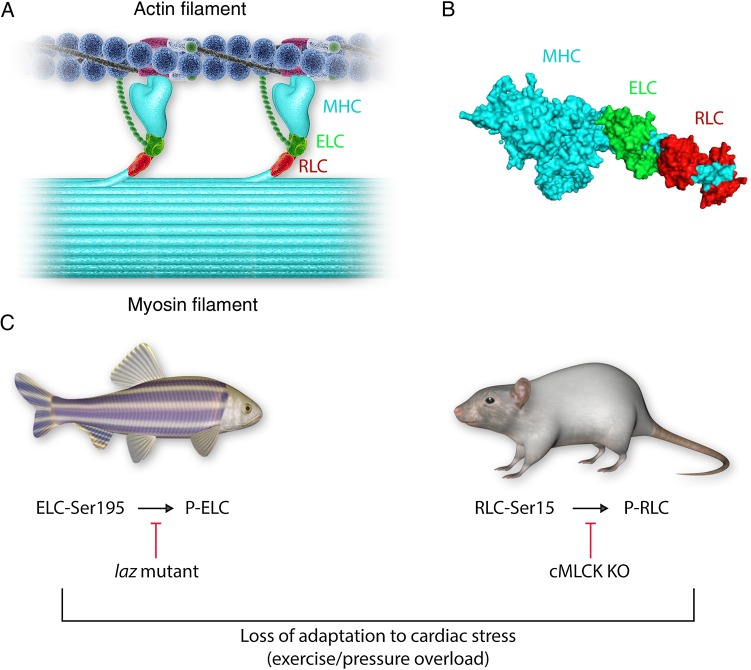
This editorial refers to ‘Essential light chain S195 phosphorylation is required for cardiac adaptation under physical stress’, by L.-M. Scheid et al., pp. 44–55.
The process of ATP-dependent cyclic attachments and detachments of the myosin cross-bridges to actin-containing thin filaments forms the basis of muscle contraction.1 As such, the myosin cross-bridge is the molecular motor of the heart. It binds ATP and actin and myosin's lever arm region, supported by the regulatory light chain (RLC) and essential light chain (ELC) (Figure 1A and B), amplifies small conformational changes generated in the motor domain into the large movements needed to produce force and sarcomere shortening.2 As an EF-hand Ca2+-binding protein, the RLC contains a Ca2+–Mg2+ binding site that can be occupied by either Ca2+ or Mg2+, and a Ser15 phosphorylation site, a target for cardiac myosin light chain kinase (cMLCK) encoded by the MYLK3 gene. The ELC is also an EF-hand-like protein; however, cardiac muscle ELC has developmentally lost its ability to bind calcium. Proteomic analysis by the Van Eyk's group revealed that residue Ser195 of ELC can be phosphorylated in pharmacologically preconditioned cardiomyocytes, although no specific kinase responsible for Ser195-ELC phosphorylation has yet been identified.3 Two new articles, published in this issue of Cardiovascular Research by Massengill et al.4 and Scheid et al.,5 focus on the functional impacts of myosin RLC and ELC phosphorylation on heart function in a mouse model of heart failure designed to knockout the Mylk3 gene4 or in a zebrafish model, where inability of ELC to be phosphorylated at Ser195 in the laz+/− mutant led to contractile defects and cardiac death5 (Figure 1C). These two articles, with their elegantly executed experiments, pose an important question regarding the role of either myosin light chain phosphorylation as a mechanism for the regulation of cardiac myosin function in the healthy heart and, moreover, may well point the way to potential novel therapeutic targets to treat heart diseases.
Figure 1.
Cardiac myosin light chains in health and disease. The cardiac sarcomere contains myosin located in the thick filament and actin located in the thin filament (A). Cardiac muscle contraction is controlled by access of the myosin heads to actin binding sites on the thin filament. This process starts with the binding of calcium to troponin (positioned on the thin filament), which causes a structural repositioning of the coiled-coil tropomyosin molecule within the grooves of the double-stranded actin filament, thereby exposing actin-binding sites on the thin filament for the myosin heads to bind and develop contractile force. Moreover, it has been suggested that the N-terminus of the ELC may interact with thin-filament actin and functionally modulate thin-filament regulation and muscle contraction (A). Each myosin head is composed of the myosin heavy chain (MHC; cyan), one regulatory light chain (RLC; red), and one essential light chain (ELC; green) (B). Both myosin light chains can be phosphorylated at ELC-Ser195 and RLC-Ser-15 (C), and this process has been postulated to play a role in the normal function of the heart. Diminished phosphorylation of either light chain may negatively impact cardiac function, particularly under conditions of haemodynamic stress (C). Modulation of the light chain phosphorylation (RLC and/or ELC) may provide a pathway for the development of novel treatment strategies to combat heart failure, an entity of ever increasing clinical significance.
Phosphorylation of Ser15 on myosin RLC has been widely recognized to play an important role in cardiac muscle contraction under both normal and disease conditions.6,7 Reduced RLC phosphorylation was reported in patients with heart failure8 and also observed in experimental animal models of cardiac disease.9–11 Attenuation of RLC phosphorylation in cardiac MLCK knockout mice was shown to lead to ventricular myocyte hypertrophy, fibrosis, and dilated cardiomyopathy.12 Changes in RLC phosphorylation were observed to cause abnormal heart performance, presumably through morphological and/or myofibrillar functional alterations (change in force, myofilament calcium sensitivity, ATPase activity, cross-bridge kinetics).13–16 The work of Massengill et al.4 convincingly shows that even acute (inducible) reduction of cMLCK, an enzyme that phosphorylates RLC in the heart, leads to sarcomeric disorganization, fibrosis, and cell death, and may cause severe systolic and diastolic dysfunction and rapid progression to heart failure. Previously, the Kasahara group had elegantly demonstrated that germline cMLCK-deficient (Mylk3−/−) mice exhibit cardiac hypertrophy, but only moderate heart failure that worsens following transaortic constriction (TAC)-induced pressure overload.16 In the current study, they generated tamoxifen-inducible adult Mylk3-knockout (KO) mice and observed a rapid onset of heart failure, primarily due to systolic dysfunction and severe myocardial morphological changes.4 In both studies, the reduction of cMLCK (chronic or acute) resulted in near complete absence of myosin RLC phosphorylation. No compensatory expression of mRNA of smooth and/or skeletal muscle MLCK or Dapk3/ZIPK was observed in these mice. Interestingly, when the hearts of inducible Mylk3-KO mice were subjected to TAC at Day 4 post-tamoxifen injection, myocytes did not increase in size, suggesting that the loss of cMLCK protein does not always trigger cardiac hypertrophy, even under conditions of haemodynamic stress. Examination of cardiomyocyte Ca2+ homeostasis, important for the proper contractile function of the heart, revealed a reduction of Ca2+-amplitude concomitant with a decreased rate of Ca2+ decay, suggesting that the changes in [Ca2+] homeostasis were most likely responsible for impaired contractility and accompanying heart failure that was observed in these animals. In support of this notion, the hearts of Mylk3-KO mice displayed a significant reduction in SERCA2a mRNA, implying that the concentration or activity of the intracellular calcium pump may be decreased thereby causing impaired transport of Ca2+ into the sarcoplasmic reticulum after contraction. The authors speculated that these observed changes in intracellular Ca2+ handling in Mylk3-KO mice may ultimately modulate the activity of Ca2+-dependent kinases and phosphatases, activating calcineurin-NFAT signalling pathways. In summary, the work by Massengill et al.4 convincingly show that rapid, progressive, and profound heart failure may occur shortly after elimination of cMLCK triggering a multitude of adverse intracellular defects initiated by compromised interactions between the myosin motor and actin that lead to impaired contractile force generation. Whether these changes are due solely to compromised myosin RLC phosphorylation or due to the absence of cMLCK per se remains to be determined.4 The question that arises next is whether normalizing RLC phosphorylation by a serine to aspartic acid (S15D) phosphomimetic mutation would offset the loss of cMLCK in heart muscle. Of note, cardiac expression of a S15D phosphomimetic variant of RLC in a hypertrophic cardiomyopathy (HCM) animal model was recently shown to prevent development of overt cardiac hypertrophy in mice whose diastolic and systolic function returned to normal in a constitutive phosphorylation mouse model of HCM.11
The question of a potential protective role of myosin essential light chain phosphorylation was addressed by Scheid et al.5 Using heterozygous adult zebrafish lazy Susan mutant (lazm647) of ELC, the authors show that Ser195-ELC phosphorylation plays a pivotal role in the adaptation of cardiac function to augmented physical stress.5 In their previous work, they showed that the homozygous lazm647 zebrafish, containing C-terminally truncated ELC, resulted in severe contractile insufficiency and early embryonic death.17 Interestingly, the lazm647 defect was rescued by the expression of a phosphomimetic S195D-ELC, but not by the expression of non-phosphorylatable S195A-ELC.17 In the current study, they employed adult laz+/− heterozygous animals and examined the response to physical stress using a multitude of functional assays.5 Echocardiography evaluation showed that even under basal conditions, adult laz+/− zebrafish display signs of systolic dysfunction. Moreover, when subjected to intense physical stress, cardiac function considerably deteriorated leading to heart failure and sudden death. Mechanistically, the authors suggest that Ser195-ELC phosphorylation is critical for the normal function of the heart and its absence negatively alters acto-myosin interactions and force development (Figure 1C). Importantly, these contractile defects are substantially exacerbated under physical stress. Analysis of the functional data clearly demonstrate that following physical stress, acto-myosin sliding velocity, myosin binding cooperativity, and force generation in adult laz+/− zebrafish ventricles are all severely altered resulting in heart dysfunction. At the molecular level, the authors suggest that ELC phosphorylation may modulate cross-bridge detachment rate, and that Ser195 phosphorylation stabilizes strong cross-bridge formation and subsequent force development. It would be interesting to test whether phosphomimetic S195D-ELC could rescue the phenotype as it was shown in their previous study with homozygous lazm647.17 Along the same lines, S195D pseudo-phosphorylation of RLC mutants linked to HCM was recently shown to rescue the abnormally high Ca2+-sensitivity of force and compromised ATPase activity in ELC-exchanged porcine cardiac muscle preparations.7
Taken together, using different experimental animal models of heart failure (Figure 1C), both studies highlighted here provide novel insights into complex genotype–phenotype relationships and present an interesting new concept of cardiac adaptation to physical and/or pharmacological stress at the level of myofilaments. Highpoints of their elegantly executed research regard the potential role of myosin regulatory and essential light chain phosphorylation as a molecular mechanism to attenuate or reverse pathological heart remodelling. Future studies are needed to test whether these unique phosphorylation sites on myosin ELC and RLC can serve as potential novel therapeutic targets to battle heart disease, an entity of ever increasing clinical significance.
Funding
This work was supported by NIH Grants R01 HL123255 and R01 HL108343 to D.S.-C., and P01 HL062426 to P.P.d.T.
References
- 1.Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem 2005;71:161–193. [DOI] [PubMed] [Google Scholar]
- 2.Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 1993;261:50–58. [DOI] [PubMed] [Google Scholar]
- 3.Arrell DK, Neverova I, Fraser H, Marban E, Van Eyk JE. Proteomic analysis of pharmacologically preconditioned cardiomyocytes reveals novel phosphorylation of myosin light chain 1. Circ Res 2001;89:480–487. [DOI] [PubMed] [Google Scholar]
- 4.Massengill MT, Ashraf HM, Chowdhury RR, Chrzanowski SM, Kar J, Warren SA et al. Acute heart failure with cardiomyocyte atrophy induced in adult mice by ablation of cardiac myosin light chain kinase. Cardiovasc Res 2016;111:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheid LM, Mosqueira M, Hein S, Kossack M, Juergensen L, Mueller M et al. Essential light chain S195 phosphorylation is required for cardiac adaptation under physical stress. Cardiovasc Res 2016;111:44–55. [DOI] [PubMed] [Google Scholar]
- 6.Kamm KE, Stull JT. Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem 2011;286:9941–9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang W, Szczesna-Cordary D. Molecular mechanisms of cardiomyopathy phenotypes associated with myosin light chain mutations. J Muscle Res Cell Motil 2015;36:433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ et al. Increased ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res 2003;57:37–47. [DOI] [PubMed] [Google Scholar]
- 9.Scruggs SB, Hinken AC, Thawornkaiwong A, Robbins J, Walker LA, de Tombe PP et al. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J Biol Chem 2009;284:5097–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheikh F, Ouyang K, Campbell SG, Lyon RC, Chuang J, Fitzsimons D et al. Mouse and computational models link mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. J Clin Invest 2012;122:1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan CC, Muthu P, Kazmierczak K, Liang J, Huang W, Irving TC et al. Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice. Proc Natl Acad Sci USA 2015;112:E4138–E4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding P, Huang J, Battiprolu PK, Hill JA, Kamm KE, Stull JT. Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo. J Biol Chem 2010;285:40819–40829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Shelton JM, Richardson JA, Kamm KE, Stull JT. Myosin regulatory light chain phosphorylation attenuates cardiac hypertrophy. J Biol Chem 2008;283:19748–19756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morano I. Tuning the human heart molecular motors by myosin light chains. J Mol Med 1999;77:544–555. [DOI] [PubMed] [Google Scholar]
- 15.Kerrick WGL, Kazmierczak K, Xu Y, Wang Y, Szczesna-Cordary D. Malignant familial hypertrophic cardiomyopathy d166v mutation in the ventricular myosin regulatory light chain causes profound effects in skinned and intact papillary muscle fibers from transgenic mice. FASEB J 2009;23:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren SA, Briggs LE, Zeng H, Chuang J, Chang EI, Terada R et al. Myosin light chain phosphorylation is critical for adaptation to cardiac stress. Circulation 2012;126:2575–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meder B, Laufer C, Hassel D, Just S, Marquart S, Vogel B et al. A single serine in the carboxyl terminus of cardiac essential myosin light chain-1 controls cardiomyocyte contractility in vivo. Circ Res 2009;104:650–659. [DOI] [PubMed] [Google Scholar]