Abstract
Klebsiella pneumoniae is among the most common gram-negative bacteria that cause pneumonia. Gp96 is an endoplasmic reticulum chaperone that is essential for the trafficking and function of Toll-like receptors (TLRs) and integrins. To determine the role of gp96 in myeloid cells in host defence during Klebsiella pneumonia. Mice homozygous for the conditional Hsp90b1 allele encoding gp96 were crossed with mice expressing Cre-recombinase under control of the LysM promoter to generate LysMcre-Hsp90b1-flox mice. LysMcre-Hsp90b1-flox mice showed absence of gp96 protein in macrophages and partial depletion in monocytes and granulocytes. This was accompanied by almost complete absence of TLR2 and TLR4 on macrophages. Likewise, integrin subunits CD11b and CD18 were not detectable on macrophages, while being only slightly reduced on monocytes and granulocytes. Gp96-deficient macrophages did not release pro-inflammatory cytokines in response to Klebsiella and displayed reduced phagocytic capacity independent of CD18. LysMcre-Hsp90b1-flox mice were highly vulnerable to lower airway infection induced by K. pneumoniae, as reflected by an enhanced bacterial growth and a higher mortality rate. The early inflammatory response in Hsp90b1-flox mice was characterized by a strongly impaired recruitment of granulocytes into the lungs, accompanied by an attenuated production of proinflammatory cytokines, while the inflammatory response during late stage pneumonia was not dependent on the presence of gp96. Blocking CD18 did not reproduce the impaired host defence of LysMcre-Hsp90b1-flox mice during Klebsiella pneumonia.
These data indicate that macrophage gp96 is essential for protective immunity during gram-negative pneumonia by regulating TLR expression.
Keywords: gp96, Klebsiella, Toll-like receptors, integrins, pneumonia
INTRODUCTION
Pneumonia is responsible for an inordinate disease burden worldwide [1]. Klebsiella (K.) pneumoniae is among the most common gram-negative bacteria that can cause infection of the lower respiratory tract [2,3].
Gp96 (also known as heat shock protein 90 kDa beta member 1 [hsp90b1], grp94, endoplasmin or ERp99) is an endoplasmic reticulum (ER) chaperone that plays an essential role in the folding and function of many Toll-like receptor (TLR) and integrin family members [4–7]. TLRs are pattern recognition receptors that prominently feature in the first line of defence against infection by virtue of their capacity to recognize conserved motifs expressed by pathogens and thereby to initiate the innate immune response [8,9]. Integrins comprise a family of 24 αβ subunit heterodimers that regulate the proliferation, differentiation and migration of cells [10]. Deletion of gp96 results in the post-translational loss of multiple TLRs and integrins [4–6], suggesting that this chaperone protein occupies a central position in innate immunity.
Studies on the role of gp96 in vivo are hampered by the fact that global gp96 deficiency is not compatible with life. Recently, myeloid specific gp96 deficient mice were generated and shown to be relatively resistant to shock induced by systemic administration of the TLR4 ligand lipopolysaccharide (LPS) in vivo. Furthermore, macrophages purified from these animals displayed strongly diminished cytokine production upon stimulation with multiple TLR agonists [5].
In the present study we sought to determine the role of myeloid gp96 in host defence during gram-negative pneumonia. For this we compared LysMcre-Hsp90b1-flox and littermate control mice after infection of the lower airways with K. pneumoniae. Our results are the first to show an essential role of macrophage gp96 in protective immunity in a clinically relevant model of lower respiratory tract infection.
METHODS
Ethics statement
Experiments were carried out in accordance with the Dutch Experiment on Animals Act and approved by the Animal Care and Use Committee of the University of Amsterdam (Permit numbers: DIX100121-AR and DIX21-CF, and sub-protocol DIX101613).
Animals
Homozygous Hsp90b1fl/fl mice [5] were crossed with LysMcre mice [11] (Jackson Laboratory, Bar Harbor, ME), to generate LysMcre-Hsp90b1-flox mice. Hsp90b1fl/fl Cre negative littermates were used as controls. All mice lines were backcrossed at least eight times to a C57Bl/6 background and age- and sex matched when used in experiments. C57BL/6 mice were purchased from Jackson Laboratory. All mice were used at 8–10 wk of age.
Antibodies
PerCP-conjugated anti-CD115, FITC-conjugated anti-CD45, Fixable Viability dye eFluor 780, PerCP-conjugated anti-CD64, PE-conjugated anti-TLR2, PE-conjugated anti-TLR4/MD2, PE-conjugated anti-CD29 and all matching isotypes were purchased from eBioscience (San Diego, CA). Alexa647-conjugated anti-Siglec-F, PE-conjugated anti-CD11b, FITC-conjugated anti-GR-1, APC-conjugated anti-streptavidin Ab, PE-conjugated anti-CD18 (clone C71/16) and Fc Block were obtained from BD Biosciences (Bedford, MA). Biotinylated anti-CD31 Ab was obtained from Biolegend (San Diego, CA). Rat anti-mouse anti-gp96 Ab was purchased from Enzo Life Sciences (Farmingdale, NY) and anti-rat IgG HRP from Santa Cruz (Dallas, TX). Biotinylated anti-F4/80 was obtained from Bio-Connect (Huissen, The Netherlands). CD18 blocking ab (purified NA/LE rat anti-mouse CD18; clone: GAME-46) and matching control Ab for in vivo studies were purchased from BD Biosciences.
Isolation, purification and identification of primary cells
Alveolar macrophages were isolated after bronchoalveolar lavage (BAL) with 10 mL PBS; peritoneal macrophages were harvested after peritoneal lavage with 5 mL PBS. Macrophages were seeded in flat bottomed 96 well cell culture plates (Greiner bio-one, Alphen a/d Rijn, Netherlands) in RPMI medium (Gibco, Bleiswijk, The Netherlands) containing 10% FBS and antibiotics (Penicillin/Streptomycin) and allowed to adhere overnight prior to analysis, stimulation or phagocytosis. For ex vivo whole blood stimulation, and to obtain granulocytes and monocytes for DNA and protein analysis, blood was collected in tubes containing EDTA or heparin by heart puncture. For whole blood stimulations, 100 µL of heparinized blood was pipetted in a 96 well U-bottom cell culture plate (Greiner). To purify granulocytes and monocytes for DNA and protein analysis, erythrocytes in EDTA blood were lysed with an ammonium chloride containing buffer; monocytes were identified as CD11b+/GR-1dim/CD115+, granulocytes as CD11b+/GR-1high/CD115−, and the fraction of CD11b− cells with a low Forward and Side Scatter pattern were identified as lymphoid cells [12]. Cells were sorted on a FACSAria II cell sorting machine (BD Biosciences).
Quantitative PCR
Deletion efficiency of Hsp90b1 was determined after extracting DNA from purified cells using the Nucleospin Blood Kit (Machery Nagel, Düren, Germany). The residual amount of the “floxed” region of Hsp90b1 in various primary cells of LysMcre-Hsp90b1-flox and littermate control mice was quantified using primer sequences 5’- GAGGAGCTTAGACTCGGGATTG-3’ (forward) and 5’- GCCCCAAGAACGTTCGAGAA-3’ (reverse) normalized to Socs3 with primer sequences 5’-ACCTTTCTTATCCGCGACAG-3’ (forward) and 5’- TGCACCAGCTTGAGTACACAG-3’ (reverse) in a SybrGreen reaction on a LightCycler System (LC480, Roche Applied Science, Mannheim, Germany). The amount of remaining “floxed” Hsp90b1 region was calculated using the 2−deltaCt (ΔΔCt) method using the amount of genomic DNA from littermate mice for the no-deletion control. The deletion efficiency was calculated as (1 − residual Hsp90b1-flox) × 100.
Western blot and flow cytometry analyses
Details are provided in Supplementary materials and Methods online
Stimulation of macrophages
Alveolar and peritoneal macrophages were seeded in flat bottomed 96 well cell culture plates (Greiner Bio-one) at a density of approximately 30 000 and 50 000 respectively per well in complete RPMI and left to adhere overnight. Cells were stimulated for 20 h with the indicated concentrations of heat-killed K. pneumonia, LPS derived from Klebsiella pneumoniae (Sigma) or Pam3CSK4 (InvivoGen, Toulouse, France) diluted in RPMI complete medium in a final volume of 200 µl.
Phagocytosis assay
For phagocytosis, FITC labelled heat killed K. pneumoniae was added to alveolar or peritoneal macrophages (at a bacterium:cell ratio of 100:1) for 1 h at 37° C of at 4° C as a control. To stop phagocytosis, samples were put on ice and non-phagocytized bacteria were washed away with ice cold PBS. Cells were then incubated for one min with Trypan Blue Solution (0.4%, Gibco) to quench extracellular fluorescence caused by bacteria that might have adhered to the outside of cells. The phagocytic index was calculated by multiplying the gMFI of FITC positive cells with the percentage of FITC positive cells. Final phagocytic index numbers were calculated by subtracting phagocytic index of 4° C samples.
Induction of pneumonia and sampling of organs
Pneumonia was induced by intranasal inoculation with ~900 or ~9 000 CFUs) of K. pneumoniae serotype 2 (ATCC 43816; American Type Culture Collection, Manassas, VA). Mice were euthanized after 6 or 24 h of infection and organs were harvested and processed exactly as described [14–16]. Survival was monitored in separate experiments.
Histopathology
Histologic examination of lungs was performed as described [14,15]. In brief, lungs were fixed in 10% buffered formalin, and embedded in paraffin. Sections of 4 µm thickness were stained with H&E and analysed by a pathologist unaware of the groups as described earlier. To score lung inflammation and damage, the entire lung surface was analysed with respect to the following parameters: bronchitis, oedema, interstitial inflammation, intra-alveolar inflammation, pleuritis, endothelialitis and percentage of the lung surface demonstrating confluent inflammatory infiltrate. Each parameter was graded 0–4, with 0 being ‘absent’ and 4 being ‘severe’
Assays
TNF-α, IL-1β, IL-6, CXCL1, CXCL2, CXCL5, CCL2, CCL20, Lipocalin-2, G-CSF and GM-CSF were measured by ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis
Data are expressed as box-and-whisker diagrams depicting the smallest observation, lower quartile, median, upper quartile, and largest observation unless indicated otherwise. Differences between control and LysMcre-Hsp90b1-flox mice were analysed by Mann Whitney U test. Serial data were tested by Kruskal-Wallis test, followed by Mann-Whitney tests. Survival curves are depicted as Kaplan-Meier plots and compared using log-rank test. These analyses were done using GraphPad Prism (San Diego, CA). P < 0.05 was considered statistically significant.
RESULTS
Characterization of LysMcre-Hsp90b1-flox mice
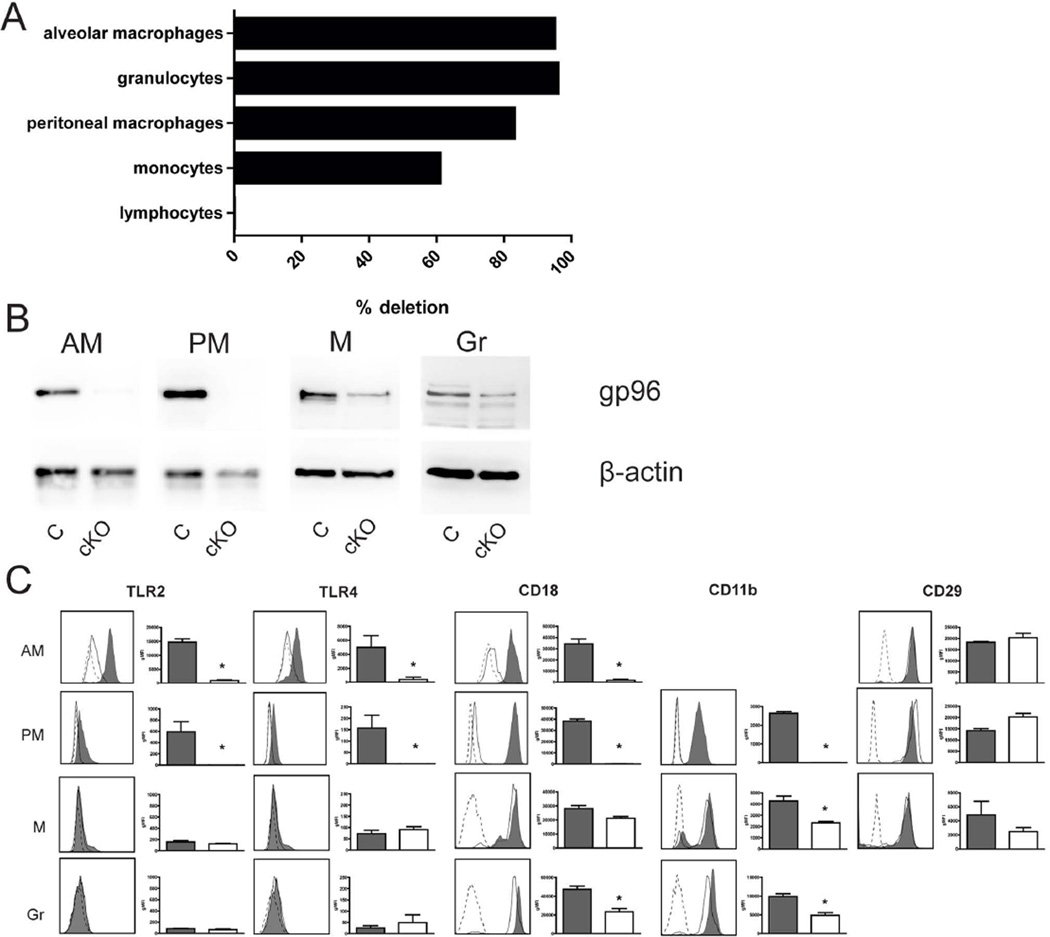
Cre-mediated Hsp90b1 deletion in LysMcre-Hsp90b1-flox mice was highly efficient in alveolar macrophages, granulocytes and peritoneal macrophages; monocytes showed partial deletion, while Hsp90b1 expression was unaffected in lymphocytes (Figure 1A). In accordance, gp96 protein was not detectable in alveolar or peritoneal macrophages derived from LysMcre-Hsp90b1-flox mice, while partial depletion of gp96 was found in monocytes (Figure 1B). Despite highly efficient Cre-mediated deletion of Hsp90b1 in granulocytes, only partial depletion of gp96 protein was observed in these cells, which is in accordance with the original characterization of LysMcre-Hsp90b1-flox mice, and has been attributed to the short life span of granulocytes in combination with a relatively long half-life of gp96 [5].
Figure 1. Characterization of LysMcre-Hsp90b1-flox mice.
DNA of primary cells derived from control and LysMcre-Hsp90b1-flox mice (n = 4 per group) was isolated and LysMcre-mediated deletion efficiency of Hsp90b1 was calculated by using control mice as reference (A). Expression of the gp96 protein isolated from control and LysMcre-Hsp90b1-flox mice (n = 4 per group, pooled) was examined by western blot, using β-actin as a loading control (B). Surface expression of TLRs and integrin family members on alveolar macrophages (CD45+/CD64+/ SiglecF+ cells), peritoneal macrophages (F4/80+ cells), monocytes (Gr1dim/ CD115+cells) and granulocytes (Gr1+/ CD115− cells) was assessed by flow cytometry (n=4 or 5 per group) (C). For each examined TLR or integrin, a representative graph (left) and geomean fluorescence intensity (gMFI) per group (right) is shown. Open histograms with dotted lines, shaded histograms/bars and open histograms/white bars represent isotype controls, control cells and LysMcre-Hsp90b1-flox cells respectively. AM (alveolar macrophages), PM (peritoneal macrophages), M (monocytes), Gr (granulocytes), C (control mice), cKO (conditional knockout mice, LysMcre-Hsp90b1-flox mice). Data in bars are expressed as mean (±SE) and compared using the Mann-Whitney test. * p< 0.05.
Since gp96 is a master chaperone for TLRs [4,5], we analysed LysMcre-Hsp90b1-flox macrophages, monocytes and granulocytes for the expression of TLR2 and TLR4 (previously found important for host defence against Klebsiella [14]) by flow cytometry (Figure 1C). LysMcre-Hsp90b1-flox alveolar and peritoneal macrophages displayed a virtually complete absence of TLR2 and TLR4 expression. TLR expression was very low on blood monocytes and granulocytes of both mouse strains. Cell-surface expression of many integrin subunits is dependent on gp96, including the α subunit CD11b and the β subunit CD18 [7]. In accordance, LysMcre-Hsp90b1-flox macrophages displayed very low surface expression of CD11b and CD18, while expression of the gp96 independent integrin subunit CD29 [7] was unaffected (Figure 1C). LysMcre-Hsp90b1-flox monocytes and granulocytes showed modestly reduced expression of gp96 dependent integrin subunits.
Together, these data indicate that LysMcre-Hsp90b1-flox mice display a virtually complete functional deficiency of gp96 in macrophages with a modestly reduced gp96 function in monocytes and granulocytes.
LysMcre-Hsp90b1-flox macrophages are hyporesponsive to K. pneumoniae
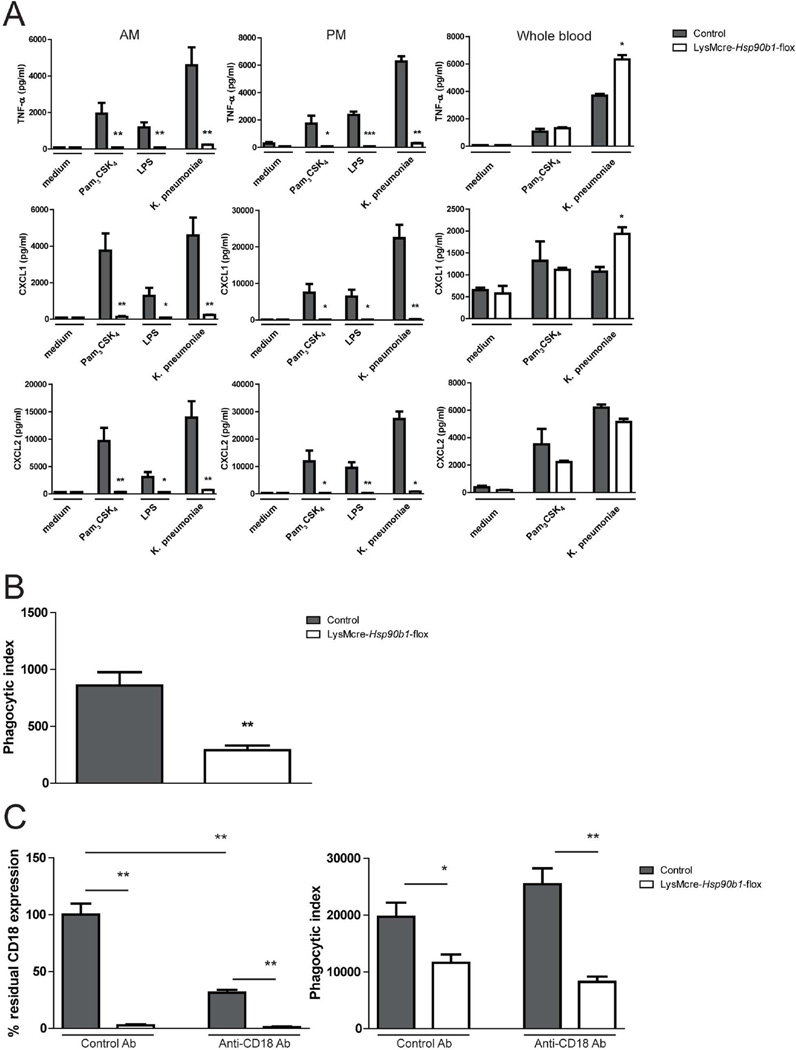
To investigate the role of gp96 in cellular responsiveness, we stimulated alveolar and peritoneal macrophages with heat killed Klebsiella, the TLR4 agonist LPS or the TLR2 agonist Pam3CSK4 (Figure 2A). Both alveolar and peritoneal macrophages isolated from LysMcre-Hsp90b1-flox mice failed to release TNF-α, CXCL1 and CXCL2 upon incubation with either one of these stimuli, while control macrophages produced high amounts of these proinflammatory mediators. Alveolar macrophages deficient in gp96 also showed a reduced capacity to phagocytose Klebsiella (Figure 2B). Because CD18 integrin complexes have been described to be important in phagocytosis [17,18], control and LysMCre-Hsp90b1-flox peritoneal macrophages were incubated with control or anti-CD18 antibody prior to phagocytosis. Although pretreatment with anti-CD18 antibody significantly reduced expression of this integrin on control macrophages, this did not lead to impaired phagocytosis (Figure 2C). These results demonstrate that LysMCre-Hsp90b1-flox macrophages are impaired in their ability to produce inflammatory mediators and to phagocytose Klebsiella, whereby the latter effect is independent of reduced CD18 expression.
Figure 2. LysMcre-Hsp90b1-flox macrophages are hypo-responsive to K. pneumoniae.
Alveolar macrophages (30 000 cells/well) and peritoneal macrophages (50 000 cells/well) from control and LysMcre-Hsp90b1-flox mice were stimulated with Pam3CSK4 (1 µg/ml), LPS derived from K. pneumoniae (100 ng/ml) or heat killed K. pneumoniae (2e7 CFU/ml) for 20 h after which levels of TNF-α, CXCL1 and CXCL2 in supernatant were determined (A). To assess phagocytic ability, alveolar macrophages (100 000 cells/well) were incubated with FITC labeled heat killed K. pneumoniae (in a bacterium:cell ratio of 100:1) (B). To determine the role of CD18 during phagocytosis, peritoneal macrophages were pretreated with 10ug/ml control or anti-CD18 antibody 30 min before incubating with FITC labelled heat killed K. pneumoniae (C). After pretreatment, residual CD18 expression was determined by flow cytometry (C, left). Phagocytic index was calculated by multiplying the gMFI of FITC positive cells with the percentage FITC positive cells, and subtracting the phagocytic index of 4°C control samples (B and C, right). Data shown represent means and variation amongst cells from four different mice stimulated separately. Comparison using the Mann-Whitney test. * p< 0.05, ** p< 0.01.
LysMcre-Hsp90b1-flox mice have a strongly impaired host defense against K. pneumoniae in vivo
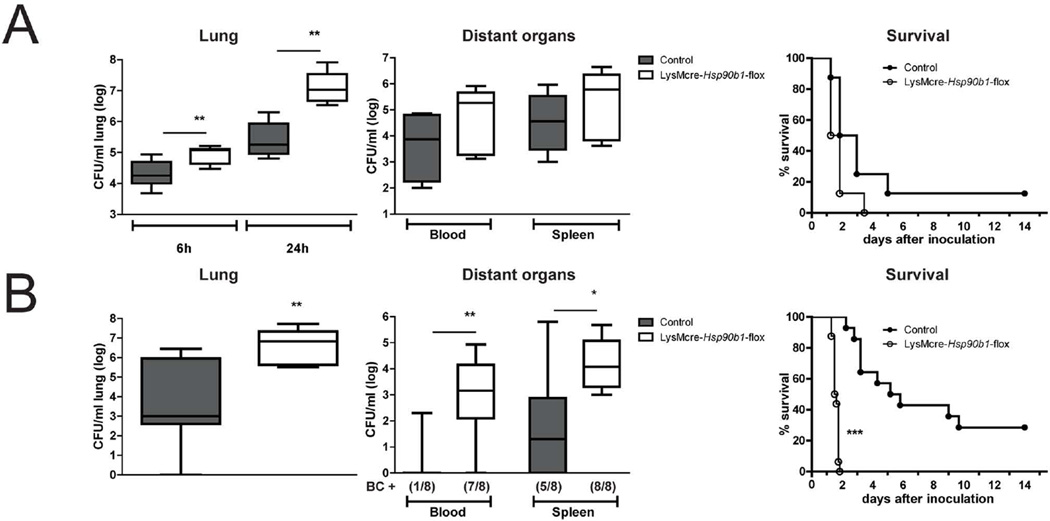
LysMcre-Hsp90b1-flox and control mice were infected intranasally with ~9 000 CFUs of K. pneumoniae. LysMcre-Hsp90b1-flox mice had higher bacterial loads in their lungs compared to control mice, a difference that already was present at 6 h and increased to almost 100-fold at 24 h (P < 0.01, Figure 3A). Cultures of distant organs harvested at 6 hours did not show bacterial growth; remarkably, at 24 h post infection bacterial loads in blood and spleen were not different between mouse strains. Using the same bacterial dose, we performed an observational study to determine the impact of gp96 deficiency on survival (Figure 3A). Although LysMcre-Hsp90b1-flox mice all died shortly after 24 h of infection, the infection was also rapidly fatal in most control mice, which led us to believe that the infectious challenge might have been too high to reveal a detrimental effect of gp96 deficiency on survival. Thus, we repeated the observational study using a 10-fold lower bacterial inoculum (~900 CFUs, Figure 3B). Whereas all control mice were still alive after 48 h, all LysMcre-Hsp90b1-flox mice died during the first two days (P < 0.0001). In accordance, in a separate experiment using this lower bacterial dose LysMcre-Hsp90b1-flox mice had much higher bacterial burdens in their lungs at 24 h after infection than control mice (P < 0.01, Figure 3B); at this time point most LysMcre-Hsp90b1-flox mice had positive blood (7 of 8) and spleen (8 of 8) cultures, while Klebsiella could be cultured from blood of 1 of 8, and from spleen of 5 of 8 of control mice (P < 0.01 and P < 0.05 respectively). Together these data suggest that macrophage deletion of gp96 results in a strongly impaired defence during Klebsiella pneumonia.
Figure 3. Impaired bacterial defense and survival in LysMcre-Hsp90b1-flox mice during Klebsiella pneumonia.
Control and LysMcre-Hsp90b1-flox mice were infected intranasally with ~9 000 CFUs (A) or ~900 CFUs (B) of K. pneumoniae. In mice infected with high dose Klebsiella (7–8 mice per group), bacterial loads in lung, blood and spleen were determined 6 and 24 h after infection. Survival of control (white symbols) and LysMcre-Hsp90b1-flox (black symbols) mice (8 mice per group) is shown in Kaplan-Meier curves (A). In mice infected with low dose Klebsiella, bacterial loads in lung, blood and spleen were determined 24 h after infection. Survival of control (white symbols) and LysMcre-Hsp90b1-flox (black symbols) (14–16 mice per group) is shown in Kaplan-Meier curves (B). BC+ = number of positive blood cultures. Bacterial loads are expressed as box-and-whisker diagrams depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. Survival curves were compared using the Log-Rank test. Bacterial load group mice were compared to control mice using the Kruskal-Wallis and Mann-Whitney tests. * p < 0.05, ** p < 0.01, *** p < 0.001.
LysMcre-Hsp90b1-flox mice demonstrate an attenuated early inflammatory response in the lung after infection with Klebsiella
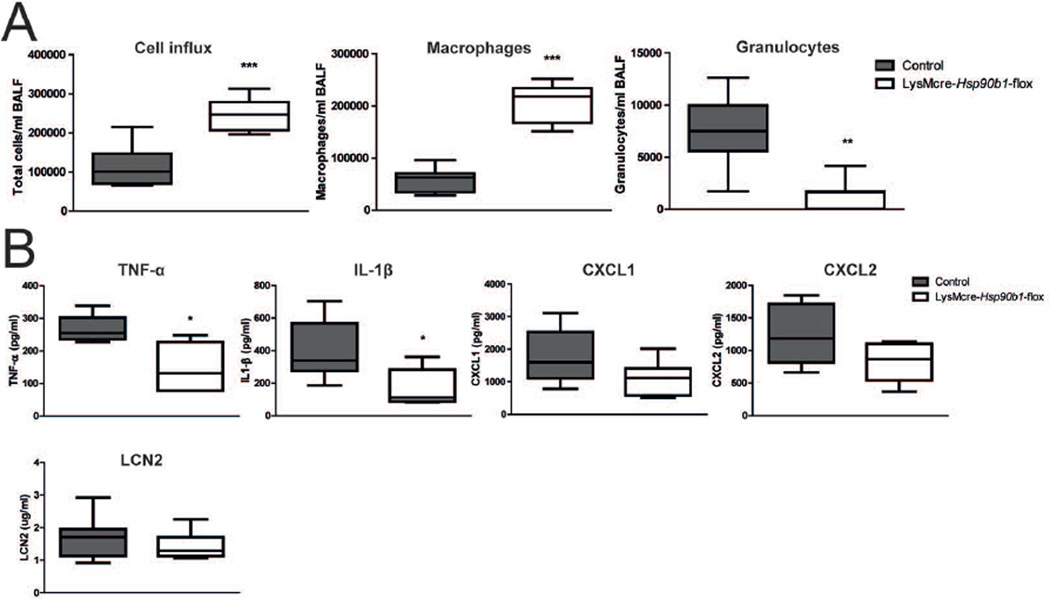
To obtain insight in the mechanism by which LysMcre-Hsp90b1-flox mice are compromised in their defence against Klebsiella in the lungs, we examined the early inflammatory response in the airways. At 6 hours after infection LysMcre-Hsp90b1-flox mice had higher cell numbers in their bronchoalveolar lavage (BAL) fluid than control mice (P < 0.001), which was caused by higher macrophage numbers (P < 0.001, Figure 4A). Differences in macrophage numbers after infection were not caused by enhanced influx since uninfected LysMcre-Hsp90b1-flox mice also had more macrophages in their BAL fluid relative to control mice (supplementary Figure 1A); considering that macrophage numbers in lung tissue, as determined by flow cytometry, were not different between mouse strains (Supplementary Figure 1B), this result likely can be explained by a better ‘lavage-ability’ of gp96 deficient macrophages due to reduced integrin expression. Importantly, however, granulocyte recruitment upon infection with Klebsiella was strongly impaired in LysMcre-Hsp90b1-flox mice (P < 0.01 versus control mice, Figure 4A). We next measured cytokines and chemokines implicated in granulocyte recruitment in lungs early after infection with Klebsiella (Figure 4B). LysMcre-Hsp90b1-flox mice demonstrated lower tumor necrosis factor (TNF)-α and interleukin (IL)-1β levels, while CXCL1 and CXCL2 levels were not significantly different between groups. Considering the important role for lipocalin-2 in host defence during Klebsiella pneumonia [19], we also measured the pulmonary levels of this mediator, and found no difference between mouse strains (Figure 4B). These results suggest that LysMcre-Hsp90b1-flox mice show a defective granulocyte influx after infection with Klebsiella via the airways at least in part due to a diminished release of macrophage derived mediators.
Figure 4. Early inflammatory response in lungs of LysMcre-Hsp90b1-flox mice is attenuated after infection with Klebsiella.
Total number of cells, macrophages and granulocytes were determined in bronchoalveolar lavage fluid (BALF) of control and LysMcre-Hsp90b1-flox mice (8 mice per group) 6 h after infection with ~9000 CFUs of K. pneumoniae (A). Cytokine and chemokine levels were measured in lung homogenates of these mice (B). Data are expressed as box-and-whisker diagrams depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. Comparisons using the Mann-Whitney test. * p < 0.05, ** p < 0.01, *** p < 0.001.
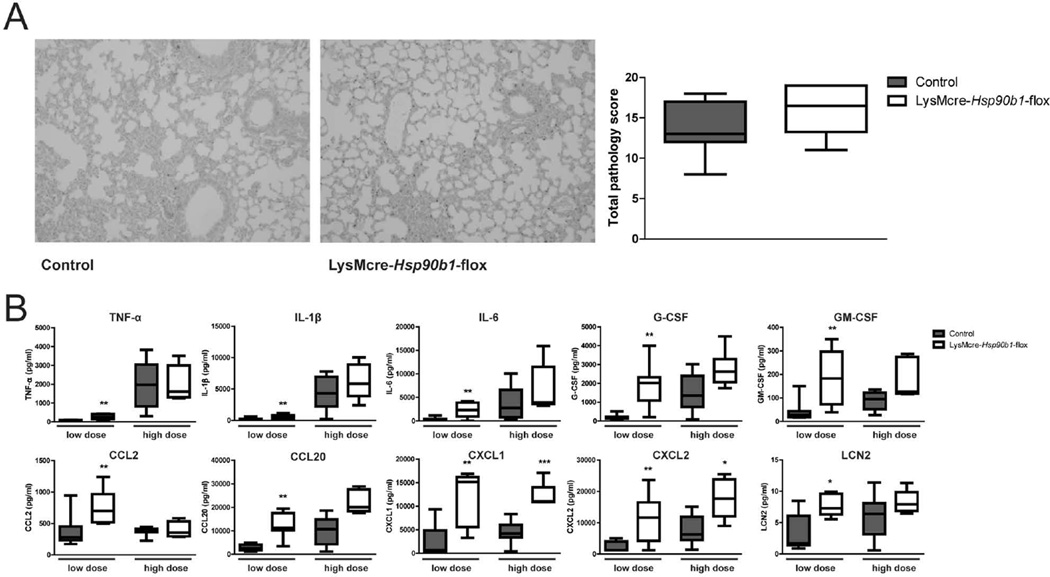
Myeloid gp96 is not required for the inflammatory response during late stage pneumonia
We next investigated whether macrophage gp96 is required for lung inflammation during late stage pneumonia. At 24 h after infection with ~9 000 Klebsiella CFU, both lung tissue slides from LysMcre-Hsp90b1-flox and control mice displayed signs of severe pneumonia. The extent of lung pathology did not differ between groups (Figure 5A). To obtain further insight in the role of myeloid gp96 in lung inflammation during severe pneumonia, we measured lung levels of several inflammatory mediators (Figure 5B). LysMcre-Hsp90b1-flox mice overall had higher concentrations of these mediators in their lungs, significantly so for CXCL1 and CXCL2. We also measured the levels of inflammatory proteins in lungs harvested from LysMcre-Hsp90b1-flox and control mice 24 h after infection with ~900 Klebsiella CFU (Figure 5B). Similar to the results obtained after infection with the higher dose, lung levels of inflammatory mediators were higher in LysMcre-Hsp90b1-flox mice, significantly so for IL-6, G-CSF, GM-CSF, CCL2, CCL20, CXCL1, CXCL2 and lipocalin-2 (LCN2). Together these data indicate that in spite of the strongly diminished responsiveness of gp96 deficient macrophages, LysMcre-Hsp90b1-flox mice can mount a profound inflammatory response in their lungs during late stage pneumonia. These results suggest that in the presence of high bacterial loads (and thus a potent proinflammatory stimulus) gp96 is not essential anymore for abundant cytokine and chemokine production in the lung.
Figure 5. Myeloid gp96 is not required for the inflammatory response during late stage pneumonia.
Representative lung histology slides of control and LysMcre-Hsp90b1-flox mice and total pathology scores determined 24 h after infection as described in Methods section (A). Inflammatory mediators were measured in lung homogenates 24 h after infection with ~900 (“low dose”) or 9 000 (high dose) CFUs of K. pneumoniae (B). Data are expressed as box-and-whisker diagrams depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. Comparisons using the Mann-Whitney test. * p < 0.05, ** p < 0.01, *** p < 0.001.
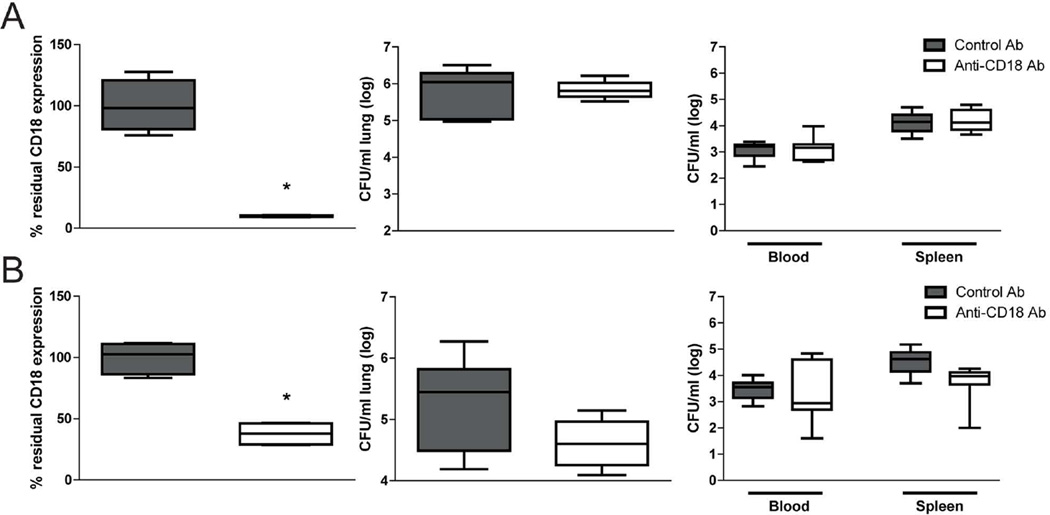
Anti-CD18 treatment does not affect host defence against K. pneumoniae
Gp96 deletion resulted in an almost complete disappearance of CD18 expression on macrophages, with a more modest effect on granulocyte CD18 (Figure 1). To obtain insight into the possible role of reduced CD18 expression in the impaired host defence against K. pneumoniae in LysMcre-Hsp90b1-flox mice, wild-type mice were treated with a CD18 antibody of which the capacity to block CD18 function was established previously [20,21] via the airways (targeting alveolar macrophages) or intravenously (targeting circulating granulocytes). Mice were euthanized 24 h after infection with ~9 000 Klebsiella CFU, i.e., at the time point at which LysMcre-Hsp90b1-flox mice showed much higher bacterial loads than control mice (Figure 3). We established the efficacy of anti-CD18 blockade by flow cytometry using an anti-CD18 antibody different from the one used in vivo, but binding the same site. Alveolar macrophages harvested from mice treated with anti-CD18 via the airways had 10% residual CD18 expression relative to mice administered with a control antibody (Figure 6A). Blood granulocytes from mice treated with anti-CD18 intravenously had 38% residual CD18 expression (Figure 6B). Hence, these two routes of anti-CD18 administration reproduced the extent of CD18 deficiency in LysMcre-Hsp90b1-flox mice. However, neither route of anti-CD18 treatment influenced bacterial loads in lungs or distant organs (Figure 6A,B). Taken together, these results do help argue against a role for gp96 mediated CD18 expression in host defence during Klebsiella pneumonia, although they do not exclude a role for low CD18 expression in the responses studied. (AQ: Please check that your original meaning is retained)
Figure 6. Pretreatment with anti-CD18 antibody does not impair bacterial defense.
C57BL/6 mice (n=8 per group) were treated intranasally (A) or intravenously (B) with 50 µg/mouse or 30 µg/mouse respectively of control or anti-CD18 antibody 30 min prior to intranasal infection with ~9000 CFUs of K. pneumoniae. To determine success of the pretreatment, residual CD18 expression was assessed on alveolar macrophages (A) and granulocytes (B). Bacterial loads in lung, blood and spleen were determined 24 h after infection. Data are expressed as box-and-whisker diagrams depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. Comparisons using the Mann-Whitney test. * p < 0.05.
DISCUSSION
Gp96 is one of the most abundant glycoproteins in the ER and serves as a chaperone supervising the correct folding and modification of proteins, a process known as “ER quality control” [22]. TLRs and integrins are important client proteins of gp96, as reflected by their strongly reduced surface expression on gp96 deficient cells [4–7]. Here. We have generated myeloid specific gp96 deficient (LysMcre-Hsp90b1-flox) mice, which upon cell-specific analysis of gp96 protein levels and surface expression of gp96 dependent TLRs and integrins appeared to lack gp96 predominantly in macrophages. We then used an established model of lower respiratory tract infection caused by the common human pathogen K. pneumoniae [14,15,23] to show that LysMcre-Hsp90b1-flox mice have a strongly impaired host defence during severe pneumonia, as reflected by an enhanced bacterial growth accompanied by a reduced survival. Our results further reveal that this enhanced susceptibility most likely is caused by a reduced TLR dependent responsiveness of alveolar macrophages resulting in a diminished cytokine-mediated recruitment of neutrophils and diminished phagocytosis.
Macrophages harvested from LysMcre-Hsp90b1-flox mice were virtually unresponsive to Klebsiella, as reflected by an almost complete absence of TNF-α, CXCL1 and CXCL2 release, which corresponded with a strongly reduced surface expression of TLR2 and TLR4, receptors involved in cellular recognition of this bacterium [14]. These data are in agreement with earlier investigations that reported attenuated cytokine production by gp96 deficient macrophages [5] and B cells [4] upon exposure to purified TLR ligands. Previous studies examined the role of TLRs and the common TLR adaptor myeloid differentiation primary response gene (MyD)88 expressed by different cell types in protective immunity during infection with K. pneumoniae. TLR2 and TLR4 expressed by hematopoietic cells were found important for host defence against Klebsiella pneumonia [14], while the reduced resistance of TLR9 deficient (Tlr9−/−) mice could be restored by adoptive transfer of wild type bone-marrow derived dendritic cells [24]. Using the Cre-lox recombination system, we found that myeloid but not endothelial cell expression of MyD88 is essential for host defence after infection with Klebsiella [23]. While these studies highlight the importance of myeloid MyD88 during Klebsiella pneumonia, several questions remained unanswered. First, our earlier investigations involved MyD88 elimination in all myeloid cells, comprising both monocytes/macrophages and granulocytes [23]. Second, MyD88 not only serves as a TLR adaptor protein (mediating signalling of all TLRs except TLR3) but also for the IL-1R1 signalling pathway [25]. Since gp96 is indirectly responsible for correct functioning of TLR mediated responses but does not involve IL-1R1 signalling [4,5], we attempted to address these unanswered questions by making use of LysMcre-Hsp90b1-flox mice, in which according to our characterization gp96 protein is not detectable in alveolar and peritoneal macrophages, while partial expression is found in monocytes and granulocytes. The almost complete absence of TLR2 and TLR4 expression on macrophages confirmed the role of gp96 as a master chaperone for these receptors [5]. CD18 and CD11b expression were almost absent in LysMcre-Hsp90b1-flox macrophages while largely preserved in LysMcre-Hsp90b1-flox monocytes and granulocytes. Hence, (functional) gp96 deficiency is principally restricted to macrophages in LysMcre-Hsp90b1-flox mice. By administration of a blocking anti-CD18 antibody via the airways or intravenously we showed that reduction of available CD18 to an extent similar to that detected in LysMcre-Hsp90b1-flox mice could not reproduce the increased bacterial growth observed in these conditional knockout animals, suggesting that the effect of gp96 deficiency on CD18 dependent integrins likely does not play a significant role in host defence during Klebsiella pneumonia. Taken together, these data support that deficient TLR signalling by macrophages drives the hyper-susceptible phenotype of LysMcre-Hsp90b1-flox mice. These results therefore add important knowledge to our earlier findings with LysMcre-Myd88-flox mice, which suffered from a global myeloid cell deficiency of both TLR and IL-1R1 signalling [23].
Macrophage gp96 deficiency had a more severe impact on host defence during infection with the lower (~900 CFU) Klebsiella dose. Indeed, after instillation of the higher (~9000 CFU) inoculum bacterial growth was accelerated at the primary site of infection in LysMcre-Hsp90b1-flox mice, but the spread to distant organs was unaltered when compared with control mice. In contrast, after administration of the lower Klebsiella dose, the infection disseminated much easier in LysMcre-Hsp90b1-flox mice, indicating that up to a certain bacterial threshold macrophage TLR signalling is essential for containment of the infection to the lungs. Our results suggest two distinct mechanisms contributing to the impaired defence against Klebsiella in the airways of LysMcre-Hsp90b1-flox mice: a reduced capacity of alveolar macrophages to respond to this bacterium by releasing pro-inflammatory cytokines, and a diminished ability of these cells to phagocytose Klebsiella. The reduced lung levels of TNF-α and IL-1β in LysMcre-Hsp90b1-flox mice may jeopardize an adequate innate immune response by providing a less potent chemotactic gradient for neutrophil influx to the site of infection [26–29], an important defensive mechanism in pneumonia [30]. In particular, adequate TNF-α release early after invasion of Klebsiella of the lower airways has been shown of utmost importance for protective immunity [31,32]. In contrast to the TNF-α and IL-1β levels, LCN2, an anti-microbial peptide known to be TLR4 dependent and expressed by mouse tracheal epithelial cells [19,33], was not diminished in LysMcre-Hsp90b1-flox mice compared to control mice. This confirms previous findings that LCN2 is mainly produced by epithelial cells and not macrophages [19].
Besides TLRs, another important group of client proteins for gp96 include integrin subunits [4–7]. There are 26 integrin subunits that can form 24 different integrin pairs, of which 14 have been shown to be gp96 dependent [7,34]. The β2 subunit (CD18) combines with four different integrin subunits to form integrins that are expressed by monocytes/macrophages (CD11a/CD18, CD11b/CD18, CD11c/CD18 and CD11d/CD18) and granulocytes (CD11a/CD18, CD11b/CD18 and CD11d/CD18), all of which are gp96 dependent [7,17,34]. As expected, almost no CD18 and CD11b expression could be detected on alveolar and peritoneal macrophages of LysMcre-Hsp90b1-flox mice, while substantial expression of these integrin molecules remained detectable on monocytes and granulocytes, reflecting the extent of gp96 deletion in these cells. Our experiments using a blocking anti-CD18 argue against a role for CD18 dependent integrins in host defence during Klebsiella pneumonia. In addition, while the integrins CD11b/CD18 (complement receptor 3, CR3) and CD11c/CD18 (p150, 95) have been implicated in phagocytosis, the reduced phagocytic ability displayed by LysMcre-Hsp90b1-flox mice when exposed to Klebsiella could not be explained due to diminished CD18 expression, considering that a blocking anti-CD18 antibody did not impact on phagocytosis. Of note, TLRs are known to be involved in the phagocytosis of microbial pathogens [35–37], suggesting that the observed impaired phagocytosis in gp96 deficient macrophages is the result of inadequate TLR signalling rather than reduced expression of CD18 and CD18 related integrin subunits.
Our study is limited in that it does not provide information on the regulation of gp96 expression during pneumonia. In the setting of rheumatoid arthritis, expression of gp96 was found to be increased in synovial tissue and to correlate with disease activity [38].
In conclusion, our data indicate that macrophage gp96 is crucial for protective immunity during lower airway infection caused by K. pneumoniae. In preliminary experiments we found no evidence for such a protective role of gp96 in the respiratory epithelium (using crossings of Hsp90b1-flox mice with mice expressing Cre recombinase controlled by the Clara-cell specific CC10 promoter). Together these data place macrophage TLR signalling in the centre of an adequate host defence in the airways after infection with a clinically relevant human pathogen.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Regina de Beer, Sanne Terpstra, Saskia Bots, Marieke ten Brink and Joost Daalhuisen (Center of Experimental and Molecular Medicine), Onno de Boer (Department of pathology) and Berend Hooijbrink, (Flow Cytometry Facility, Department of Cell Biology) from the Academic Medical Center, Amsterdam the Netherlands for expert technical assistance.
FUNDING
Adam A. Anas and Miriam H.P. van Lieshout were supported by a grant from the Academic Medical Center Graduate School of Medical Sciences (http://www.amc.nl/web/Onderwijs/PhD/Graduate-School.htm). Zihai Li was supported by NIH grants (AI070603 and AI077283). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
STATEMENT OF AUTHOR CONTRIBUTIONS
AAA conceived and carried out experiments. AAA, AFdV, CvtV and TvdPoll conceived and analysed data. AJH, MHPvL, JWJvH, SF and ZL analysed data. All authors were involved in writing the paper and had final approval of the submitted and published versions.
- Methods: additional information describing methods used for Western Blot and Flow Cytometry.
- Results: Supplementary figure 1
REFERENCES
- 1.Mizgerd JP. Respiratory Infection and the Impact of Pulmonary Immunity on Lung Health and Disease. Am J Respir Crit Care Med. 2012;186:824–829. doi: 10.1164/rccm.201206-1063PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podschun R, Ullmann U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clinical Microbiology Reviews. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care–associated pneumonia*: Results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 4.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Liu B, Dai J, et al. Heat Shock Protein gp96 Is a Master Chaperone for Toll-like Receptors and Is Important in the Innate Function of Macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Li Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. ed) 2008 doi: 10.1182/blood-2008-03-143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staron M, Yang Y, Liu B, et al. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood. 2010;115:2380–2390. doi: 10.1182/blood-2009-07-233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai T, Akira S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Moresco EMY, LaVine D, Beutler B. Toll-like receptors. Current Biology. 2011;21:R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 10.Hogg N, Patzak I, Willenbrock F. The insider's guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11:416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 11.Clausen BE, Burkhardt C, Reith W, et al. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 12.de Bruin AM, Libregts SF, Valkhof M, et al. IFNgamma induces monopoiesis and inhibits neutrophil development during inflammation. Blood. 2012;119:1543–1554. doi: 10.1182/blood-2011-07-367706. [DOI] [PubMed] [Google Scholar]
- 13.Misharin AV, Morales-Nebreda L, Mutlu GM, et al. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. American journal of respiratory cell and molecular biology. 2013;49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wieland CW, van Lieshout MHP, Hoogendijk AJ, et al. Host defence during Klebsiella pneumonia relies on haematopoietic-expressed Toll-like receptors 4 and 2. European Respiratory Journal. 2011;37:848–857. doi: 10.1183/09031936.00076510. [DOI] [PubMed] [Google Scholar]
- 15.van Lieshout MH, Blok DC, Wieland CW, et al. Differential roles of MyD88 and TRIF in hematopoietic and resident cells during murine gram-negative pneumonia. J Infect Dis. 2012;206:1415–1423. doi: 10.1093/infdis/jis505. [DOI] [PubMed] [Google Scholar]
- 16.Achouiti A, Vogl T, Urban CF, et al. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog. 2012;8:e1002987. doi: 10.1371/journal.ppat.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzone A, Ricevuti G. Leukocyte CD11/CD18 integrins: biological and clinical relevance. Haematologica. 1995;80:161–175. [PubMed] [Google Scholar]
- 18.Uotila LM, Aatonen M, Gahmberg CG. Integrin CD11c/CD18 α-Chain Phosphorylation Is Functionally Important. Journal of Biological Chemistry. 2013;288:33494–33499. doi: 10.1074/jbc.C113.497446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan YR, Liu JS, Pociask DA, et al. Lipocalin 2 Is Required for Pulmonary Host Defense against Klebsiella Infection. The Journal of Immunology. 2009;182:4947–4956. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Driessens MH, van Hulten P, Zuurbier A, et al. Inhibition and stimulation of LFA-1 and Mac-1 functions by antibodies against murine CD18. Evidence that the LFA-1 binding sites for ICAM-1, -2, and -3 are distinct. Journal of leukocyte biology. 1996;60:758–765. doi: 10.1002/jlb.60.6.758. [DOI] [PubMed] [Google Scholar]
- 21.Ridger VC, Wagner BE, Wallace WAH, et al. Differential Effects of CD18, CD29, and CD49 Integrin Subunit Inhibition on Neutrophil Migration in Pulmonary Inflammation. The Journal of Immunology. 2001;166:3484–3490. doi: 10.4049/jimmunol.166.5.3484. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Li Z. Roles of heat shock protein gp96 in the ER quality control: redundant or unique function? Molecules and cells. 2005;20:173–182. [PubMed] [Google Scholar]
- 23.van Lieshout MHP, Anas AA, Florquin S, et al. Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis. PLoS Pathog. 2014;10:e1004368. doi: 10.1371/journal.ppat.1004368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhan U, Lukacs NW, Osterholzer JJ, et al. TLR9 Is Required for Protective Innate Immunity in Gram-Negative Bacterial Pneumonia: Role of Dendritic Cells. The Journal of Immunology. 2007;179:3937–3946. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 25.Wesche H, Henzel WJ, Shillinglaw W, et al. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 26.Lukacs NW, Strieter RM, Chensue SW, et al. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. The Journal of Immunology. 1995;154:5411–5417. [PubMed] [Google Scholar]
- 27.Mizgerd JP. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Seminars in Immunology. 2002;14:123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 28.Jones MR, Simms BT, Lupa MM, et al. Lung NF-κB Activation and Neutrophil Recruitment Require IL-1 and TNF Receptor Signaling during Pneumococcal Pneumonia. The Journal of Immunology. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams MR, Azcutia V, Newton G, et al. Emerging mechanisms of neutrophil recruitment across endothelium. Trends in immunology. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinton LJ, Mizgerd JP. Dynamics of Lung Defense in Pneumonia: Resistance, Resilience, and Remodeling. Annual Review of Physiology. 2015;77:407–430. doi: 10.1146/annurev-physiol-021014-071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laichalk LL, Kunkel SL, Strieter RM, et al. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infection and Immunity. 1996;64:5211–5218. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laichalk LL, Bucknell KA, Huffnagle GB, et al. Intrapulmonary Delivery of Tumor Necrosis Factor Agonist Peptide Augments Host Defense in Murine Gram-Negative Bacterial Pneumonia. Infection and Immunity. 1998;66:2822–2826. doi: 10.1128/iai.66.6.2822-2826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo B-H, Carman CV, Springer TA. Structural Basis of Integrin Regulation and Signaling. Annual review of immunology. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blander JM, Medzhitov R. Regulation of Phagosome Maturation by Signals from Toll-Like Receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 36.Doyle SE, O'Connell RM, Miranda GA, et al. Toll-like Receptors Induce a Phagocytic Gene Program through p38. The Journal of experimental medicine. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong L, Sun L, Zhang H, et al. An Essential Role for RIG-I in Toll-like Receptor-Stimulated Phagocytosis. Cell Host & Microbe. 2009;6:150–161. doi: 10.1016/j.chom.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Huang Q-Q, Sobkoviak R, Jockheck-Clark AR, et al. Heat Shock Protein 96 Is Elevated in Rheumatoid Arthritis and Activates Macrophages Primarily via TLR2 Signaling. The Journal of Immunology. 2009;182:4965–4973. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.