Abstract
The stability of 41 selected breath constituents in three types of polymer sampling bags, Tedlar, Kynar, and Flexfilm, was investigated using solid phase microextraction and gas chromatography mass spectrometry. The tested molecular species belong to different chemical classes (hydrocarbons, ketones, aldehydes, aromatics, sulphurs, esters, terpenes, etc.) and exhibit close-to-breath low ppb levels (3–12 ppb) with the exception of isoprene, acetone and acetonitrile (106 ppb, 760 ppb, 42 ppb respectively). Stability tests comprised the background emission of contaminants, recovery from dry samples, recovery from humid samples (RH 80% at 37 °C), influence of the bag’s filling degree, and reusability. Findings yield evidence of the superiority of Tedlar bags over remaining polymers in terms of background emission, species stability (up to 7 days for dry samples), and reusability. Recoveries of species under study suffered from the presence of high amounts of water (losses up to 10%). However, only heavier volatiles, with molecular masses higher than 90, exhibited more pronounced losses (20–40%). The sample size (the degree of bag filling) was found to be one of the most important factors affecting the sample integrity. To sum up, it is recommended to store breath samples in pre-conditioned Tedlar bags up to 6 hours at the maximum possible filling volume. Among the remaining films, Kynar can be considered as an alternative to Tedlar; however, higher losses of compounds should be expected even within the first hours of storage. Due to the high background emission Flexfilm is not suitable for sampling and storage of samples for analyses aiming at volatiles at a low ppb level.
1. Introduction
Preservation of the sample integrity during sampling and sample storage is probably one of the most demanding challenges in analytical chemistry. Different phenomena accompanying these phases of analysis like, e.g., background emission of pollutants, losses and interactions between sample constituents irreversibly modify the original sample composition and consequently distort the final results of analyses. This is particularly true in the case of exhaled breath analysis. Ultra-low concentrations of volatile organic breath constituents (from low ppb to low ppt), presence of highly reactive species and high humidity inducing wet chemistry make breath samples particularly vulnerable to all problems related to storage.1–6 Despite availability of real-time techniques such as proton transfer reaction mass spectrometry (PTR-MS),7–14 or selected ion flow tube mass spectrometry (SIFT-MS),15,16 gas chromatography mass spectrometry (GC-MS) remains the gold standard for the analysis of breath constituents.1–3,5,17 Since GC-MS analysis of exhaled air is usually coupled with a time-consuming sample pre-concentration method (e.g., solid phase microextraction (SPME), sorbent trapping, or needle traps (NTD)), sample storage is an inherent part of the analytical procedure. In this context the selection of the optimal storage conditions for breath samples is of particular importance.
Currently, Tedlar (PVF, polyvinyl fluoride) is one of the most popular and commonly accepted materials for collecting gaseous samples in general and breath gas samples in particular.1,3,18–26 This is due to its moderate price, inertness, relatively good durability, and reusability. A number of studies have investigated the storage of breath constituents in polymer bags. Nevertheless, the majority of them focused on a limited number of species at levels much higher than the ones observed in breath, or dealt with a single analyte related to a specific disease or disorder.19–21,27,28 For example, Groves and Zellers27 tested the influence of high humidity on the recovery of 6 breath-related compounds at the ppm level. The observed differences between dry and wet matrices were smaller than 10%. Steeghs et al.20 investigated the stabilities of 7 species (methanol, acetaldehyde, acetone, isoprene, benzene, toluene and styrene) at approximately 100 ppb level over a period of 72 hours in black-layered Tedlar. The results evidenced good recovery (better than 80%) of acetone, isoprene, acetaldehyde and benzene over this storage period. A more detailed study involving 12 breath species at levels of 70–85 ppb was performed by Beauchamp et al.19 After 10 h of storage, the observed losses were smaller than 20%.
The main goals of this study were the investigation and comparison of stabilities of selected breath constituents in three types of polymers bags, Tedlar, Kynar, and Flexfilm, as well as the identification of optimal storage conditions for breath samples. The 41 selected C3–C10 species represented different chemical classes (hydrocarbons, ketones, aldehydes, aromatics, sulphurs, esters, terpenes, heterocyclics, etc.) and exhibited close-to-breath low ppb concentrations. The tests comprised the background emission of pollutants, recovery from dry and humid (RH 100% at 32 °C) samples over a period of 7 days, and the influence of sample size (degree of bag filling) on sample stability. Finally, the effectiveness of the cleaning protocol was examined as a crucial factor for bag reusability. Gas chromatography with mass spectrometric detection coupled with solid phase microextraction (SPME) as the pre-concentration method was selected as the analytical tool during all experiments. In the framework of the present study SPME exhibits some advantages over alternative pre-concentration methods (e.g., sorbent trapping) like ease of operation, good sensitivity, excellent reproducibility, full automation and relatively small extraction dependence on humidity.29 Finally, this pre-concentration method requires relatively small amounts of sample volume (10–20 ml) to perform extraction. The latter feature was particularly beneficial during this study, as the initial volumes of the samples in the bags remained almost unaffected during experiments. Alternative techniques (e.g., SPE) are more time- and effort-consuming and usually require much larger sample volumes.
2. Experimental
2.1. Sampling bags
Within this study, three types of sampling bags were compared with respect to the stability of breath constituents:
3 l in volume transparent Tedlar (PVF – polyvinyl fluoride) bags (SKC Inc., USA) equipped with a single polypropylene valve (dimensions when deflated: 26 cm × 24.5 cm, film thickness: 50 μm).
3 l in volume SamplePro Flexfilm bags (unknown polymer – trade secret of SKC Inc., USA) equipped with a single polypropylene valve (dimensions when deflated: 21 cm × 41.5 cm, film thickness: 76 μm).
3 l in volume Kynar (PVDF – polyvinylidene difluoride) bags (SKC Inc., USA) equipped with a single polypropylene valve (dimensions when deflated: 26 cm × 28.5 cm, film thickness: 50.8 μm).
All bags were new and flushed five times with high-purity nitrogen (type 6.0 – 99.9999%) directly before their use.
2.2. Chemicals and standards
Multi-compound test gas mixtures as well as calibration mixtures were prepared from pure liquid or gaseous substances. The majority of them were purchased from Sigma-Aldrich (Vienna, Austria): n-butane (99%), n-pentane (99.8%), n-hexane (99%), n-octane (99.8%), n-decane (99%), isobutane (99%), 3-methyl pentane (99%), 2-butene E and Z (99%), 2-pentene E and Z (99%), 1-hexene (97%), methylcyclopentane (97%), α-pinene (98%), (+)-3-carene (98.5%), p-cymene (99%), D-limonene (99%), eucalyptol (99%), benzene (99.8%), toluene (99.8%), p-xylene (99%), o-xylene (99%), acetone (99.8%), 2-butanone (99.5%), 2-pentanone (99%), 4-heptanone (97%), 2-butenone (99%), propanal (97%), 2-methyl propanal (99.5%), butanal (99%), hexanal (98%), octanal (99%), 2-methyl-2-propenal (95%), furan (99%), 2-methyl furan (99%), 2,5-dimethyl furan (99%), thiophene (99%), 3-methyl thiophene (98%), methyl acetate (99.5%), ethyl acetate (99.9%), n-propyl acetate (98%), methyl methacrylate (99%), dimethyl selenide (99%), ethyl ether (99.7%), pyrimidine (99%) and acetonitrile (99.8%). Moreover, 2-methyl pentane (99.5%), 4-methyl heptane (97%), isoprene (99%), ethylbenzene (99.8%), dimethyl sulfide (99%), 2-methyl-1-pentene (99.5%) and n-butyl acetate (99.7%) were obtained from Fluka (Switzerland), whereas, 2,4-dimethyl heptane (95%), 2,4-dimethyl-1-heptene (94%) and 4-methyl octane (97.5%) were provided by Chemsampco (USA). 3-Methyl furan (98%) was purchased from Acros Organic (Belgium) and methyl propyl sulfide (98%) from SAFC (USA).
The standard mixtures were prepared in two steps. Firstly, multi-compound primary standards were prepared in 1 l glass bulbs (Supelco, Canada). Prior to the use, each bulb was thoroughly cleaned with methanol and dried at 70 °C for at least 12 h. Then, the bulb was evacuated using a vacuum membrane pump and approximately 1 μl of a liquid (or 0.5 ml of gaseous) analyte was injected through a rubber septum. Next, the bulb was heated to 60 °C for 30 min to ensure complete evaporation and subsequently balanced to ambient pressure with high-purity nitrogen (6.0 – 99.9999%). The final calibration or test mixtures were prepared by transferring appropriate volumes of primary standard with Hamilton syringes into sampling bags filled in advance with predefined amounts of high-purity nitrogen. Calibration curves were obtained on the basis of triplicate analyses of 7 mixtures. Humid test mixtures were prepared in an analogous way as dry samples; however, during the last step polymer bags were filled with humid zero-air produced by means of a generator GasLab (Breitfuss Messtechnik, Germany). The GasLab unit comprises an integrated zero-air generator and a humidification module enabling the preparation of gas mixtures at predefined humidity levels. To avoid water condensation and to mimic conditions during breath sampling, the transfer line and polymer bags were maintained at 37 °C during the filling procedure. In all cases the gas volumes in the polymer bags were measured using an EL-FLOW F201CV digital mass flow controller (Bronkhorst hightech B.V., Netherlands).
A great majority of human breath constituents exhibit very low concentration levels ranging from ppt to several ppb.1–4,22 Consequently, an effort has been made to investigate stabilities of breath compounds at levels close to the ones observed in real samples. Effectively, the multi-compound test mixture contained analytes with concentrations falling within the range of 3–12 ppb. The three exceptions were acetone (720 ppb) and isoprene (106 ppb) exhibiting higher physiological levels in human breath,23,24 as well as acetonitrile (42 ppb) showing higher LOD for the applied analytical method. The range of volume fractions used during calibration and validation of the analytical method as well as the compounds’ concentration levels in the multi-compound test mixture are presented in Table 1.
Table 1.
Retention times Rt (min), LODs (ppb), RSDs (%), coefficients of variation (R2), linear ranges (ppb) of compounds under study and levels of species in the multicompound test mixture. Compounds are ordered with respect to retention time
| VOC | CAS | Rt [min] | Test mixture level [ppb] | RSD [%] | LOD [ppb] | R2 | Linear range [ppb] | Quantifier ion |
|---|---|---|---|---|---|---|---|---|
| Isobutane | 75-28-5 | 10.90 | 6 | 6.7 | 0.32 | 0.998 | 1–30 | 43 |
| 2-Butene, (E) | 624-64-6 | 11.01 | — | 1.8 | 0.3 | 0.995 | 1–17 | 56 |
| 2-Butene, (Z) | 590-18-1 | 11.11 | — | 3 | 0.3 | 0.994 | 1–22 | 56 |
| Acetonitrile | 75-05-8 | 11.44 | 42 | 7.4 | 4 | 0.999 | 12–62 | 41 |
| n-Butane | 106-97-8 | 11.85 | 6.2 | 4.3 | 0.19 | 0.987 | 0.63–25 | 43 |
| Furan | 110-00-9 | 13.39 | 12 | 2.1 | 0.22 | 0.999 | 0.6–22 | 68 |
| Propanal | 123-38-6 | 13.51 | 22 | 2.6 | 0.6 | 0.997 | 2–45 | 58 |
| Acetone | 67-64-1 | 13.65 | 720 | 2.6 | 0.74 | 0.999 | 3–1000 | 58 |
| Dimethyl sulfide | 75-18-3 | 14.33 | 10 | 1.5 | 0.1 | 0.999 | 0.3–30 | 62 |
| Methyl acetate | 79-20-9 | 15.06 | 12 | 2.2 | 0.14 | 0.999 | 0.4–25 | 43 |
| Ethyl ether | 60-29-7 | 15.94 | 8 | 1.2 | 0.29 | 0.999 | 1–20 | 74 |
| Isoprene | 78-79-5 | 16.10 | 106 | 1.3 | 0.1 | 0.999 | 0.5–175 | 67 |
| 2-Pentene, (E) | 646-04-8 | 16.32 | 8 | 1.6 | 0.1 | 0.999 | 0.4–10 | 55 |
| 2-Pentene, (Z) | 627-20-3 | 16.48 | 5 | 2.5 | 0.14 | 0.998 | 0.3–6 | 55 |
| n-Pentane | 109-66-0 | 16.57 | 6.2 | 1.6 | 0.11 | 0.996 | 0.4–25 | 43 |
| Dimethyl selenide | 593-79-3 | 16.76 | 10 | 3 | 0.23 | 0.998 | 0.6–12.2 | 95 |
| 2-Propenal, 2-methyl- | 78-85-3 | 16.98 | — | 1 | 0.11 | 0.998 | 0.4–29 | 70 |
| Propanal, 2-methyl- | 78-84-2 | 17.25 | — | 6.5 | 0.26 | 0.997 | 0.8–15.7 | 43 |
| 3-Buten-2-one | 78-94-4 | 17.59 | — | 5 | 0.19 | 0.998 | 0.6–23 | 55 |
| Butanal | 123-72-8 | 18.03 | — | 3 | 0.4 | 0.988 | 1.2–12 | 72 |
| Furan, 2-methyl- | 534-22-5 | 18.10 | 7 | 2 | 0.1 | 0.998 | 0.3–18 | 82 |
| 2-Butanone | 78-93-3 | 18.20 | 9 | 7 | 0.13 | 0.997 | 0.4–36 | 43 |
| Furan, 3-methyl- | 930-27-8 | 18.39 | — | 3 | 0.15 | 0.997 | 0.4–20 | 82 |
| Ethyl acetate | 141-78-6 | 18.96 | 8 | 2.2 | 0.13 | 0.996 | 0.4–17 | 43 |
| Thiophene | 110-02-1 | 19.93 | 9 | 3.3 | 0.15 | 0.999 | 0.45–21 | 84 |
| 1-Pentene, 2-methyl- | 763-29-1 | 19.95 | — | 3.2 | 0.1 | 0.999 | 0.3–15 | 56 |
| Pentane, 2-methyl- | 107-83-5 | 20 | 9.5 | 1 | 0.18 | 0.999 | 0.55–11 | 43 |
| Pentane, 3-methyl- | 96-14-0 | 20.19 | — | 1.5 | 0.1 | 0.999 | 0.4–12 | 57 |
| 1-Hexene | 592-41-6 | 20.22 | 9 | 1.7 | 0.2 | 0.999 | 0.6–10 | 56 |
| Benzene | 71-43-2 | 20.38 | 12 | 3.8 | 0.3 | 0.998 | 1–36 | 78 |
| Cyclopentane, methyl- | 96-37-7 | 20.45 | — | 9 | 0.1 | 0.991 | 0.3–11 | 56 |
| n-Hexane | 110-54-3 | 20.70 | 6.2 | 1.6 | 0.12 | 0.995 | 0.4–25 | 57 |
| Pyrimidine | 289-95-2 | 21.70 | 10 | 9 | 0.1 | 0.972 | 0.4–28 | 80 |
| 2-Pentanone | 107-87-9 | 21.98 | 8 | 2.2 | 0.1 | 0.998 | 0.4–24 | 43 |
| Furan, 2,5-dimethyl- | 625-86-5 | 22.04 | 7.5 | 1.4 | 0.08 | 0.999 | 0.3–15 | 96 |
| Methyl methacrylate | 80-62-6 | 22.11 | — | 1.6 | 0.11 | 0.999 | 0.4–18 | 69 |
| n-Propyl acetate | 109-60-4 | 22.71 | — | 1.3 | 0.15 | 0.999 | 0.5–17 | 43 |
| Methyl propyl sulfide | 3877-15-4 | 22.73 | 6 | 2.1 | 0.04 | 0.996 | 0.2–30 | 61 |
| Thiophene, 3-methyl- | 616-44-4 | 24.00 | 6.5 | 4.2 | 0.1 | 0.996 | 0.3–22 | 97 |
| Toluene | 108-88-3 | 24.30 | 12 | 2.9 | 0.1 | 0.993 | 0.3–30 | 91 |
| Hexanal | 66-25-1 | 25.76 | 5 | 9 | 0.4 | 0.996 | 1.2–10 | 56 |
| n-Butyl acetate | 123-86-4 | 26.21 | 6 | 2.1 | 0.4 | 0.995 | 1.2–12 | 56 |
| Heptane, 4-methyl- | 589-53-7 | 26.76 | — | 2.8 | 0.24 | 0.989 | 0.6–11.6 | 43 |
| Ethylbenzene | 100-41-4 | 27.45 | — | 7 | 0.25 | 0.989 | 0.75–13 | 91 |
| n-Octane | 111-65-9 | 27.60 | 8 | 2.8 | 0.1 | 0.998 | 0.3–14 | 85 |
| p-Xylene | 106-42-3 | 27.72 | 8.5 | 8 | 0.07 | 0.986 | 0.3–18 | 91 |
| o-Xylene | 95-47-6 | 28.03 | — | 6 | 0.15 | 0.991 | 0.4–15 | 91 |
| 4-Heptanone | 123-19-3 | 28.36 | 5 | 6.3 | 0.06 | 0.978 | 0.2–17 | 71 |
| Heptane, 2,4-dimethyl- | 2213-23-2 | 28.98 | — | 5.9 | 0.1 | 0.987 | 0.3–8.8 | 85 |
| 1-Heptene, 2,4-dimethyl- | 19549-87-2 | 29.05 | — | 6 | 0.12 | 0.986 | 0.4–9 | 83 |
| Octane, 4-methyl- | 2216-34-4 | 29.76 | 7 | 3.4 | 0.2 | 0.995 | 0.6–11 | 43 |
| α-Pinene | 80-56-8 | 30.81 | 6 | 8 | 0.46 | 0.985 | 1.4–19 | 93 |
| Octanal | 124-13-0 | 31.87 | 3 | 11 | 0.3 | 0.974 | 1–17 | 84 |
| (+)-3-Carene | 498-15-7 | 32.14 | 4 | 5.3 | 0.61 | 0.954 | 1.8–12 | 93 |
| p-Cymene | 99-87-6 | 32.67 | 7 | 5.7 | 0.1 | 0.973 | 0.4–21 | 119 |
| D-Limonene | 5989-27-5 | 32.88 | 9 | 6 | 0.45 | 0.954 | 1.4–18 | 68 |
| n-Decane | 124-18-5 | 33.21 | 7 | 9 | 0.4 | 0.978 | 1.2–17 | 57 |
| Eucalyptol | 470-82-6 | 33.46 | 8.5 | 6.1 | 1 | 0.986 | 3–25 | 43 |
| n-Dodecane | 112-40-3 | 36.22 | — | 8 | 0.5 | 0.964 | 1.5–15 | 71 |
2.3. SPME procedure and chromatographic analysis
The test gas samples were taken using a 20 ml gas-tight glass syringe (Roth, Germany) equipped with a replaceable needle. Sampling was achieved manually by drawing a volume of 18 ml from the sampling bag and subsequent injection of this volume into an evacuated SPME vial (20 ml in volume, Gerstel, Germany) sealed with a 1.3 mm butyl/PTFE septum (Macherey-Nagel, Germany). To avoid loss of analytes during the sample storage in SPME vials an effort was made to analyze samples within 3 h after vial filling. The SPME procedure was carried out automatically using a multipurpose sampler MPS (Gerstel, Germany). SPME was achieved by inserting a 75 μm carboxen-polydimethylsiloxane (CAR–PDMS) fiber (Supelco, Canada) into the vial and exposing it to its content for 10 minutes at 37 °C. Immediately after extraction, the fiber was introduced into the inlet of the gas chromatograph where the sorbed VOCs were thermally desorbed at 290 °C. The fiber was conditioned at 290 °C for 5 minutes prior to each analysis.
The GC-MS analyses were performed using an Agilent 7890A/5975C GC-MS system (Agilent, USA). During the fiber desorption, the split/splitless inlet operated in a splitless mode (1 min), followed by the split mode at a ratio of 1 : 20. The analytes under study were separated using a PoraBond Q column (25 m × 0.32 mm, film thickness 5 μm, Varian, USA) working in a constant flow mode of helium at 1.4 ml min−1. The column temperature program was as follows: 40 °C for 2 min, increase to 260 °C at a rate of 7 °C min−1, held at 260 °C for 7 min. The mass spectrometer worked in a SCAN mode with an associated m/z range set from 20 to 200. The quadrupole, ion source and transfer line were kept at 150 °C, 230 °C and 280 °C, respectively.
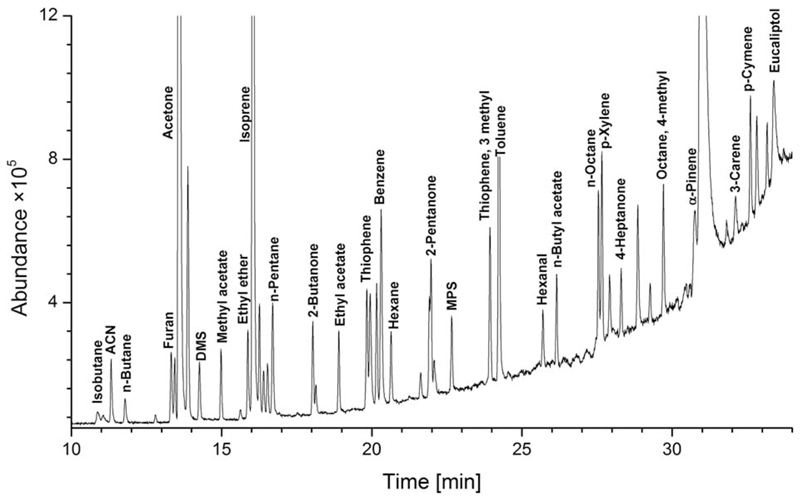
The identification of compounds was performed in two steps. First, the peak spectrum was checked against the NIST mass spectral library. Next, the NIST identification was confirmed by the retention times obtained on the basis of standards prepared from pure compounds. Peak integration was based on extracted ion chromatograms. The retention times of the investigated compounds for the applied chromatographic parameters as well as the ions used for the integration are presented in Table 1. An exemplary chromatogram from a test mixture analysis is presented in Fig. 1.
Fig. 1.
An exemplary chromatogram from a test mixture analysis.
2.4. Sampling bags tests
2.4.1. Background test
To identify contaminants emitted by the polymer films 3 new bags of each type were filled with 2000 ml of high-purity nitrogen (corresponding to approximately 67% of their nominal volume), stored at room temperature for 24 hours and analyzed after certain time periods. The time intervals of the measurements were defined as follows: the first sampling was carried out immediately after filling the bag, subsequent ones after 6, 12 and 24 h of storage. Additionally, blank (nitrogen) and laboratory air measurements were performed.
Next, an additional cleaning procedure was applied to check if it is possible to reduce contaminant emission from the bags under study. For this purpose, after five-fold flushing bags were filled with nitrogen and heated overnight (approximately 12 h) in an oven at 50 °C to induce potential contaminant desorption from the polymer film or from the valves. Next, bags were again flushed five times with nitrogen and the aforementioned background test procedure was repeated; however, in this case only 3 samplings were performed, immediately after filling, and after 6 and 24 hours of storage.
2.4.2. Dry standard stability test
To investigate the stability of breath species, a 41-component test mixture was prepared using the aforementioned procedure and injected into the tested polymer bags. The nominal levels of all compounds in the test mixture are presented in Table 1. To study the influence of different (film) surface-to-(sample) volume ratios (SA : V) on the sample integrity, three bags of each type of film were filled with different volumes of standard mixture: 2.4, 1.2 and 0.6 l (i.e., 80, 40, and 20% of the maximum capacity). Due to some differences in the film dimensions these volumes corresponded to SA : V ratio values of 53, 106, and 212 m−1 for Tedlar bags, 73, 145, and 291 m−1 for Flexfilm bags, and 62, 124, 247 m−1 for Kynar bags respectively. All bags were pre-conditioned and filled at the same time with the same test mixture and were stored at room temperature (24 °C) exposed to daylight. The stability of the test gas was monitored over a period of 7 days with the time instants for drawing the samples defined as follows: the first sample was taken approximately 10 minutes after the bag filling and the next ones after 6, 24, 48, 72, 126 and 168 hours of storage.
To confirm repeatability, the stability test was repeated for the test gas mixture volume of 2.4 l with the same sampling protocol, however, with three bags of each type being involved.
2.4.3. Humid standard stability test
The stability of compounds under study in humid matrices was investigated using a test mixture having water content similar to breath leaving the upper airways, i.e., RH of 100% at 32 °C.30 Three new bags from each type of film were filled with 2.4 l of humid test mixture and sampled immediately after filling and after 6, 24 and 48 h of storage. To avoid condensation and to mimic the sampling of real breath samples (having body temperature) during filling all bags were heated to 37 °C. However, during the experiment they were stored at room temperature. The duration of the experiment was restricted to 2 days as water vapor permeates relatively easily through all tested polymer films, and after a few hours sample humidity reaches ambient levels.19,31,32 Additionally, one bag of each type was sampled after 2 and 4 hours to study the evolution of the VOC concentrations during the first hours of storage, when the humidity still remains elevated.
2.4.4. Reusability test
The reusability test was focused on studying the effectiveness of the bag cleaning protocol developed during one of our previous studies.21 Polymer bags involved in the dry standard stability test (i.e., containing the test mixture for 7 days) were used during the test. Firstly, bags were flushed five times with high purity nitrogen to remove remainings of the test gas. Next, all bags were filled with 2 l of nitrogen and conditioned at 50 °C for approximately 12 h to remove volatiles of interest from the bags’ material (film, valve etc.). After heating, the bags were again rinsed five times, filled with 2 l of high-purity nitrogen and stored at room temperature for 24 hours. The effectiveness of the applied cleaning protocol was checked by comparing the levels of test mixture VOCs before and after this time of storage.
3. Results and discussion
3.1. Method validation
Limits of detection (LODs) were calculated using the mean value of the blank responses and their standard deviations obtained on the basis of 10 blank measurements33 and are presented in Table 1. The limit of quantification (LOQ) was defined as three times the LOD. The relative standard deviations (RSDs) were calculated on the basis of five consecutive analyses of standard mixtures. The calculated RSDs varied from 1% to 9% and were recognised as satisfactory for the aims of this study. The system response was found to be linear within the investigated concentration ranges, as shown in Table 1, with coefficients of variation ranging from 0.954 to 0.999.
3.2. Background test
All volatiles found to be emitted by the investigated polymer sampling bags are summarised in Tables 2–4. The presented concentrations are the mean values of VOC levels in three bags. The emission rates were calculated for unconditioned bags assuming that the contaminants are emitted by the polymer film.
Table 2.
Contaminants emitted by Flexfilm bags [ppb]. Compounds are ordered with respect to increasing retention time. ʺ—ʺ denotes that the VOC was not detected, whereas ʺ<LOQʺ stands for VOC level below LOQ
| New bag sampling time [h] |
Preconditioned bag sampling time [h] |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOC | CAS | 0 | 6 | 12 | 24 | 0 | 6 | 24 | VOC emission × 10−12 [g × h−1 × cm−2] | ||||||
| Carbonyl sulfide (COS) | 463-58-1 | Not quantified | |||||||||||||
| Acetaldehyde | 75-07-0 | Not quantified | |||||||||||||
| 2-Butene, (E) | 624-64-6 | — | 1.6 | 2.0 | 2.3 | — | — | 0.6 | 0.39 | ||||||
| 2-Butene, (Z) | 590-18-1 | — | 3.6 | 6.7 | 12.2 | — | 0.8 | 3.6 | 1.53 | ||||||
| n-Butane | 106-97-8 | 1.6 | 6.2 | 11.1 | 19.1 | <LOQ | 3.7 | 8.1 | 2.62 | ||||||
| Propanal | 123-38-6 | <LOQ | 1.4 | 2.1 | 2.4 | — | — | — | 0.48 | ||||||
| Acetone | 67-64-1 | 14 | 75 | 102 | 140 | — | 9.9 | 19 | 25.7 | ||||||
| Carbon disulfide (CS2) | 75-15-0 | Not quantified | |||||||||||||
| Methyl acetate | 79-20-9 | <LOQ | <LOQ | 0.3 | 0.4 | — | — | — | 0.1 | ||||||
| n-Pentane | 109-66-0 | 0.4 | 2.0 | 3.4 | 5.5 | 0.4 | 1.7 | 3.4 | 0.99 | ||||||
| 2-Propenal, 2-methyl- | 78-85-3 | <LOQ | <LOQ | 0.3 | 0.4 | — | — | <LOQ | 0.1 | ||||||
| 3-Buten-2-one | 78-94-4 | <LOQ | <LOQ | 0.6 | 0.6 | — | <LOQ | <LOQ | 0.19 | ||||||
| Butanal | 123-72-8 | <LOQ | 2.2 | 3.6 | 4.5 | — | 1.3 | 1.3 | 1.01 | ||||||
| 2-Butanone | 78-93-3 | 0.5 | 1.8 | 2.9 | 4.1 | — | 0.6 | 1.0 | 0.85 | ||||||
| Pentane, 2-methyl- | 107-83-5 | <LOQ | 0.7 | 1.1 | 1.8 | <LOQ | 1.4 | 3.0 | 0.41 | ||||||
| 1-Hexene | 592-41-6 | <LOQ | 0.9 | 1.4 | 2.2 | <LOQ | 0.8 | 1.6 | 0.49 | ||||||
| Pentane, 3-methyl- | 96-14-0 | <LOQ | 1.1 | 2.0 | 3.1 | 0.4 | 1.7 | 3.8 | 0.68 | ||||||
| Cyclopentane, methyl- | 96-37-7 | 1.7 | 6.6 | 11.1 | 17.3 | 2.0 | 9.2 | 18.7 | 3.78 | ||||||
| n-Hexane | 110-54-3 | 3.8 | 17.1 | 28.6 | 44.8 | 4.8 | 22.7 | 45.6 | 10 | ||||||
| Methyl methacrylate | 80-62-6 | 1.6 | 6.7 | 10.1 | 14.6 | 0.4 | 1.4 | 2.6 | 4.22 | ||||||
| Toluene | 108-88-3 | <LOQ | 1.2 | 1.8 | 2.4 | <LOQ | 0.3 | 0.5 | 0.67 | ||||||
| Heptane, 4-methyl- | 589-53-7 | — | <LOQ | 1.2 | 2.0 | — | 1.2 | 2.4 | 0.53 | ||||||
| Heptane, 2,4-dimethyl- | 2213-23-2 | 1.9 | 8.9 | 14.8 | 23.2 | 3.4 | 14.8 | 30.4 | 7.76 | ||||||
| 1-Heptene, 2,4-dimethyl- | 19549-87-2 | — | <LOQ | 0.6 | 0.8 | <LOQ | 0.5 | 1.1 | 0.26 | ||||||
| Octane, 4-methyl- | 2216-34-4 | <LOQ | 2.8 | 4.5 | 6.8 | 1.0 | 4.1 | 8.6 | 2.37 | ||||||
| Caprolactam | 105-60-2 | Not quantified | |||||||||||||
| Dodecane | 112-40-3 | <LOQ | 3.3 | 5.3 | 6.4 | 2.0 | 3.3 | 5.2 | 3.51 | ||||||
Table 4.
Contaminants emitted by Tedlar bags [ppb]. Compounds are ordered with respect to the increasing retention time. ʺ—ʺ denotes that the VOC was not detected, whereas ʺ<LOQʺ stands for VOC level below LOQ
| New bag sampling time [h] |
Preconditioned bag sampling time [h] |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| VOC | CAS | 0 | 6 | 12 | 24 | 0 | 6 | 24 | VOC emission × 10−12 [g × h−1 × cm−2] |
| Carbonyl sulfide (COS) | 463-58-1 | Not quantified | |||||||
| Acetonitrile | 75-05-8 | <LOQ | 14.2 | 17.3 | 18.9 | — | — | — | 4.23 |
| Carbon disulfide (CS2) | 75-15-0 | Not quantified | |||||||
| n-Hexane | 110-54-3 | — | — | — | 0.5 | — | — | — | 0.15 |
| Acetamide, N,N-dimethyl- | 127-19-5 | Not quantified | |||||||
| 2-Propyl acetate, 1-methoxy- | 108-65-6 | Not quantified | |||||||
| Heptane, 2,4-dimethyl- | 2213-23-2 | — | 0.5 | 0.8 | 1.3 | — | 0.3 | 0.4 | 0.5 |
| Octane, 4-methyl- | 2216-34-4 | <LOQ | 0.8 | 1.0 | 1.5 | — | <LOQ | <LOQ | 0.82 |
| Phenol | 108-95-2 | Not quantified | |||||||
A total of 27 compounds were emitted by Flexfilm bags. The most dominant chemical classes were hydrocarbons with 14 and aldehydes with four species, respectively. Amongst the remaining compounds, there were three ketones, two esters, two volatile sulphur compounds, one aromatic and one amide. Sulphur compounds (COS and CS2) were found to be produced by all three types of bags and seem to be emitted by rubber parts of the sampling valves.21 As the emission of these two species was investigated thoroughly in our previous paper21 they were not quantified within this study. Acetone was the most abundant compound with the concentration level reaching 140 ppb after 24 hours of storage. Apart from acetone, high concentrations were observed for some hydrocarbons (2-butene, n-butane, methylcyclopentane, n-hexane, 2,4-dimethyl heptane). 75% of the contaminants were detected shortly after filling the Flexfilm bag. Six hours later all of them were present in the bags at levels of several ppb. In the context of breath gas analysis this emission can be considered as significant. Pre-conditioning of Flexfilm bags reduced the emission of aldehydes, ketones and esters by 50–80%; however, the emission rates of hydrocarbons remained unchanged (with the exception of 2-butene and toluene). Acetone background was particularly improved with levels spreading around 20 ppb after conditioning and 1 day of storage. Nevertheless, despite conditioning considerable concentrations of contaminants could be found after several hours of storage. Repeating the pre-conditioning step (data not shown) further improved the background of all species apart from hydrocarbons.
Kynar bags released 21 species. The predominant chemical class was aromatics with four compounds. Apart from them, there were two hydrocarbons, two volatile sulphur compounds (COS and CS2), three aldehydes, three ketones and three esters, one CFC, one halide and one nitro compound. In the case of unconditioned Kynar bags the great majority of contaminants could be detected in small amounts (usually below 1 ppb) after 6 hours of storage. At the end of the experiment the highest levels were noted for acetone and toluene, 33 and 9.4 ppb respectively. The applied pre-conditioning method was found to be very efficient in the case of Kynar bags. After cleaning only five species (2-butanone, toluene, p-xylene, COS and CS2) could be detected in the Kynar bag samples after 6 hours of storage. After 1 day several additional contaminants were found in the Kynar bags in detectable amounts, however, their levels were below the LOQs of the analytical method. Taking into account the good results of the pre-conditioning protocol it can be surmised that additional cleaning/s could further reduce the contaminants emission to the levels acceptable for breath analysis. Conversely, conditioning promoted the emission of sulphur species – COS and CS2 – which is consistent with the findings of our previous paper21 indicating rubber parts of polymer bags (o-ring, septum) as potential sources of these species.
Only 9 compounds were found to be emitted by the Tedlar film: three hydrocarbons (n-hexane, 2,4-dimethylheptane and 4 methyl octane), two volatile sulphur compounds (COS and CS2), N,N-dimethylacetamide, phenol, acetonitrile and 1-methoxy-2-propyl acetate. N,N-Dimethylacetamide and phenol are commonly known and well documented contaminants in Tedlar bags.19,34 Amongst the quantified species the highest levels were noted for acetonitrile (19 ppb). 2,4-Dimethylheptane and 4-methyl octane could be detected within few hours of storage, however, n-hexane was found only at the end of the experiment. Like in the case of Kynar bags pre-conditioning considerably improved the background emission. However, small amounts of 2,4-dimethylheptane and 4-methyl octane could still be detected after 6 hours of storage.
Several compounds identified as contaminants in the tested bags were found also in room air at low ppb levels. Hence, permeation from room air might be a possible source of pollution, e.g., for acetone, 2-butanone, n-butane, n-pentane, methyl acetate, toluene, and p-xylene. However, the fact that several other species having similar physicochemical properties were detected in room air but not in bag air favours emissions from the polymer film as the main source of contamination. For example, 2-methyl butane – a hydrocarbon very similar to n-pentane – was present in room air at levels of several ppb but was not detected in the bag content during the background test.
3.3. Dry standard stability test
The stability of test mixture compounds in tested polymer bags over a period of one week is presented in Table 5. A compound’s concentration was considered stable when its level was higher than 80% of its initial value.
Table 5.
Stability of selected breath constituents in Tedlar, Kynar and Flexfilm sampling bags for the dry matrix. Vf–filling volume in %. The recoveries exceeding 100% reflect the emission of pollutants
| Recovery in Flexfilm bag [%] |
Recovery in Kynar bag [%] |
Recovery in Tedlar bag [%] |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOC | Vf[%] | 6 h | 24 h | 48 h | 72 h | 126 h | 168 h | 6 h | 24 h | 48 h | 72 h | 126 h | 168 h | 6 h | 24 h | 48 h | 72 h | 126 h | 168 h |
| n-Butane | 20 | 180 | 424 | 714 | 1020 | 1621 | 1978 | 113 | 107 | 109 | 131 | 130 | 95 | 94 | 92 | 90 | 88 | 85 | |
| 40 | 159 | 296 | 454 | 607 | 963 | 0 | 93 | 94 | 89 | 85 | 86 | 84 | 93 | 95 | 94 | 91 | 94 | 81 | |
| 80 | 142 | 238 | 352 | 471 | 693 | 840 | 112 | 113 | 103 | 106 | 104 | 104 | 103 | 102 | 97 | 93 | 94 | 93 | |
| n-Pentane | 20 | 120 | 176 | 210 | 296 | 412 | 486 | 94 | 91 | 91 | 84 | 82 | 92 | 93 | 89 | 85 | 79 | 76 | |
| 40 | 111 | 132 | 162 | 193 | 260 | 0 | 96 | 92 | 84 | 82 | 78 | 77 | 99 | 93 | 88 | 81 | 85 | 80 | |
| 80 | 98 | 113 | 137 | 154 | 170 | 205 | 112 | 106 | 105 | 98 | 96 | 90 | 102 | 100 | 91 | 86 | 86 | 81 | |
| n-Hexane | 20 | 215 | 472 | 721 | 992 | 1448 | 1723 | 103 | 101 | 100 | 129 | 125 | 99 | 97 | 95 | 90 | 82 | 87 | |
| 40 | 179 | 347 | 528 | 673 | 1019 | 0 | 92 | 91 | 88 | 81 | 83 | 75 | 94 | 96 | 90 | 90 | 93 | 82 | |
| 80 | 152 | 263 | 387 | 495 | 729 | 855 | 111 | 114 | 107 | 103 | 103 | 107 | 106 | 104 | 95 | 94 | 96 | 92 | |
| n-Octane | 20 | 95 | 89 | 81 | 78 | 69 | 67 | 91 | 86 | 83 | 74 | 75 | 99 | 91 | 89 | 86 | 82 | 78 | |
| 40 | 97 | 91 | 85 | 85 | 81 | 0 | 93 | 92 | 88 | 84 | 81 | 78 | 98 | 95 | 88 | 85 | 86 | 80 | |
| 80 | 93 | 92 | 89 | 84 | 80 | 80 | 108 | 107 | 102 | 102 | 98 | 84 | 98 | 91 | 91 | 87 | 86 | 82 | |
| n-Decane | 20 | 82 | 71 | 59 | 50 | 56 | 40 | 95 | 78 | 73 | 64 | 57 | 86 | 78 | 69 | 57 | 50 | 50 | |
| 40 | 96 | 92 | 69 | 59 | 55 | 0 | 98 | 83 | 78 | 71 | 63 | 49 | 92 | 85 | 76 | 71 | 62 | 57 | |
| 80 | 106 | 92 | 83 | 70 | 62 | 56 | 102 | 91 | 94 | 87 | 85 | 76 | 100 | 92 | 89 | 85 | 84 | 70 | |
| Isobutane | 20 | 96 | 88 | 88 | 95 | 96 | 99 | 90 | 93 | 79 | 92 | 86 | 107 | 100 | 86 | 83 | 86 | 84 | |
| 40 | 99 | 93 | 86 | 69 | 80 | 0 | 99 | 96 | 79 | 77 | 74 | 80 | 101 | 90 | 88 | 74 | 77 | 74 | |
| 80 | 89 | 88 | 69 | 72 | 75 | 74 | 100 | 85 | 85 | 82 | 76 | 89 | 98 | 99 | 82 | 84 | 87 | 80 | |
| Pentane, 2-methyl- | 20 | 102 | 132 | 138 | 149 | 187 | 203 | 102 | 100 | 89 | 92 | 88 | 94 | 81 | 84 | 83 | 83 | 81 | |
| 40 | 91 | 92 | 98 | 95 | 104 | 0 | 101 | 98 | 93 | 84 | 88 | 83 | 89 | 92 | 82 | 85 | 85 | 72 | |
| 80 | 87 | 86 | 87 | 85 | 89 | 97 | 110 | 102 | 101 | 95 | 98 | 88 | 99 | 97 | 86 | 88 | 89 | 93 | |
| Octane, 4-methyl- | 20 | 124 | 178 | 231 | 263 | 332 | 358 | 100 | 99 | 104 | 105 | 104 | 105 | 125 | 123 | 147 | 148 | 179 | |
| 40 | 110 | 140 | 162 | 166 | 222 | 0 | 98 | 103 | 93 | 105 | 103 | 108 | 91 | 101 | 112 | 110 | 125 | 107 | |
| 80 | 110 | 107 | 140 | 131 | 170 | 169 | 99 | 104 | 102 | 103 | 103 | 89 | 102 | 103 | 100 | 104 | 111 | 112 | |
| 2-Pentene, (E) | 20 | 94 | 93 | 83 | 80 | 82 | 81 | 88 | 75 | 63 | 48 | 37 | 89 | 93 | 88 | 84 | 83 | 81 | |
| 40 | 93 | 81 | 85 | 80 | 80 | 0 | 93 | 87 | 73 | 63 | 44 | 30 | 97 | 94 | 90 | 87 | 86 | 82 | |
| 80 | 89 | 84 | 87 | 85 | 85 | 82 | 109 | 105 | 97 | 88 | 72 | 58 | 98 | 95 | 89 | 88 | 87 | 78 | |
| 2-Pentene, (Z) | 20 | 103 | 94 | 85 | 96 | 82 | 80 | 85 | 77 | 64 | 53 | 41 | 91 | 93 | 90 | 84 | 82 | 80 | |
| 40 | 113 | 105 | 104 | 99 | 104 | 93 | 90 | 75 | 66 | 50 | 36 | 96 | 92 | 91 | 85 | 84 | 83 | ||
| 80 | 94 | 88 | 93 | 88 | 88 | 85 | 109 | 105 | 95 | 89 | 76 | 65 | 99 | 93 | 89 | 89 | 87 | 82 | |
| 1-Hexene | 20 | 96 | 127 | 154 | 172 | 253 | 285 | 99 | 88 | 77 | 78 | 75 | 96 | 92 | 89 | 85 | 83 | 80 | |
| 40 | 99 | 112 | 129 | 138 | 173 | 94 | 88 | 85 | 76 | 74 | 68 | 92 | 91 | 84 | 87 | 88 | 80 | ||
| 80 | 97 | 103 | 111 | 115 | 135 | 145 | 111 | 107 | 101 | 96 | 93 | 88 | 97 | 94 | 83 | 85 | 81 | 84 | |
| Isoprene | 20 | 93 | 87 | 80 | 70 | 70 | 65 | 85 | 77 | 69 | 57 | 47 | 88 | 90 | 86 | 82 | 79 | 77 | |
| 40 | 95 | 83 | 83 | 77 | 77 | 93 | 88 | 76 | 71 | 60 | 50 | 97 | 92 | 89 | 86 | 84 | 81 | ||
| 80 | 91 | 86 | 87 | 83 | 80 | 77 | 109 | 105 | 98 | 92 | 83 | 75 | 101 | 102 | 93 | 94 | 96 | 89 | |
| Benzene | 20 | 85 | 80 | 69 | 65 | 58 | 53 | 90 | 77 | 63 | 54 | 54 | 92 | 85 | 79 | 74 | 68 | 65 | |
| 40 | 93 | 86 | 82 | 71 | 71 | 88 | 80 | 71 | 65 | 61 | 56 | 88 | 86 | 79 | 77 | 74 | 69 | ||
| 80 | 93 | 87 | 83 | 73 | 76 | 70 | 105 | 99 | 91 | 84 | 81 | 70 | 97 | 91 | 82 | 79 | 81 | 79 | |
| Toluene | 20 | 85 | 78 | 67 | 61 | 56 | 51 | 81 | 66 | 60 | 52 | 46 | 90 | 81 | 73 | 64 | 59 | 55 | |
| 40 | 87 | 84 | 79 | 70 | 66 | 88 | 79 | 71 | 63 | 56 | 52 | 90 | 84 | 78 | 72 | 66 | 62 | ||
| 80 | 92 | 88 | 82 | 77 | 73 | 67 | 100 | 94 | 85 | 78 | 70 | 64 | 95 | 91 | 85 | 82 | 78 | 73 | |
| p-Xylene | 20 | 80 | 72 | 55 | 49 | 42 | 40 | 71 | 55 | 48 | 40 | 39 | 84 | 69 | 64 | 52 | 45 | 43 | |
| 40 | 89 | 78 | 74 | 63 | 63 | 0 | 76 | 69 | 56 | 51 | 48 | 38 | 87 | 79 | 68 | 62 | 59 | 53 | |
| 80 | 86 | 83 | 76 | 70 | 64 | 52 | 94 | 84 | 73 | 68 | 58 | 49 | 91 | 88 | 82 | 76 | 72 | 67 | |
| Acetone | 20 | 95 | 95 | 92 | 94 | 92 | 90 | 71 | 58 | 53 | 39 | 37 | 89 | 79 | 71 | 65 | 55 | 52 | |
| 40 | 90 | 93 | 90 | 89 | 92 | 73 | 59 | 47 | 43 | 32 | 26 | 90 | 82 | 77 | 72 | 67 | 61 | ||
| 80 | 94 | 91 | 91 | 88 | 86 | 86 | 97 | 82 | 70 | 62 | 51 | 39 | 103 | 97 | 92 | 88 | 84 | 81 | |
| 2-Butanone | 20 | 123 | 133 | 158 | 210 | 217 | 228 | 87 | 80 | 80 | 72 | 71 | 89 | 79 | 76 | 69 | 55 | 56 | |
| 40 | 121 | 139 | 155 | 152 | 226 | 82 | 72 | 60 | 60 | 56 | 52 | 102 | 96 | 89 | 83 | 77 | 65 | ||
| 80 | 105 | 122 | 131 | 123 | 140 | 163 | 103 | 83 | 81 | 74 | 67 | 62 | 99 | 92 | 86 | 85 | 79 | 76 | |
| 2-Pentanone | 20 | 86 | 74 | 65 | 60 | 55 | 47 | 64 | 42 | 34 | 24 | 20 | 89 | 78 | 65 | 61 | 55 | 47 | |
| 40 | 89 | 86 | 79 | 75 | 70 | 71 | 53 | 40 | 33 | 23 | 19 | 91 | 82 | 72 | 70 | 65 | 59 | ||
| 80 | 94 | 92 | 81 | 82 | 76 | 70 | 96 | 78 | 59 | 54 | 43 | 36 | 100 | 93 | 84 | 78 | 81 | 77 | |
| 4-Heptanone | 20 | 86 | 65 | 51 | 42 | 29 | 26 | 42 | 27 | 22 | 10 | 12 | 82 | 64 | 55 | 51 | 38 | 31 | |
| 40 | 87 | 78 | 66 | 60 | 51 | 59 | 38 | 26 | 20 | 16 | 13 | 89 | 73 | 69 | 60 | 55 | 49 | ||
| 80 | 92 | 87 | 85 | 68 | 60 | 53 | 82 | 60 | 44 | 38 | 24 | 21 | 100 | 83 | 86 | 80 | 76 | 70 | |
| Propanal | 20 | 132 | 174 | 208 | 233 | 278 | 299 | 84 | 75 | 75 | 71 | 67 | 96 | 86 | 83 | 76 | 70 | 67 | |
| 40 | 118 | 154 | 175 | 194 | 239 | 85 | 78 | 76 | 71 | 66 | 66 | 94 | 89 | 83 | 80 | 76 | 71 | ||
| 80 | 108 | 121 | 134 | 142 | 156 | 166 | 109 | 98 | 90 | 91 | 85 | 75 | 99 | 93 | 87 | 85 | 81 | 81 | |
| Hexanal | 20 | 104 | 86 | 121 | 139 | 125 | 145 | 72 | 130 | 115 | 98 | 92 | 101 | 100 | 95 | 94 | 91 | 85 | |
| 40 | 103 | 101 | 135 | 114 | 129 | 92 | 78 | 78 | 73 | 72 | 72 | 103 | 101 | 98 | 95 | 93 | 91 | ||
| 80 | 100 | 107 | 123 | 98 | 76 | 126 | 155 | 140 | 115 | 126 | 110 | 125 | 99 | 98 | 99 | 103 | 88 | 95 | |
| Octanal | 20 | 101 | 95 | 96 | 80 | 65 | 82 | 72 | 63 | 54 | 45 | 61 | 98 | 66 | 69 | 45 | 50 | 51 | |
| 40 | 113 | 85 | 87 | 109 | 80 | 79 | 45 | 63 | 52 | 40 | 51 | 83 | 87 | 85 | 61 | 55 | 50 | ||
| 80 | 132 | 99 | 95 | 90 | 85 | 89 | 110 | 93 | 86 | 80 | 53 | 53 | 101 | 88 | 77 | 64 | 64 | 52 | |
| Furan | 20 | 93 | 97 | 97 | 100 | 106 | 107 | 85 | 70 | 60 | 46 | 37 | 86 | 86 | 79 | 75 | 70 | 64 | |
| 40 | 90 | 97 | 99 | 102 | 111 | 86 | 81 | 74 | 63 | 54 | 47 | 93 | 87 | 84 | 79 | 76 | 71 | ||
| 80 | 98 | 94 | 92 | 92 | 92 | 92 | 107 | 101 | 93 | 87 | 78 | 67 | 97 | 93 | 88 | 86 | 84 | 81 | |
| Furan, 2-methyl- | 20 | 88 | 76 | 70 | 65 | 58 | 53 | 77 | 59 | 49 | 34 | 27 | 93 | 82 | 76 | 71 | 66 | 62 | |
| 40 | 92 | 88 | 83 | 80 | 78 | 87 | 74 | 65 | 56 | 42 | 34 | 103 | 96 | 90 | 83 | 81 | 76 | ||
| 80 | 95 | 89 | 84 | 79 | 76 | 73 | 106 | 89 | 87 | 76 | 65 | 56 | 97 | 87 | 78 | 84 | 81 | 78 | |
| Furan, 2,5-dimethyl- | 20 | 89 | 74 | 57 | 44 | 29 | 22 | 66 | 42 | 26 | 12 | 7 | 91 | 80 | 67 | 62 | 54 | 47 | |
| 40 | 86 | 81 | 68 | 58 | 46 | 86 | 68 | 47 | 29 | 13 | 6 | 96 | 86 | 75 | 72 | 66 | 59 | ||
| 80 | 99 | 91 | 82 | 77 | 67 | 59 | 106 | 93 | 72 | 59 | 37 | 22 | 101 | 92 | 82 | 77 | 79 | 72 | |
| Methyl acetate | 20 | 89 | 86 | 79 | 68 | 73 | 71 | 65 | 53 | 47 | 42 | 44 | 81 | 79 | 68 | 64 | 57 | 53 | |
| 40 | 94 | 88 | 83 | 79 | 81 | 71 | 61 | 50 | 41 | 29 | 22 | 92 | 85 | 79 | 74 | 67 | 63 | ||
| 80 | 93 | 88 | 86 | 84 | 80 | 72 | 99 | 85 | 72 | 63 | 47 | 38 | 99 | 92 | 87 | 84 | 80 | 77 | |
| Ethyl acetate | 20 | 91 | 80 | 67 | 66 | 54 | 48 | 70 | 54 | 47 | 34 | 29 | 89 | 80 | 73 | 67 | 58 | 53 | |
| 40 | 89 | 76 | 74 | 71 | 69 | 79 | 57 | 54 | 46 | 37 | 32 | 100 | 94 | 87 | 79 | 74 | 67 | ||
| 80 | 93 | 84 | 87 | 78 | 77 | 71 | 100 | 87 | 76 | 67 | 58 | 52 | 98 | 93 | 83 | 84 | 80 | 78 | |
| n-Butyl acetate | 20 | 84 | 82 | 66 | 64 | 52 | 59 | 71 | 59 | 51 | 45 | 38 | 86 | 78 | 69 | 57 | 50 | 50 | |
| 40 | 92 | 86 | 81 | 75 | 64 | 82 | 69 | 60 | 52 | 43 | 39 | 92 | 85 | 76 | 71 | 62 | 57 | ||
| 80 | 93 | 89 | 82 | 78 | 72 | 69 | 97 | 83 | 69 | 64 | 53 | 41 | 101 | 93 | 86 | 82 | 79 | 73 | |
| Dimethyl sulfide | 20 | 87 | 73 | 59 | 50 | 36 | 30 | 69 | 45 | 30 | 16 | 9 | 90 | 86 | 80 | 73 | 67 | 61 | |
| 40 | 88 | 76 | 63 | 55 | 46 | 80 | 75 | 63 | 50 | 39 | 31 | 89 | 86 | 80 | 77 | 71 | 69 | ||
| 80 | 93 | 83 | 78 | 73 | 65 | 61 | 104 | 95 | 85 | 76 | 63 | 55 | 97 | 89 | 85 | 84 | 82 | 79 | |
| Methyl propyl sulfide | 20 | 89 | 76 | 64 | 56 | 44 | 37 | 73 | 47 | 31 | 14 | 7 | 93 | 83 | 79 | 73 | 65 | 59 | |
| 40 | 86 | 84 | 75 | 66 | 58 | 85 | 74 | 59 | 48 | 35 | 25 | 91 | 88 | 82 | 76 | 73 | 69 | ||
| 80 | 95 | 89 | 77 | 77 | 68 | 66 | 104 | 96 | 79 | 73 | 60 | 52 | 95 | 91 | 85 | 84 | 81 | 78 | |
| Thiophene | 20 | 77 | 70 | 58 | 54 | 45 | 40 | 75 | 61 | 52 | 39 | 31 | 86 | 76 | 65 | 59 | 50 | 47 | |
| 40 | 82 | 79 | 73 | 65 | 60 | 83 | 72 | 63 | 55 | 46 | 40 | 87 | 80 | 74 | 68 | 62 | 51 | ||
| 80 | 86 | 84 | 78 | 74 | 68 | 62 | 101 | 93 | 84 | 77 | 68 | 62 | 99 | 100 | 82 | 84 | 79 | 80 | |
| Thiophene, 3-methyl- | 20 | 73 | 56 | 43 | 38 | 29 | 24 | 64 | 45 | 37 | 27 | 20 | 83 | 66 | 55 | 48 | 39 | 34 | |
| 40 | 83 | 71 | 60 | 53 | 42 | 77 | 61 | 50 | 42 | 33 | 27 | 86 | 75 | 65 | 59 | 52 | 46 | ||
| 80 | 89 | 78 | 71 | 64 | 57 | 51 | 96 | 83 | 71 | 63 | 52 | 46 | 99 | 93 | 85 | 80 | 74 | 69 | |
| (+)-3-Carene | 20 | 85 | 71 | 61 | 54 | 42 | 37 | 88 | 84 | 58 | 48 | 42 | 83 | 84 | 80 | 71 | 57 | 60 | |
| 40 | 97 | 89 | 88 | 76 | 67 | 92 | 80 | 79 | 64 | 46 | 36 | 94 | 91 | 95 | 85 | 77 | 62 | ||
| 80 | 104 | 98 | 93 | 80 | 68 | 66 | 91 | 92 | 91 | 84 | 76 | 69 | 93 | 82 | 88 | 83 | 76 | 67 | |
| α-Pinene | 20 | 93 | 82 | 78 | 68 | 58 | 60 | 101 | 95 | 75 | 65 | 57 | 99 | 87 | 84 | 85 | 81 | 88 | |
| 40 | 86 | 89 | 77 | 76 | 70 | 99 | 90 | 86 | 80 | 67 | 56 | 93 | 95 | 92 | 87 | 84 | 78 | ||
| 80 | 99 | 81 | 92 | 82 | 86 | 81 | 115 | 103 | 107 | 100 | 83 | 75 | 101 | 99 | 90 | 94 | 89 | 88 | |
| p-Cymene | 20 | 77 | 59 | 45 | 38 | 29 | 27 | 67 | 50 | 40 | 34 | 26 | 83 | 75 | 63 | 56 | 44 | 43 | |
| 40 | 85 | 77 | 67 | 58 | 50 | 79 | 65 | 54 | 48 | 39 | 30 | 93 | 87 | 75 | 67 | 63 | 51 | ||
| 80 | 98 | 87 | 78 | 68 | 59 | 54 | 89 | 76 | 69 | 63 | 52 | 46 | 98 | 93 | 85 | 81 | 78 | 63 | |
| D-Limonene | 20 | 82 | 67 | 57 | 51 | 39 | 41 | 79 | 60 | 50 | 36 | 30 | 81 | 82 | 75 | 70 | 54 | 53 | |
| 40 | 87 | 83 | 75 | 71 | 58 | 86 | 77 | 63 | 54 | 39 | 28 | 96 | 92 | 75 | 80 | 71 | 59 | ||
| 80 | 107 | 98 | 90 | 82 | 68 | 66 | 99 | 79 | 82 | 76 | 54 | 41 | 103 | 100 | 95 | 92 | 86 | 69 | |
| Eucalyptol | 20 | 81 | 86 | 70 | 33 | 29 | 36 | 80 | 69 | 63 | 57 | 58 | 96 | 125 | 138 | 142 | 123 | 122 | |
| 40 | 74 | 82 | 71 | 65 | 63 | 91 | 86 | 80 | 83 | 75 | 87 | 106 | 108 | 113 | 111 | 109 | 88 | ||
| 80 | 112 | 107 | 95 | 101 | 90 | 84 | 105 | 96 | 94 | 102 | 87 | 82 | 98 | 97 | 102 | 101 | 102 | 85 | |
| Ethyl ether | 20 | 94 | 89 | 82 | 70 | 73 | 69 | 86 | 82 | 79 | 72 | 67 | 88 | 90 | 88 | 85 | 82 | 78 | |
| 40 | 96 | 86 | 83 | 80 | 78 | 94 | 90 | 84 | 83 | 76 | 73 | 96 | 92 | 89 | 85 | 87 | 81 | ||
| 80 | 88 | 86 | 88 | 84 | 80 | 79 | 106 | 103 | 98 | 93 | 91 | 87 | 94 | 90 | 89 | 87 | 86 | 79 | |
| Acetonitrile | 20 | 47 | 31 | 27 | 34 | 22 | 17 | 57 | 34 | 31 | 12 | 7 | 57 | 38 | 27 | 24 | 16 | 11 | |
| 40 | 69 | 42 | 27 | 32 | 24 | 45 | 26 | 18 | 12 | 8 | 2 | 64 | 47 | 38 | 31 | 23 | 17 | ||
| 80 | 74 | 50 | 43 | 36 | 27 | 28 | 66 | 39 | 22 | 12 | 4 | 6 | 87 | 65 | 56 | 49 | 38 | 33 | |
| Dimethyl selenide | 20 | 79 | 65 | 46 | 44 | 30 | 26 | 37 | 12 | 4 | 2 | 1 | 89 | 77 | 65 | 51 | 40 | 31 | |
| 40 | 82 | 64 | 54 | 45 | 37 | 78 | 53 | 29 | 17 | 7 | 3 | 96 | 86 | 76 | 64 | 61 | 48 | ||
| 80 | 87 | 81 | 73 | 71 | 64 | 59 | 96 | 74 | 55 | 40 | 23 | 15 | 96 | 89 | 84 | 78 | 73 | 66 | |
| Pyrimidine | 20 | 45 | 26 | 18 | 18 | 12 | 10 | 47 | 27 | 25 | 14 | 13 | 53 | 31 | 21 | 16 | 11 | 10 | |
| 40 | 52 | 40 | 30 | 24 | 17 | 36 | 19 | 13 | 11 | 6 | 5 | 65 | 42 | 29 | 25 | 17 | 14 | ||
| 80 | 80 | 58 | 42 | 38 | 30 | 25 | 56 | 33 | 17 | 17 | 10 | 8 | 82 | 66 | 50 | 42 | 32 | 28 | |
For all bags the stability of the compounds of interest was strongly correlated with the volume of the test sample filled into the bag. Regardless of the chemical class of a compound, its recovery was significantly better when the sampling bag was filled up to 80% of its nominal volume. For the majority of all species, SA : V ratios below 100 m−1 provided good recoveries even after 7 days of storage. Samples with the highest surface-to-volume ratios (above 200 m−1) were stable only for several hours. This finding is not surprising, as the area of the bag materials (polymer film, valve, etc.) having contact with the sample for all SA : V ratios remained the same and its potential for interactions with the sample constituents was comparable. Consequently, large samples containing higher masses of the investigated species were more resistant to losses during storage. Additionally, samples stored at lower SA : V ratios were less susceptible to the emission of contaminants, as can be seen in Table 5. For example, in Flexfilm bags after 24 hours of storage the concentration of n-butane remained stable when the bag was filled up to 80% of its nominal volume, whereas in the bags filled up to only 20% of its nominal volume its concentration increased two-fold. Thus, it is strongly recommended to collect the largest possible volume of the sample in order to provide the optimal conditions for the preservation of its integrity.
Considerable differences were found for the stabilities of compounds in different polymer bags. Since the superiority of lower SA : V ratios of stored samples is undeniable, further discussion of the compound recoveries will refer to bags filled up to 80% (2.4 l) with the test mixture, unless otherwise stated.
3.3.1. Aliphatic hydrocarbons
The stabilities of hydrocarbons tested within this study suffered significantly from the background emission in Flexfilm and Kynar bags. Despite pre-conditioning, levels of numerous species tended to increase rapidly (even within 6 hours of storage). This phenomenon was particularly pronounced for Flexfilm bags, confirming the finding of the background tests. For HCs found not to be emitted by bag materials, good recoveries were noted even after 3 days of storage. Due to the much lower background emission Kynar bags provided better stability of HCs. The majority of species from this chemical class remained stable up to 3 days of storage (when filled up to 80% of maximum volume). Nevertheless, the risk of contamination considerably limits the applicability of Kynar and Flexfilm bags during breath studies aiming at hydrocarbons at low ppb levels. Hydrocarbons stored in Tedlar bags exhibited excellent recoveries over the whole investigated storage period, even for higher SA : V ratios. Only heavier hydrocarbons (e.g., n-decane) showed higher losses. Specifically, unsaturated hydrocarbons were much better preserved in Tedlar bags than in the other ones. Interestingly, in Kynar and Flexfilm bags the drop of isoprene levels (initial value of 106 ppb) was accompanied by the increase of 2-methyl-2-propenal, 3-buten-2-one and 3-methylfuran – species known to be the products of isoprene degradation in the atmosphere.35 For example, after 7 days concentrations of these species in Kynar bags filled with 2.4 l of test mixture were 12, 11, and 1.8 ppb, respectively. In Tedlar bags this effect was much less evident. In the case of this film only 3-buten-2-one was found to be produced (1 ppb after 1 day, 3 ppb after 7 days). Perhaps the presence of Kynar and Flexfilm films promotes the degradation of isoprene. As a result, due to the good background and excellent recoveries Tedlar bags seem to be the best choice for sampling and storage of breath hydrocarbons.
3.3.2. Aromatic hydrocarbons
In Kynar and Flexfilm bags the stability of the studied aromatics was relatively poor. In general, acceptable recoveries were observed only up to 24 hours of storage. In samples having higher SA : V ratios losses were pronounced even within the first hours of storage. In Tedlar bags recovery of species from this class was over 80% at the end of the investigated period, however, only in bags filled up to 80% of the maximum capacity. In all cases the values of recovery tended to decrease with increasing molecular mass of a compound.
3.3.3. Ketones
Recovery of ketones in Kynar bags was unsatisfactory. Their levels rapidly dropped below the arbitrarily chosen threshold of 80%. Even acetone having an initial concentration of 720 ppb followed this pattern. The apparently better stability of 2-butanone can easily be explained by its background emission from the Kynar film. Consequently, ketones stored in Kynar bags should be analysed within several hours after sampling. Much better recoveries were observed in Flexfilm bags, with characteristic drops related to the molecular mass of the compound. Once more Tedlar bags provided the best storage conditions for the discussed species. Apart from 4-heptanone, all ketones were stable for up to 7 days of storage. These results and the fact that Tedlar bags do not exhibit ketone release render this material optimal for the storage of species from this chemical category.
3.3.4. Aldehydes
Flexfilm bags were found to be inappropriate for the storage of aldehydes. Background emission significantly affected their initial concentrations. The 7 day monitoring period even revealed the emission of additional aldehydes (e.g., n-heptanal) not being detected during the 24 hour background test. Nevertheless, n-octanal stored in Flexfilm bags exhibited the best stability. In Kynar and Tedlar bags the stability of all tested aldehydes was comparable (up to 3 days).
For all remaining compounds (e.g., sulphurs, esters, terpenes) the superiority of storage in Tedlar bags is undisputed. For species with molecular mass up to 90 Tedlar bags provided good stability for up to 7 days of storage. Recoveries of heavier species were better than 80% only within 3–4 days. In Kynar and Flexfilm bags losses of these analytes were more evident and usually exceeded 20% in samples stored longer than one day. The stability of pyrimidine, acetonitrile and dimethyl selenide was especially poor. In all cases acetonitrile concentrations rapidly dropped even within the first several hours of storage. This finding is consistent with previous studies evidencing huge losses of this compound during storage due to the permeation through the polymer film.19 Its slightly better recovery in Tedlar bags can be explained by a background emission. A similar progression of stability was noted for pyrimidine with losses being acceptable only for Tedlar bags within 6 hours of storage. Dimethyl selenide showed good recoveries only in Tedlar bags.
3.4. Humid standard stability test
The comparison of recoveries of volatiles for dry and humid test mixtures is presented in Table 6. It must be stressed here that water permeates relatively easily through all tested materials and consequently, sample humidity remains elevated only for several hours of storage19,31,32 and subsequently reaches an ambient level. The contrary holds true for dry samples that exhibit ambient levels of water vapour after a few hours of storage. Consequently, the humid standard stability test was restricted to a period of 48 h only.
Table 6.
Comparison of recoveries of volatiles under study for dry and humid test mixtures in Tedlar, Kynar and Flexfilm bags
| Recovery from Flexfilm bag [%] |
Recovery from Kynar bag [%] |
Recovery from Tedlar bag [%] |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOC | 2 h | 4 h | 6 h | 24 h | 48 h | 2 h | 4 h | 6 h | 24 h | 48 h | 2 h | 4 h | 6 h | 24 h | 48 h | |
| n-Butane | Humid | 105 | 106 | 108 | 115 | 123 | 105 | 99 | 99 | 95 | 91 | 105 | 106 | 68 | 99 | 95 |
| Dry | 115 | 130 | 131 | 202 | 214 | 107 | 101 | 110 | 103 | 85 | 100 | 103 | 103 | 101 | 88 | |
| n-Pentane | Humid | 103 | 104 | 105 | 109 | 114 | 105 | 104 | 103 | 89 | 85 | 103 | 104 | 73 | 99 | 100 |
| Dry | 103 | 105 | 109 | 128 | 153 | 101 | 100 | 100 | 78 | 77 | 96 | 98 | 99 | 96 | 95 | |
| n-Hexane | Humid | 103 | 104 | 104 | 111 | 117 | 99 | 101 | 88 | 79 | 76 | 103 | 102 | 81 | 98 | 100 |
| Dry | 126 | 151 | 188 | 412 | 583 | 103 | 103 | 99 | 80 | 81 | 102 | 98 | 99 | 102 | 97 | |
| n-Octane | Humid | 90 | 92 | 93 | 92 | 91 | 101 | 101 | 100 | 90 | 86 | 90 | 92 | 93 | 92 | 91 |
| Dry | 99 | 101 | 99 | 101 | 94 | 103 | 98 | 98 | 87 | 73 | 95 | 98 | 98 | 99 | 95 | |
| n-Decane | Humid | 61 | 61 | 61 | 60 | 57 | 82 | 89 | 82 | 82 | 66 | 71 | 61 | 60 | 60 | 57 |
| Dry | 84 | 101 | 92 | 90 | 79 | 103 | 97 | 97 | 68 | 52 | 100 | 116 | 104 | 114 | 104 | |
| Isobutane | Humid | 102 | 100 | 89 | 73 | 78 | 101 | 105 | 100 | 99 | 83 | 100 | 98 | 89 | 71 | 76 |
| Dry | 100 | 98 | 96 | 87 | 94 | 102 | 100 | 94 | 78 | 84 | 94 | 88 | 89 | 86 | 76 | |
| Pentane, 2-methyl- | Humid | 80 | 79 | 79 | 92 | 84 | 104 | 104 | 102 | 92 | 89 | 80 | 79 | 70 | 92 | 84 |
| Dry | 100 | 91 | 101 | 115 | 124 | 101 | 98 | 100 | 82 | 75 | 100 | 98 | 97 | 100 | 95 | |
| Octane, 4-methyl- | Humid | 96 | 98 | 99 | 106 | 104 | 98 | 98 | 95 | 87 | 90 | 96 | 98 | 79 | 106 | 104 |
| Dry | 93 | 108 | 105 | 158 | 189 | 104 | 98 | 96 | 84 | 81 | 103 | 98 | 100 | 103 | 110 | |
| 2-Pentene, (E) | Humid | 91 | 91 | 90 | 90 | 85 | 104 | 101 | 97 | 84 | 42 | 91 | 91 | 92 | 90 | 89 |
| Dry | 98 | 98 | 98 | 93 | 83 | 101 | 99 | 99 | 60 | 44 | 96 | 99 | 98 | 98 | 96 | |
| 2-Pentene, (Z) | Humid | 90 | 102 | 92 | 92 | 93 | 106 | 102 | 97 | 88 | 49 | 90 | 100 | 98 | 92 | 93 |
| Dry | 99 | 100 | 98 | 93 | 100 | 94 | 105 | 103 | 57 | 53 | 87 | 86 | 88 | 84 | 87 | |
| 1-Hexene | Humid | 99 | 100 | 100 | 104 | 109 | 104 | 102 | 99 | 90 | 78 | 99 | 100 | 73 | 76 | 79 |
| Dry | 102 | 98 | 109 | 145 | 168 | 101 | 98 | 98 | 72 | 65 | 101 | 98 | 99 | 98 | 97 | |
| Isoprene | Humid | 104 | 104 | 105 | 102 | 99 | 104 | 102 | 99 | 86 | 64 | 98 | 99 | 105 | 97 | 94 |
| Dry | 100 | 99 | 98 | 93 | 96 | 100 | 97 | 96 | 60 | 50 | 97 | 98 | 99 | 99 | 95 | |
| Benzene | Humid | 87 | 86 | 85 | 83 | 81 | 98 | 96 | 94 | 86 | 76 | 87 | 86 | 88 | 83 | 81 |
| Dry | 96 | 88 | 95 | 92 | 85 | 98 | 95 | 95 | 74 | 63 | 90 | 97 | 96 | 95 | 92 | |
| Toluene | Humid | 90 | 89 | 89 | 87 | 84 | 97 | 96 | 93 | 94 | 87 | 90 | 89 | 88 | 87 | 84 |
| Dry | 96 | 93 | 95 | 86 | 85 | 97 | 92 | 92 | 80 | 64 | 93 | 96 | 96 | 93 | 84 | |
| p-Xylene | Humid | 86 | 88 | 89 | 82 | 79 | 94 | 90 | 89 | 85 | 74 | 86 | 88 | 89 | 82 | 79 |
| Dry | 97 | 96 | 94 | 86 | 79 | 98 | 93 | 91 | 66 | 54 | 80 | 95 | 95 | 90 | 80 | |
| Acetone | Humid | 99 | 100 | 100 | 98 | 99 | 97 | 92 | 89 | 80 | 66 | 99 | 100 | 97 | 98 | 99 |
| Dry | 99 | 97 | 97 | 91 | 88 | 92 | 86 | 84 | 61 | 57 | 98 | 96 | 95 | 93 | 81 | |
| 2-Butanone | Humid | 96 | 98 | 97 | 99 | 102 | 106 | 94 | 100 | 100 | 76 | 96 | 98 | 84 | 95 | 97 |
| Dry | 115 | 129 | 131 | 140 | 151 | 96 | 93 | 95 | 66 | 71 | 101 | 105 | 89 | 85 | 97 | |
| 2-Pentanone | Humid | 91 | 93 | 95 | 85 | 83 | 88 | 83 | 78 | 72 | 43 | 91 | 93 | 95 | 85 | 83 |
| Dry | 99 | 91 | 97 | 92 | 84 | 94 | 84 | 84 | 52 | 37 | 100 | 99 | 96 | 96 | 88 | |
| 4-Heptanone | Humid | 87 | 87 | 89 | 64 | 67 | 87 | 87 | 89 | 64 | 67 | 87 | 87 | 89 | 64 | 67 |
| Dry | 90 | 100 | 99 | 93 | 86 | 90 | 100 | 99 | 93 | 86 | 90 | 100 | 99 | 93 | 86 | |
| Propanal | Humid | 97 | 98 | 98 | 97 | 96 | 104 | 104 | 101 | 97 | 97 | 97 | 98 | 93 | 97 | 96 |
| Dry | 101 | 102 | 101 | 95 | 89 | 98 | 96 | 96 | 76 | 76 | 100 | 104 | 97 | 95 | 84 | |
| Hexanal | Humid | 101 | 95 | 96 | 96 | 109 | 101 | 89 | 95 | 103 | 92 | 100 | 94 | 97 | 96 | 95 |
| Dry | 102 | 104 | 102 | 98 | 85 | 116 | 119 | 113 | 85 | 58 | 89 | 100 | 103 | 98 | 90 | |
| Octanal | Humid | 69 | 92 | 69 | 93 | 76 | 83 | 53 | 50 | 72 | 49 | 81 | 92 | 59 | 93 | 76 |
| Dry | 94 | 102 | 101 | 99 | 71 | 105 | 106 | 130 | 86 | 58 | 102 | 98 | 89 | 81 | 73 | |
| Furan | Humid | 90 | 90 | 90 | 88 | 88 | 103 | 103 | 99 | 90 | 77 | 90 | 90 | 93 | 88 | 88 |
| Dry | 99 | 97 | 97 | 94 | 88 | 98 | 96 | 95 | 68 | 62 | 99 | 97 | 95 | 97 | 87 | |
| Furan, 2-methyl- | Humid | 90 | 91 | 86 | 85 | 81 | 101 | 95 | 93 | 85 | 58 | 90 | 91 | 90 | 85 | 81 |
| Dry | 96 | 97 | 95 | 93 | 89 | 99 | 96 | 93 | 60 | 49 | 98 | 96 | 96 | 97 | 92 | |
| Furan, 2,5-dimethyl- | Humid | 93 | 91 | 90 | 79 | 65 | 98 | 89 | 81 | 68 | 23 | 93 | 91 | 92 | 79 | 65 |
| Dry | 99 | 96 | 98 | 90 | 83 | 99 | 92 | 94 | 39 | 18 | 98 | 98 | 97 | 95 | 88 | |
| Methyl acetate | Humid | 90 | 90 | 90 | 86 | 84 | 96 | 93 | 89 | 82 | 68 | 90 | 90 | 92 | 86 | 84 |
| Dry | 97 | 97 | 95 | 90 | 88 | 90 | 88 | 86 | 55 | 54 | 97 | 96 | 96 | 93 | 87 | |
| Ethyl acetate | Humid | 92 | 92 | 91 | 87 | 86 | 98 | 95 | 93 | 87 | 76 | 92 | 92 | 93 | 87 | 86 |
| Dry | 95 | 95 | 95 | 92 | 90 | 96 | 92 | 90 | 64 | 59 | 99 | 97 | 99 | 93 | 89 | |
| n-Butyl acetate | Humid | 87 | 92 | 93 | 73 | 78 | 89 | 85 | 81 | 74 | 55 | 87 | 92 | 91 | 85 | 78 |
| Dry | 102 | 103 | 98 | 92 | 94 | 97 | 94 | 91 | 53 | 52 | 98 | 98 | 101 | 95 | 82 | |
| Dimethyl sulfide | Humid | 92 | 92 | 91 | 86 | 80 | 100 | 101 | 104 | 94 | 84 | 92 | 92 | 96 | 86 | 80 |
| Dry | 96 | 94 | 95 | 91 | 91 | 94 | 96 | 94 | 63 | 74 | 98 | 97 | 97 | 97 | 90 | |
| Methyl propyl sulfide | Humid | 94 | 95 | 93 | 87 | 81 | 104 | 103 | 98 | 90 | 69 | 94 | 95 | 98 | 87 | 81 |
| Dry | 96 | 92 | 97 | 92 | 83 | 98 | 93 | 94 | 85 | 75 | 99 | 96 | 95 | 97 | 92 | |
| Thiophene | Humid | 89 | 89 | 88 | 83 | 79 | 100 | 97 | 96 | 86 | 77 | 89 | 89 | 88 | 83 | 79 |
| Dry | 95 | 88 | 90 | 85 | 81 | 96 | 93 | 91 | 69 | 63 | 100 | 97 | 96 | 92 | 87 | |
| Thiophene, 3-methyl- | Humid | 87 | 86 | 85 | 77 | 70 | 94 | 90 | 87 | 79 | 63 | 87 | 86 | 87 | 77 | 70 |
| Dry | 94 | 87 | 89 | 78 | 73 | 94 | 88 | 86 | 58 | 49 | 94 | 94 | 94 | 88 | 81 | |
| (+)-3-Carene | Humid | 82 | 82 | 83 | 76 | 69 | 96 | 92 | 90 | 86 | 58 | 82 | 82 | 80 | 76 | 69 |
| Dry | 96 | 96 | 91 | 90 | 83 | 99 | 93 | 93 | 72 | 46 | 101 | 96 | 96 | 104 | 98 | |
| α-Pinene | Humid | 91 | 93 | 95 | 85 | 89 | 92 | 105 | 99 | 92 | 68 | 91 | 93 | 87 | 85 | 89 |
| Dry | 94 | 97 | 83 | 94 | 90 | 110 | 100 | 102 | 90 | 57 | 101 | 108 | 104 | 100 | 97 | |
| p-Cymene | Humid | 68 | 69 | 69 | 64 | 59 | 88 | 85 | 84 | 79 | 68 | 68 | 69 | 65 | 64 | 59 |
| Dry | 90 | 94 | 88 | 86 | 73 | 96 | 85 | 86 | 72 | 58 | 109 | 105 | 104 | 112 | 98 | |
| d-Limonene | Humid | 68 | 72 | 75 | 66 | 63 | 88 | 85 | 81 | 76 | 36 | 68 | 72 | 72 | 66 | 63 |
| Dry | 95 | 97 | 91 | 88 | 80 | 102 | 90 | 91 | 48 | 32 | 100 | 95 | 96 | 100 | 90 | |
| Eucalyptol | Humid | 88 | 84 | 84 | 45 | 57 | 88 | 84 | 84 | 45 | 57 | 88 | 84 | 84 | 45 | 57 |
| Dry | 93 | 107 | 101 | 105 | 96 | 93 | 107 | 101 | 105 | 96 | 93 | 107 | 101 | 105 | 96 | |
| Ethyl ether | Humid | 90 | 90 | 92 | 89 | 87 | 106 | 106 | 102 | 94 | 88 | 90 | 90 | 96 | 89 | 87 |
| Dry | 98 | 96 | 97 | 91 | 93 | 100 | 97 | 96 | 68 | 72 | 96 | 98 | 98 | 99 | 96 | |
| Acetonitrile | Humid | 72 | 63 | 64 | 59 | 42 | 73 | 62 | 56 | 54 | 30 | 72 | 63 | 69 | 59 | 42 |
| Dry | 84 | 80 | 77 | 62 | 43 | 69 | 56 | 52 | 26 | 15 | 87 | 81 | 78 | 64 | 50 | |
| Dimethyl selenide | Humid | 90 | 91 | 90 | 80 | 70 | 107 | 99 | 89 | 80 | 25 | 90 | 91 | 94 | 80 | 70 |
| Dry | 94 | 99 | 96 | 93 | 88 | 102 | 97 | 94 | 37 | 18 | 92 | 98 | 98 | 96 | 84 | |
For the majority of compounds the difference between recoveries in dry samples and humid samples was smaller than 10%, which is in good agreement with the results obtained by Groves and Zellers.27 Nevertheless, species in humid samples exhibited usually slightly poorer stability. In general, recovery differences tended to increase with increasing molecular masses of the compounds. For the heaviest species investigated within this study (n-decane, eucalyptol, D-limonene, p-cymene, α-pinene), they amounted to 20–40%, thus significantly reducing the safe storage time. Interestingly, in Flexfilm and Kynar bags the presence of large amounts of water reduced the emission of contaminants. Most probably water condensing and permeating through polymer films forms a kind of barrier protecting samples from background emission of pollutants. The same water layer seems to induce higher losses of less volatile and more soluble species tending to go into the liquid phase. Consequently, a rapid drop in the concentrations of hydrophilic compounds is observed during the first hours of their storage. Amongst the remaining volatiles acetonitrile was especially sensitive to the presence of water with losses of 30% already after 2 hours of storage. The humidity influence is relatively similar for all bag materials tested. To sum up, high humidity is a crucial factor considerably reducing safe storage time of breath constituents. Since the recoveries of compounds heavier than 90 drop significantly during the first hours of storage, it is recommended to analyse breath samples within six hours of storage.
3.5. Reusability test
The applied cleaning protocol was found to be efficient. In Tedlar bags after 24 hours of storage of pure nitrogen only 4 compounds from the tested ones were detected: 2-butanone, 3-methylthiophene, hexanal and p-xylene. However, their levels were below the LOQs of the applied method. The same number of species was found in Kynar bags: acetonitrile, 3-methyl-thiophene, n-octane and octanal. Amongst them acetonitrile exhibited quantified levels spreading around 4 ppb. Cleaning of Flexfilm bags was more difficult. Excluding species known to be released, six artifacts from the test mixture were detected in these bags after storage for one day: acetonitrile, acetone, pyrimidine, n-octane, p-xylene, and octanal. Acetone showed an average concentration of 18 ppb, whereas acetonitrile levels reached a mean value of 6 ppb. Additional cleaning cycles might be necessary to further remove remainings of the previous sample.
4. Conclusions
In general, several valuable pieces of information on the storage of breath gas samples (as well as other samples containing species at the ppb level) in polymer bags can be extracted from the results of this study.
Firstly, the background emission of pollutants is one of the most important factors when selecting the optimal polymer. High contaminants release distorts the original sample composition already during sampling (bag filling). In the context of breath research aiming at VOCs at low ppb or even ppt levels, Tedlar bags with only nine identified contaminants seem to be the best choice. However, two-fold pre-conditioning of bags before usage is highly recommended. On the other hand, Kynar and particularly Flexfilm were found to emit numerous pollutants (mainly hydrocarbons) detectable immediately, or after few hours of storage at ppt to ppb levels. Preconditioning, even when repeated for several times was not efficient in the case of Flexfilm bags; consequently, this type of material is only suitable for studies aiming at much higher levels of VOCs (e.g., at the ppm level). Due to the quite effective cleaning Kynar could be considered as an alternative to Tedlar, however, it must be remembered that even repeated conditioning does not guarantee the reduction of contaminant emission to a safe level. Finally, it must be underlined that within this study due to the chromatographic limitations only C3–C12 contaminants were monitored, thus the emission of heavier pollutants cannot be excluded.
Secondly, in the case of all tested materials the recovery of volatiles strongly depends on the degree of bag filling (i.e., on the polymer surface-to-(sample) volume ratio (SA : V)). The recoveries of the investigated species in bags with low SA : V values (below 100 m−1) were satisfactory up to 7 days of storage. The increase of the SA : V ratio values above 200 decreases the storage time with acceptable recovery (>80%) by a factor of 3–6. This finding is not surprising as the VOC levels in smaller samples (containing smaller masses of species) are more vulnerable to losses related to sorption or permeation. Additionally, samples in bags filled up to 80% of their maximum volume were less affected by the background emission of contaminants. Consequently, if breath samples are to be stored in polymer bags it is strongly recommended to collect a sample volume as large as possible.
Stability comparisons of the analytes under study in the three polymer bags demonstrated the supremacy of Tedlar over remaining films. In the case of a dry test mixture, recoveries from Tedlar bags (when filled up to 80% of nominal volume) were excellent even after one week of storage. Nevertheless, this safe storage time decreases with the increase of the VOC’s molecular mass. For Kynar the storage times for which an acceptable stability of the tested VOCs can be expected are generally shorter. For this type of bag low ppb VOCs should be analyzed within one day of storage. Nevertheless, Kynar is not suitable for storage of some classes of compounds like ketones (poor recovery), or hydrocarbons (high background emission, or polymer dependent decomposition as it may be the case for isoprene). The suitability of Flexfilm bags for storing breath C3–C10 species is very limited. Although the losses of compounds under study in this polymer were lower than in Kynar bags, the levels of pollutants (hydrocarbons, aldehydes, ketones) were especially high, thereby considerably affecting sample integrity.
High humidity also affects the species’ recoveries. For the majority of compounds stabilities in humid air were up to 10% lower than in a dry matrix. Higher losses (20–40%) detectable even shortly after the bags filling were observed for volatiles with molecular mass above 110. Consequently, in order to reduce losses of heavier species it is advised to analyze breath samples within 6 hours after sampling.
All tested polymers can be reused. The applied cleaning protocol was found to be quite efficient for the removal of artifacts from a previous sample. Nonetheless, to provide maximum security the cleaning procedure proposed here should be repeated at least two times.
In the context of reusability one important factor was not investigated within this study, namely the ageing effect of the polymer film. It was demonstrated in our previous paper21 that used polymer bags with a scratched film exhibit poorer recoveries for sulphur compounds; a similar effect is expected for other classes of species. Therefore, an effort must be made to protect the polymer film and control its quality during usage.
Finally, it must be stressed that due to limitations of the analytical method applied, only C3–C10 volatiles were tested within the study. For heavier or for more reactive species problems arising from sample storage can be much more apparent and demand additional studies.
Table 3.
Contaminants emitted by Kynar bags [ppb]. Compounds are ordered with respect to increasing retention time. ʺ—ʺ denotes that the VOC was not detected, whereas ʺ<LOQʺ stands for VOC level below LOQ
| New bag sampling time [h] |
Pre-conditioned bag sampling time [h] |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOC | CAS | 0 | 6 | 12 | 24 | 0 | 6 | 24 | VOC emission × 10−12 [g × h−1 × cm−2] | ||||||
| Carbonyl sulfide (COS) | 463-58-1 | Not quantified | |||||||||||||
| Acetone | 67-64-1 | 2.7 | 41.4 | 29.4 | 32.8 | — | — | — | 11 | ||||||
| Carbon disulfide (CS2) | 75-15-0 | Not quantified | |||||||||||||
| Trimethylsilyl fluoride | 420-56-4 | Not quantified | |||||||||||||
| Methyl acetate | 79-20-9 | — | <LOQ | 0.4 | 0.5 | — | — | — | 0.14 | ||||||
| n-Pentane | 109-66-0 | — | — | — | 0.5 | — | — | <LOQ | 0.08 | ||||||
| 2-Propenal, 2-methyl- | 78-85-3 | — | <LOQ | 0.4 | 0.4 | — | — | — | 0.12 | ||||||
| Propanal, 2-methyl- | 78-84-2 | <LOQ | 1.3 | 1.6 | 1.9 | — | — | <LOQ | 0.59 | ||||||
| 2-Butanone | 78-93-3 | 0.5 | 2.3 | 3.6 | 4.3 | <LOQ | 0.5 | 0.7 | 1.19 | ||||||
| Ethyl acetate | 141-78-6 | <LOQ | 1.0 | 1.4 | 1.8 | — | — | <LOQ | 0.59 | ||||||
| 1,2-Dichlorohexafluoropropane | 661-97-2 | Not quantified | |||||||||||||
| n-Hexane | 110-54-3 | — | <LOQ | <LOQ | 0.4 | — | — | <LOQ | 0.12 | ||||||
| Propane, 2-nitro- | 79-46-9 | Not quantified | |||||||||||||
| 2-Pentanone | 107-87-9 | — | <LOQ | <LOQ | <LOQ | — | — | — | 0.09 | ||||||
| n-Propyl acetate | 109-60-4 | <LOQ | 0.6 | 0.8 | 1.0 | — | — | — | 0.43 | ||||||
| Toluene | 108-88-3 | 1.2 | 5.6 | 7.4 | 9.4 | — | 0.3 | 0.4 | 3.44 | ||||||
| Hexanal | 66-25-1 | — | — | 1.1 | 1.1 | — | — | — | 0.34 | ||||||
| 1,3-Dioxane, 4,4-dimethyl- | 766-15-4 | Not quantified | |||||||||||||
| Ethylbenzene | 100-41-4 | — | <LOQ | 1.0 | 1.4 | — | — | — | 0.51 | ||||||
| p-Xylene | 106-42-3 | — | 0.5 | 1.0 | 1.5 | — | 0.3 | 0.4 | 0.48 | ||||||
| o-Xylene | 95-47-6 | — | <LOQ | 0.4 | 0.5 | — | — | — | 0.22 | ||||||
Acknowledgements
P.M., J.K., and K.U. gratefully acknowledge support from the Austrian Science Fund (FWF) under grant no. Y330 and P24736-B23. We appreciate funding from the Austrian Federal Ministry for Transport, Innovation and Technology (BMVIT/BMWA, project 836308, KIRAS). We greatly appreciate the generous support of the government of Vorarlberg, Austria.
References
- 1.Amann A, Corradi M, Mazzone P, Mutti A. Expert Rev Mol Diagn. 2011;11:207–217. doi: 10.1586/erm.10.112. [DOI] [PubMed] [Google Scholar]
- 2.Amann A, Smith D. Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring. World Scientific; New Jersey: 2005. [Google Scholar]
- 3.Bajtarevic A, Ager C, Pienz M, Klieber M, Schwarz K, Ligor M, Ligor T, Filipiak W, Denz H, Fiegl M, Hilbe W, et al. BMC Cancer. 2009;9:348. doi: 10.1186/1471-2407-9-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miekisch W, Schubert JK, Noeldge-Schomburg GF. Clin Chim Acta. 2004;347:25–39. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Phillips M, Altorki N, Austin JH, Cameron RB, Cataneo RN, Kloss R, Maxfield RA, Munawar MI, Pass HI, Rashid A, Rom WN, et al. Clin Chim Acta. 2008;393:76–84. doi: 10.1016/j.cca.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poli D, Carbognani P, Corradi M, Goldoni M, Acampa O, Balbi B, Bianchi L, Rusca M, Mutti A. Respir Res. 2005;6:71. doi: 10.1186/1465-9921-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King J, Kupferthaler A, Frauscher B, Hackner H, Unterkofler K, Teschl G, Hinterhuber H, Amann A, Hogl B. Physiol Meas. 2012;33:413–428. doi: 10.1088/0967-3334/33/3/413. [DOI] [PubMed] [Google Scholar]
- 8.King J, Mochalski P, Unterkofler K, Teschl G, Klieber M, Stein M, Amann A, Baumann M. Biochem Biophys Res Commun. 2012;423:526–530. doi: 10.1016/j.bbrc.2012.05.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koc H, King J, Teschl G, Unterkofler K, Teschl S, Mochalski P, Hinterhuber H, Amann A. J Breath Res. 2011;5:037102. doi: 10.1088/1752-7155/5/3/037102. [DOI] [PubMed] [Google Scholar]
- 10.King J, Unterkofler K, Teschl G, Teschl S, Koc H, Hinterhuber H, Amann A. J Math Biol. 2011;63:959–999. doi: 10.1007/s00285-010-0398-9. [DOI] [PubMed] [Google Scholar]
- 11.King J, Koc H, Unterkofler K, Mochalski P, Kupferthaler A, Teschl G, Teschl S, Hinterhuber H, Amann A. J Theor Biol. 2010;267:626–637. doi: 10.1016/j.jtbi.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 12.King J, Kupferthaler A, Unterkofler K, Koc H, Teschl S, Teschl G, Miekisch W, Schubert J, Hinterhuber H, Amann A. J Breath Res. 2009;3:027006. doi: 10.1088/1752-7155/3/2/027006. [DOI] [PubMed] [Google Scholar]
- 13.King J, Mochalski P, Kupferthaler A, Unterkofler K, Koc H, Filipiak W, Teschl S, Hinterhuber H, Amann A. Physiol Meas. 2010;31:1169–1184. doi: 10.1088/0967-3334/31/9/008. [DOI] [PubMed] [Google Scholar]
- 14.King J, Unterkofler K, Teschl G, Teschl S, Mochalski P, Koc H, Hinterhuber H, Amann A. J Breath Res. 2012;6:016005. doi: 10.1088/1752-7155/6/1/016005. [DOI] [PubMed] [Google Scholar]
- 15.Endre ZH, Pickering JW, Storer MK, Hu WP, Moorhead KT, Allardyce R, McGregor DO, Scotter JM. Physiol Meas. 2011;32:115–130. doi: 10.1088/0967-3334/32/1/008. [DOI] [PubMed] [Google Scholar]
- 16.Spanel P, Smith D. Mass Spectrom Rev. 2011;30:236–267. doi: 10.1002/mas.20303. [DOI] [PubMed] [Google Scholar]
- 17.Ligor M, Ligor T, Bajtarevic A, Ager C, Pienz M, Klieber M, Denz H, Fiegl M, Hilbe W, Weiss W, Lukas P, et al. Clin Chem Lab Med. 2009;47:550–560. doi: 10.1515/CCLM.2009.133. [DOI] [PubMed] [Google Scholar]
- 18.Buszewski B, Ulanowska A, Ligor T, Denderz N, Amann A. Biomed Chromatogr. 2008;23:551–556. doi: 10.1002/bmc.1141. [DOI] [PubMed] [Google Scholar]
- 19.Beauchamp J, Herbig J, Gutmann R, Hansel A. J Breath Res. 2008;2:046001. doi: 10.1088/1752-7155/2/4/046001. [DOI] [PubMed] [Google Scholar]
- 20.Steeghs MM, Cristescu SM, Harren FJ. Physiol Meas. 2007;28:73–84. doi: 10.1088/0967-3334/28/1/007. [DOI] [PubMed] [Google Scholar]
- 21.Mochalski P, Wzorek B, Sliwka I, Amann A. J Chromatogr B: Anal Technol Biomed Life Sci. 2009;877:189–196. doi: 10.1016/j.jchromb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Ligor T, Ligor M, Amann A, Ager C, Bachler M, Dzien A, Buszewski B. J Breath Res. 2008;2:046006. doi: 10.1088/1752-7155/2/4/046006. [DOI] [PubMed] [Google Scholar]
- 23.Kushch I, Arendacka B, Stolc S, Mochalski P, Filipiak W, Schwarz K, Schwentner L, Schmid A, Dzien A, Lechleitner M, Witkovsky V, et al. Clin Chem Lab Med. 2008;46:1011–1018. doi: 10.1515/CCLM.2008.181. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz K, Pizzini A, Arendacka B, Zerlauth K, Filipiak W, Schmid A, Dzien A, Neuner S, Lechleitner M, Scholl-Burgi S, Miekisch W, et al. J Breath Res. 2009;3:027003. doi: 10.1088/1752-7155/3/2/027003. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz K, Filipiak W, Amann A. J Breath Res. 2009;3:027002. doi: 10.1088/1752-7155/3/2/027002. [DOI] [PubMed] [Google Scholar]
- 26.Erhart S, Amann A, Haberlandt E, Edlinger G, Schmid A, Filipiak W, Schwarz K, Mochalski P, Rostasy K, Karall D, Scholl-Burgi S. J Breath Res. 2009;3:016004. doi: 10.1088/1752-7155/3/1/016004. [DOI] [PubMed] [Google Scholar]
- 27.Groves WA, Zellers ET. Am Ind Hyg Assoc J. 1996;57:257–263. doi: 10.1080/15428119691014981. [DOI] [PubMed] [Google Scholar]
- 28.Gilchrist FJ, Razavi C, Webb AK, Jones AM, Spanel P, Smith D, Lenney W. J Breath Res. 2012;6:036004. doi: 10.1088/1752-7155/6/3/036004. [DOI] [PubMed] [Google Scholar]
- 29.Pawliszyn J. Solid Phase Microextraction: Theory and Practice. Wiley-VCH, Inc; New York: 1997. [Google Scholar]
- 30.McFadden ER, Jr, Pichurko BM, Bowman HF, Ingenito E, Burns S, Dowling N, Solway J. J Appl Physiol. 1985;58:564–570. doi: 10.1152/jappl.1985.58.2.564. [DOI] [PubMed] [Google Scholar]
- 31.Cariou S, Guillot JM. Anal Bioanal Chem. 2006;384:468–474. doi: 10.1007/s00216-005-0177-4. [DOI] [PubMed] [Google Scholar]
- 32.Beghi S, Guillot JM. J Chromatogr A. 2006;1127:1–5. doi: 10.1016/j.chroma.2006.05.102. [DOI] [PubMed] [Google Scholar]
- 33.Huber W. Accredit Qual Assur. 2003;8:213–217. [Google Scholar]
- 34.Trabue SL, Anhalt JC, Zahn JA. J Environ Qual. 2006;35:1668–1677. doi: 10.2134/jeq2005.0370. [DOI] [PubMed] [Google Scholar]
- 35.Dibble TS. J Phys Chem A. 1999;103:8559–8565. [Google Scholar]