mechanisms operate in determining orientation discrimination: the strongest is gravity-based, and normally dominates. However, when gravity is unavailable, a head-based oblique effect emerges. As the orientation of the head also determines the orientation of the retina, this could reflect asymmetries in orientation tuning mechanisms in early retinotopic visual cortex (Furmanski & Engel, 2000) and beyond (Liu & Pettigrew, 2003; Orban & Vogels, 1998; Wang, Ding, & Yunokuchi, 2003).
We model the data assuming that two separate processes combine to determine orientation sensitivity, one allocentric (gravity-based), and the other egocentric (head-based). For simplicity, we assumed that the sensitivity of these mechanisms (SH and SG, respectively) have a sinusoidal pattern on log coordinates maximal at 0° and 90° and minimal at ±45° (see dashed curves of Figure 4). Mathematically, this can be formulated by the following equations:
| (1) |
| (2) |
θH and θG are the stimulus angles defined in head and gravitational coordinates, respectively, and αH, αG, and b are free parameters adjusted to fit the data. αH and αG determine the amplitude of the olique effect, and b determines the baseline sensitivity at ±45°. The three free parameters were chosen independently when fitting alternative models to the data. There is no real justification for the assumption of sinusoidally modulated sensitivity, and it is probably wrong in detail, but without some simplification there would be too many parameters to constrain.
Figure 4.
Comparison of optimal fusion and compulsory fusion model fits to data. Model predictions for four experimental conditions: supine (A–B), upright (C–D), upright with tilted head, either by 30° (E–F) or by 45° (G–H). Dotted and dashed lines represent sensitivity for the head-based and gravity-based systems fitted separately for the two classes of models. Light blue shows the predictions for a system operating an optimal fusion of the two mechanisms assigning a fixed weight according to the sensitivity of the two sources of information. Red shows the predictions for a system operating a compulsory fusion of the systems assigning a fixed weight of 0.7 to the gravity-based information. Please note that in A and B, the resulting model prediction is equal to the head-based system; the dashed head-based system is not visible because the model prediction is overlaid on top. The Pisa dataset is shown with filled symbols, and the York dataset with empty symbols. Stars indicate stimuli, which are vertical or horizontal on the retina (head-eye). Vertical lines indicate gravitational vertical and horizontal. Best fits of the models have been obtained with values of b =−1.15, αH = 0.6, and αG = 0.7 for the optimal fusion and b = −1.085, αH = 0.6, and αG = 0.7 for the compulsory fusion.
How should the two sources of information combine? We simulated two standard methods: optimal fusion, in which the two pieces of information are combined in a statistically optimal way, and compulsory fusion, in which they combine algebraically, even if to disadvantage (Hillis, Ernst, Banks, & Landy, 2002).
When performing a linear combination of two independent sources of information, the resulting variance is always a linear combination of the two. In our case, where the two mechanisms are orientation in gravitational coordinates and orientation in head–eye coordinates, respectively, with variances and the overall variance is given by:
| (3) |
Different strategies can be used to assign weights. The optimal fusion has variable weights, proportional to the reliability for each stimulus, in which σ2 is the variance noise in each system (Landy, Banks, & Knill, 2011).
| (4) |
| (5) |
This method of combination is considered optimal in that it minimizes the variance of the combined estimate. This can be confirmed mathematically by substituting Equations 4 and 5 into Equation 3 as follows:
| (6) |
As a consequence, in an optimal fusion model the combination can only improve performance; it can never be worse than the better of the two systems alone.
The alternative model assumes compulsory fusion, where the two mechanisms are always combined with a given weight regardless of stimulus orientation, whether advantageous or not. In this case the system noise equates simply with Equation 3.
In our simulation of the compulsory fusion model, the weight of gravity-based and head-based mechanisms are fixed. To simulate the general trend of the data, we assume a greater weight for the gravity than the head-based mechanism. Without any specific information in the literature to guide our choice as the dominance of the head-based mechanism varies with many factors, such as line length (Orban et al., 1984), we assume that the gravity-based system is about twice as effective as the head-based system, leading to weights wG = 0.7 and wH = 0.3. As a final assumption, we assume that there exists a higher level intrinsic noise affecting all orientations, set to 1° (Orban et al., 1984); consequently, even if the information available is perfect, the visual system has a limiting resolution that does not allow it to perform a task with maximum precision (Burr & Wijesundra, 1991).
Figure 4 displays best fitting functions for the optimal fusion (light blue) and the compulsory fusion (red) models, optimizing the free parameters, b, αH and αG of Equations 1 and 2, which determine the floor and peaks of the sinusoidal-like sensitivities independently for each of the two models. Dashed lines represent sensitivity curves for the two sources of information (gravity-based and head-based). In Figure 4A and B the resulting model predictions are essentially equal to the head-based system, because the task was performed in a supine position. In Figure 4C and D the head is vertical so the two functions are aligned, whereas in Figure 4E through H, the head is tilted either 30° or 45°, so the function peaks are 30° or 45° out of phase, respectively.
In all conditions the optimal fusion essentially surfs over the top of both curves, while the compulsory fusion model lies between the two single mechanism predictions, closer to the stronger gravity-based mechanism. The key differences in the predictions lie at the cardinal orientations on the retina, at −30° and +60° in Figure 4E and F, and ±45° in Figure 4G and H, showing deterioration in performance that can only be predicted by compulsory fusion of information. This compulsory fusion model predicts a reduction in sensitivity at gravitational vertical when the head is tilted, without a shift in the peak of the function—the two mechanisms work in counterphase against each other, lowering sensitivity but not displacing it. On the contrary, the optimal fusion model provides poor fits in the upright misaligned condition as it predicts a much better performance (Figure 4E). This behavior stems directly from a basic property of a system operating an optimal integration of the information available: the resulting combined performance is always better than the performance of any of the two mechanisms operating in isolation.
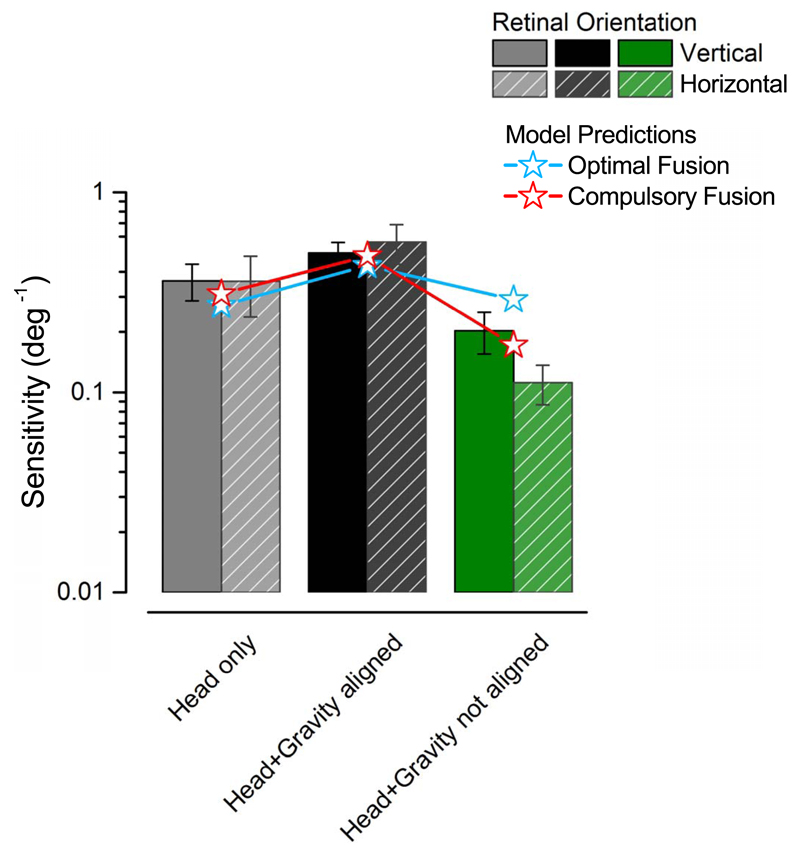
Figure 5 compares the data and model predictions for retinal stimuli on cardinal axes in different positions: supine, upright, and upright head tilted 30°. In the supine condition (grey bars), only the head-based system is at work; in the upright condition (black), both mechanisms are at work and they are aligned. When the head is tilted in an upright position (green bars) the head-eye based and the gravity-based systems are misaligned. A two-way repeated measures ANOVA examined the effects of position (three levels: supine, upright, upright head tilted) and stimulus orientation (two levels: vertical vs. horizontal) on orientation discrimination abilities. It shows a significant main effect of position, F(2, 8) = 13.8, p = 0.003, and a significant difference between upright (black) versus upright head tilted (green) positions, Bonferroni corrected t test: t(4) = 5.14, p = 0.003; and supine (gray) versus upright head tilted (green) positions, Bonferroni corrected t test: t(4) = 3.51, p = 0.024.
Figure 5.
Data for a stimulus presented along the cardinal axes of the retina (solid is vertical, striped is horizontal) in three key conditions: supine, upright and upright with the head tilted 30°. Thin lines overlay the predictions of the two methods for system integration. Only compulsory fusion predicts a drop of performance in the upright head tilted condition.
These data speak strongly in favor of compulsory fusion of the separate mechanisms: one mechanism on its own is more sensitive than two out of phase. Red and light-blue lines illustrate predictions of the two strategies for system integration. Only compulsory fusion predicts a drop of performance in the upright head tilted condition.
Discussion
The aim of this study was to examine the oblique effect of orientation discrimination under various conditions, to determine the coordinate system of the effect (allocentric or egocentric), and the possible mechanisms behind it. By measuring orientation discrimination precision at different angles of head tilt, both while seated upright and lying supine to eliminate the influence of gravity, we show that the oblique effect is anchored in both allocentric and egocentric coordinates. The strongest effect, which normally dominates, is gravity-based, with a strong superiority for vertical and horizontal stimuli, irrespective of their retinal projections. However, when the gravitational system is unavailable, a head-centered oblique effect emerges, possibly reflecting asymmetries in orientation-tuned mechanisms in early visual cortex (Furmanski & Engel, 2000).
The results are well explained by a simple model in which the gravity-based and the head-based mechanisms are combined with fixed weightings, with the gravity-based weight about double than the retinal-based (wG = 0.7; wH = 0.3). This class of model can be termed compulsory fusion, or the obligatory combination of the two mechanisms, even when the combination leads to lower sensitivity than the best mechanism alone. An optimal fusion model, on the other hand, predicts that sensitivity is always governed by the most sensitive mechanism—or a probabilistic combination of them—so it can never be worse than the action of either mechanism on its own. However, the data summarized in Figure 5 show that with the head tilted at 30°, sensitivity is two to three times worse when the subject is upright—with both gravity-based and head-based mechanisms operative—than when gravity is excluded with the subject supine. We therefore pursued the compulsory fusion idea, and showed that this model, using minimal assumptions can fit the data reasonably well. More precise assumptions would obviously lead to a better fit.
The choice of the 2:1 weighting in favor of gravity-based mechanism was somewhat arbitrary, but probably not far wrong. Previous work has shown that the oblique effect for upright subjects varies with stimulus parameters; for example, the effect follows allocentric coordinates when tested with short lines (0.5°) and retinotopic coordinates for long lines (15°). Thus, the exact weighting will vary from situation to situation, depending on line length and other factors, such as contrast and spatial frequency. We presume that the system takes this information into account when assigning weights; however, the compulsory fusion model assumes that the stimulus weights do not vary with factors such as head-tilt, even if the result is decreased precision.
Indeed, many aspects of the model were arbitrary, such as the assumption of sinusoidal modulation of log sensitivity. There is no evidence that this is the case, and close inspection of the results shows that, in detail, it is not. However, some simplifying assumptions had to be made to reduce the parameter space. The model should not be considered a precise description of the underlying mechanisms, but instead, an existence proof that the trend of the data can be captured by a simple model with two distinct mechanisms, one tuned to gravity-based coordinates, the other to retina-based coordinates, combined with appropriate, but unvarying, weights.
What neural mechanisms correspond to the two mechanisms we propose in our compulsory fusion model? The gravity-based mechanism must depend on output from the otolith organs in the inner ear, the utricle and the saccule, which sense the position of the head relative to gravity. Whereas the utricle senses left/right and forward/backward translations, the saccule senses translations in the vertical plane (up/down and forward/backward). These vestibular signals must provide an internal standard for orientation judgments (Mast, 2000). In the absence of gravity, orientation discrimination tasks become more difficult as the subject must store in memory the orientation of the first presentation and compare it with the second.
The other system is head-based. A likely candidate for increased sensitivity around the cardinal axes is the distribution and selectivity of detectors in V1. Larger neural responses for cardinal rather than oblique stimuli have been reported in the human primary visual cortex as evidence for orientation selectivity, which corresponded to subjects’ contrast detection and orientation discrimination thresholds (Furmanski & Engel, 2000). Similar effects have been reported in cat striate cortex where an increased number of cells were found to prefer cardinal over oblique orientations (Li, Peterson, & Freeman, 2003).
However, we cannot be certain that the sensitivity of the head-based mechanism comes entirely from neural tuning of retinotopic mechanisms in V1 (and beyond). As we cannot dissociate eye from head tilt, other head-centered factors could be involved. For example, the sense of the orientation of the head, obtained through kinaesthetic sensation, may provide an internal standard akin to that of gravity. We cannot dissociate eye-centered from head-centered effects because the position of the two is extremely similar, differing by only about 10% because of the ocular counter-roll (OCR; Bockisch & Halswanter, 2001).
The situation could be even more complicated. There is evidence that the activity of single units in the mammalian visual cortex can be modulated by vestibular input (Jung, Kornhuber, & da Fonseca, 1963), suggesting that head movements not only induce compensatory eye movements via the vestibular ocular reflex (VOR) and OCR, but they can also affect the activity of some V1 neurons. This agrees with studies of the cat visual cortex showing that a minority of cortical units undergo a receptive field orientation change following the spatial and not the retinal orientation of the stimulus (Denney & Adorjani, 1972; Horn & Hill, 1969).
One other possibility is that orientations are coded as deviations from the gravitational vertical and possibly horizontal. Indeed, assuming that the noise varies with deviation from vertical or horizontal, such a system could be a straightforward implementation of a gravity-based system with periodic sensitivity. This proposition fits nicely with the idea of compulsory fusion as it would be implemented with a cascade of processes; first retinotopic orientation decoding, followed by translation onto allocentric coordinates, with the overall noise being the sum of the two, similar to compulsory fusion.
Given that the oblique effect has been traditionally considered to result from orientation-tuning mechanisms in early retinotopic visual areas, the strong dependence on gravity might come as a surprise. Previous studies carried out in space, with all gravitational cues eliminated, have shown that although orientation judgments measured by reproduction of a remembered orientation depend on a multimodal frame of reference that includes gravity, an egocentric reference is sufficient to elicit the oblique effect (Lipshits, Bengoetxea, Cheron, & McIntyre, 2005). Broadly, this is in agreement with our results, highlighting the importance of both effects. We further show that gravity is more important than egocentric effects. Gravity has been shown to affect various tasks, such as the perceived stability of objects (Barnett-Cowan, Fleming, Singh, & Bülthoff, 2011), and together with visual and body-orientation cues, it has been implicated in the direction of perceptual upright (Jenkin, Jenkin, Dyde, & Harris, 2004). All this is consistent with our results endorsing its importance for the oblique effect.
To conclude, the fine sense of gravitational vertical, sensed by the vestibular system, may be fundamental to keeping vision stable in the face of continual head rotations. Furthermore, enhanced neural sensitivity for vertical and horizontal orientations may help keep the internal spatial map aligned with the world.
Acknowledgments
This work was supported by the European Research Council (FP7; Space Time and Number in the Brain; and Early Sensory Cortex Plasticity and Adaptability in Human Adults) and the Italian Ministry of Education, University and Research-Futuro in Ricerca Grant 2013 (FIRB RBFR1332DJ).
Footnotes
Commercial relationships: none.
Contributor Information
Kyriaki Mikellidou, Department of Translational Research on New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
Guido Marco Cicchini, Institute of Neuroscience, National Research Council (CNR), Pisa, Italy cicchini@in.cnr.it.
Peter G. Thompson, Department of Psychology, University of York, York, United Kingdom peter.thompson@york.ac.uk
David C. Burr, Institute of Neuroscience, National Research Council (CNR), Pisa, Pisa, Italy; Department of Neuroscience, University of Florence, Florence, Italy Dave@in.cnr.it
References
- Appelle S. Perception and discrimination as a function of stimulus orientation: The “oblique effect” in man and animals. Psychological Bulletin. 1972;78:266–278. doi: 10.1037/h0033117. [DOI] [PubMed] [Google Scholar]
- Banks MS, Stolarz SJ. The effect of head tilt on meridional differences in acuity: Implications for orientation constancy. Perception & Psychophysics. 1975;17:17–22. [Google Scholar]
- Barnett-Cowan M, Fleming RW, Singh M, Bülthoff HH. Perceived object stability depends on multisensory estimates of gravity. PloS one. 2011;6(4):e19289. doi: 10.1371/journal.pone.0019289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockisch CJ, Halswanter T. Three-dimensional eye position during static roll and pitch in humans. Vision Research. 2001;41:2127–2137. doi: 10.1016/s0042-6989(01)00094-3. [DOI] [PubMed] [Google Scholar]
- Buchanan-Smith HM, Heeley DW. Anisotropic axes in orientation perception are not retinotopically mapped. Perception. 1993;22:1389–1402. doi: 10.1068/p221389. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC. Constructing stable spatial maps of the world. Perception. 2012;41:1355–1372. doi: 10.1068/p7392. [DOI] [PubMed] [Google Scholar]
- Burr DC, Wijesundra SA. Orientation discrimination depends on spatial frequency. Vision Research. 1991;31:1449–1452. doi: 10.1016/0042-6989(91)90064-c. [DOI] [PubMed] [Google Scholar]
- Denney D, Adorjani C. Orientation specificity of visual cortical neurons after head tilt. Experimental Brain Research. 1972;14:312–317. doi: 10.1007/bf00816165. [DOI] [PubMed] [Google Scholar]
- Dyde RT, Jenkin MR, Harris LR. The subjective visual vertical and the perceptual upright. Experimental Brain Research. 2006;173:612–622. doi: 10.1007/s00221-006-0405-y. [DOI] [PubMed] [Google Scholar]
- Freeman J, Brouwer GJ, Heeger DJ, Merriam EP. Orientation decoding depends on maps, not columns. The Journal of Neuroscience. 2011;31:4792–4804. doi: 10.1523/jneurosci.5160-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmanski CS, Engel SA. An oblique effect in human primary visual cortex. Nature Neuroscience. 2000;3:535–536. doi: 10.1038/75702. [DOI] [PubMed] [Google Scholar]
- Girshick AR, Landy MS, Simoncelli EP. Cardinal rules: Visual orientation perception reflects knowledge of environmental statistics. Nature Neuroscience. 2011;14:926–932. doi: 10.1038/nn.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeley DW, Buchanan-Smith HM, Cromwell JA, Wright JS. The oblique effect in orientation acuity. Vision Research. 1997;37:235–242. doi: 10.1016/s0042-6989(96)00097-1. [DOI] [PubMed] [Google Scholar]
- Hillis JM, Ernst MO, Banks MS, Landy MS. Combining sensory information: Mandatory fusion within, but not between, senses. Science. 2002;298(5598):1627–1630. doi: 10.1126/science.1075396. [DOI] [PubMed] [Google Scholar]
- Horn G, Hill RM. Modifications of receptive fields of cells in the visual cortex occurring spontaneously and associated with bodily tilt. Nature. 1969;221(5176):186–188. doi: 10.1038/221186a0. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. The Journal of Physiology. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin HL, Jenkin MR, Dyde RT, Harris LR. Shape-from-shading depends on visual, gravitational, and body-orientation cues. Perception. 2004;33:1453–1461. doi: 10.1068/p5285. [DOI] [PubMed] [Google Scholar]
- Jung R, Kornhuber HH, da Fonseca JS. Multisensory convergence on cortical neurons: Neuronal effects of visual, acoustic and vestibular stimuli in the superior convolutions of the cat’s cortex. In: Moruzzi G, Fessard A, editors. Progress in brain research. Vol. 1. Amsterdam: Elsevier; 1963. pp. 207–240. [Google Scholar]
- Landy MS, Banks MS, Knill DC. Ideal-observer models of cue integration. In: Trommershäuser J, Körding K, Landy MS, editors. Sensory cue integration. New York: Oxford University Press; 2011. pp. 5–29. [Google Scholar]
- Li B, Peterson M, Freeman RD. Oblique effect: A neural basis in the visual cortex. Journal of Neurophysiology. 2003;90:204–217. doi: 10.1152/jn.00954.2002. [DOI] [PubMed] [Google Scholar]
- Lipshits M, Bengoetxea A, Cheron G, McIntyre J. Two reference frames for visual perception in two gravity conditions. Perception. 2005;34:545–555. doi: 10.1068/p5358. [DOI] [PubMed] [Google Scholar]
- Lipshits M, McIntyre J. Gravity affects the preferred vertical and horizontal in visual perception of orientation. NeuroReport. 1999;10:1085–1089. doi: 10.1097/00001756-199904060-00033. [DOI] [PubMed] [Google Scholar]
- Liu GB, Pettigrew JD. Orientation mosaic in barn owl’s visual Wulst revealed by optical imaging: Comparison with cat and monkey striate and extra-striate areas. Brain Research. 2003;961(1):153–158. doi: 10.1016/s0006-8993(02)03747-2. [DOI] [PubMed] [Google Scholar]
- Luyat M, Gentaz E. Body tilt effect on the reproduction of orientations: Studies on the visual oblique effect and subjective orientations. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:1002–1011. doi: 10.1037/0096-1523.28.4.1002. [DOI] [PubMed] [Google Scholar]
- Luyat M, Gentaz E, Corte TR, Guerraz M. Reference frames and haptic perception of orientation: Body and head tilt effects on the oblique effect. Perception & Psychophysics. 2001;63:541–554. doi: 10.3758/BF03194419. [DOI] [PubMed] [Google Scholar]
- Mannion DJ, McDonald JS, Clifford CWG. Discrimination of the local orientation structure of spiral Glass patterns early in human visual cortex. NeuroImage. 2009;46:511–515. doi: 10.1016/j.neuroimage.2009.01.052. [DOI] [PubMed] [Google Scholar]
- Mast FW. Human perception of verticality: Psychophysical experiments on the centrifuge and their neuronal implications. Japanese Psychological Research. 2000;42(4):194–206. doi: 10.1111/1468-5884.00146. [DOI] [Google Scholar]
- Mittelstaedt H. The subjective vertical as a function of visual and extraretinal cues. Acta Psychologica. 1986;63:63–85. doi: 10.1016/0001-6918(86)90043-0. [DOI] [PubMed] [Google Scholar]
- Orban GA, Vandenbussche E, Vogels R. Human orientation discrimination tested with long stimuli. Vision Research. 1984;24:121–128. doi: 10.1016/0042-6989(84)90097-x. [DOI] [PubMed] [Google Scholar]
- Orban GA, Vogels R. The neuronal machinery involved in successive orientation discrimination. Progress in Neurobiology. 1998;55:117–147. doi: 10.1016/s0301-0082(98)00010-0. [DOI] [PubMed] [Google Scholar]
- Peirce JW. PsychoPy—Psychophysics software in Python. Journal of Neuroscience Methods. 2007;162(1):8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW. Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics. 2008;2:1–8. doi: 10.3389/neuro.11.010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Rajimehr R, Kim BW, Ekstrom LB, Vanduffel W, Tootell RB. The radial bias: A different slant on visual orientation sensitivity in human and nonhuman primates. Neuron. 2006;51:661–670. doi: 10.1016/j.neuron.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Sclar G, Maunsell JH, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Research. 1990;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- Wang G, Ding S, Yunokuchi K. Difference in the representation of cardinal and oblique contours in cat visual cortex. Neuroscience Letters. 2003;338:77–81. doi: 10.1016/s0304-3940(02)01355-1. [DOI] [PubMed] [Google Scholar]
- Weber KB, Rosengren SM, Michels R, Sturm V, Straumann D, Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. The Journal of Physiology. 2012;590:3091–3101. doi: 10.1113/jphysiol.2011.226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH. Neuronal mechanisms of visual stability. Vision Research. 2008;48:2070–2089. doi: 10.1016/j.visres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]