Abstract
Thrombin is a highly plastic molecule whose activity and specificity are regulated by exosites 1 and 2, positively-charged domains that flank the active site. Exosite binding by substrates and cofactors regulates thrombin activity by localizing thrombin, guiding substrates, and by inducing allosteric changes at the active site. Although inter-exosite and exosite-to-active-site allostery have been demonstrated, the impact of active site ligation on exosite function has not been examined. To address this gap, we used surface plasmon resonance to determine the effects of dabigatran and argatroban, active site-directed inhibitors, on thrombin binding to immobilized γA/γA-fibrin or glycoprotein Ibα peptide via exosite 1 and 2, respectively, and thrombin binding to γA/γ′-fibrin or factor Va, which is mediated by both exosites. Whereas dabigatran attenuated binding, argatroban increased thrombin binding to γA/γA- and γA/γ′-fibrin and to factor Va. The results with immobilized fibrin were confirmed by examining the binding of radiolabeled thrombin to fibrin clots. Thus, dabigatran modestly accelerated the dissociation of thrombin from γA/γA-fibrin clots, whereas argatroban attenuated dissociation. Dabigatran had no effect on thrombin binding to glycoprotein Ibα peptide, whereas argatroban promoted binding. These findings not only highlight functional effects of thrombin allostery, but also suggest that individual active site-directed thrombin inhibitors uniquely modulate exosite function, thereby identifying potential novel mechanisms of action.
Introduction
The substrate specificity of thrombin is dependent on exosites 1 and 2, positively-charged domains that flank the active site [1,2]. These exosites modulate the reactivity of thrombin by providing initial binding sites for substrates, inhibitors or cofactors, sterically hindering other interactions, and by allosterically modifying the active site. Exosite 1 is more versatile because it (a) serves as a docking site for substrates, (b) redirects thrombin activity by binding thrombomodulin, and (c) mediates thrombin inhibition by hirudin and heparin cofactor II. In contrast, exosite 2 has a more limited role because it mainly serves to tether or localize thrombin. For example, exosite 2 contributes to thrombin binding to platelet glycoprotein (Gp) Ibα [3,4] and, by binding heparin, accelerates the inhibition of thrombin by the heparin-antithrombin complex. Exosite 2 also binds the γ′-chain of fibrinogen, thereby mediating a bivalent, high affinity interaction with the variant γA/γ′-fibrinogen [5]. In contrast, because the γA-chain lacks a thrombin binding site, thrombin binds the bulk γA/γA-fibrinogen with lower affinity solely via exosite 1 [6,7]. Both exosites of thrombin also are involved in the interaction of thrombin with factor V, and activated factor V (factor Va) retains affinity for thrombin [8–10].
Numerous studies have shown that thrombin is subject to allosteric modulation. For example, binding of Na+ to a conserved site on thrombin increases the catalytic activity of thrombin by altering the conformation of the active site [11,12]. Likewise, ligand binding to exosites 1 or 2 can induce allosteric changes at the active site and/or the reciprocal exosite. Thus, binding of the hirudin peptide [13–15], platelet PAR-1 peptide [13,16], or thrombomodulin [17,18] to exosite 1 or GpIbα [19] or prothrombin fragment 2 [14] to exosite 2 elicits conformational changes at the active site. Likewise, binding of prothrombin fragment 2, γ′-peptide (an analog of the thrombin-binding domain on the γ′-chain of fibrinogen), or HD22 (an exosite 2-binding aptamer) attenuates exosite 1-mediated thrombin interactions with fibrin and other ligands, thereby providing evidence of inter-exosite allostery [20–24]. Previous studies have demonstrated that the exosite to active site connection is bidirectional, such that ligand binding to the active site of thrombin induces reciprocal allosteric changes at the exosites [21,22]. However, the studies were performed with peptide ligands, and it remains unknown whether the same bidirectional effects occur with native ligands or in functional assays. To address this gap, we examined the effects of dabigatran, argatroban, and dansylarginine N-(3-ethyl-1,5-pentanediyl)amide (DAPA), active site-directed small molecules that inhibit thrombin with Ki values of 4.5 nM [25], 19 nM [26], and 45 nM [27], respectively, on thrombin binding to immobilized γA/γA-fibrin, γA/γ′-fibrin, factor Va, and GpIbα peptide. In addition, the effects of these inhibitors on the binding of radiolabeled thrombin to fibrin clots and its subsequent dissociation were examined.
Experimental Procedures
Materials
Reagents
Human thrombin and plasminogen-free fibrinogen were from Enzyme Research Laboratories (South Bend, IN). Fibrinogen was immunodepleted of factor XIII as described [28]. Human factors Va and XIII and DAPA were from Haematologic Technologies Inc. (Essex Junction, VT). Recombinant thrombin with Arg residues 93, 97, and 101 changed to Ala (RA-thrombin) was a generous gift from Dr. C. Esmon (Oklahoma Medical Research Foundation). Prionex was from Pentapharm (Basel, Switzerland). γA/γA- and γA/γ′-fibrinogen were isolated and characterized as previously described [7,28,29]. Batroxobin from the venom of Bathrops atrox moojeni was from Pentapharm (Basel, Switzerland). D-Phe-Pro-Arg (FPR) chloromethyl ketone was from Calbiochem (EMD Millipore, Toronto ON). Chromozym-Thrombin (Chz-Th) was from Hyphen BioMed (Neuville sur Oise, France). Active dabigatran was generously provided by Dr. J. van Ryn (Boehringer-Ingelheim, Biberach, Germany), whereas argatroban was a gift from Dr. D. Stump (Genentech, South San Francisco, CA). Recombinant hirudin was from Dade-Behring (Marburg, Germany). Chloramine T and sodium metabisulfite were from Sigma-Aldrich and Bolton-Hunter reagent was from Pierce Biotechnology (Rockford, IL). The synthetic peptide corresponding to the thrombin exosite 2-specific binding site at residues 269–286 on GpIbα, (Asp-Glu-Gly-Asp-Thr-Asp-Leu-Tyr(PO3)-Asp-Tyr(PO3)-Tyr(PO3)-Pro-Glu-Glu-Asp-Thr-Glu-Gly; GpIbα269-286ppp) was synthesized with three phosphorylated Tyr residues by Mimotopes (Minneapolis, MN) [30,31].
Radiolabeled thrombin
To 10 μl of 0.2 M sodium borate, pH 8.0, and 10 μl (1 mCi) of Na125I (McMaster University Nuclear Reactor, Hamilton, ON) was added 5 μl of 1.5 mM Bolton-Hunter reagent in DMSO. The reaction was initiated by adding 10 μl of 5 mg/ml chloramine T in 20 mM sodium phosphate, 150 mM NaCl, pH 7.4 (PBS). After 1 min at 23°C, the reaction was stopped by addition of 10 μl of 12 mg/ml sodium metabisulfite in PBS. To the reaction mixture was added 150 μg of thrombin and after incubation for 1 hr at 23°C, the reaction was terminated by addition of 100 μl of 0.2 M glycine in 0.2 M sodium borate, pH 8.0. Labeled thrombin was isolated on a PD-10 column (GE Healthcare, Baie d’Urfe, PQ), and eluted with 20 mM Tris-HCl, 150 mM NaCl, pH 7.4 (TBS) containing 0.01% Tween-80 (TBS-Tw). The specific activity of the labeled thrombin was 3.8 x 108 cpm/mg.
Active site-blocked thrombin
Thrombin (3–3.5 mg/ml) was incubated with 2-fold molar excess of FPR chloromethyl ketone at 23°C. After 30 min, absence of residual activity of 2 μM FPR-thrombin was determined at 405 nm with 200 μM Chz-Th in a Spectramax plate reader (Molecular Devices, Sunnyvale CA). The sample was washed with TBS and concentrated using an Amicon Ultra 4 ml– 3000 MW centrifugal cartridge (Millipore) [14].
Methods
SPR
Studies were performed using a Biacore T200 (GE Healthcare). γA/γA-fibrinogen, γA/γ′-fibrinogen, and factor Va were immobilized to separate flow cells of a CM5 sensor chip using the amine coupling kit from GE Healthcare [32]. Briefly, after injecting the 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride/N-hydroxysuccinimide mixture into the flow cell at a rate of 10 μl/min for 420 s, 50 μg/ml of γA/γA- or γA/γ′-fibrinogen or 28 μg/ml factor Va in 10 mM acetate buffer, pH 4.5, was injected at a rate of 5 μl/min until about 8000 response units (RU) were immobilized. High levels of protein were adsorbed because previous studies indicate that only 30% of bound fibrinogen is in an accessible orientation, and because of the large difference in molecular weight between the bound ligands and the analyte thrombin [28]. Flows cells were then washed with 1 M ethanolamine for 420 s, followed by the running buffer composed of 20 mM HEPES, 150 mM NaCl, pH 7.4 (HBS) containing 0.01% Tween-80 (HBS-Tw) and 5 mM CaCl2. To convert the immobilized fibrinogen to fibrin, flow cells were subjected to successive 60-min injections of up to 1 μM thrombin [24,28,32]. Quantitative conversion to fibrin was evidenced by saturable decline in RU following thrombin treatment [33].
To measure binding to immobilized γA/γA-fibrin, γA/γ′-fibrin or factor Va, increasing concentrations of up to 5 μM thrombin or FPR-thrombin were injected into the flow cells. To measure the effects of dabigatran, argatroban, and DAPA on thrombin binding to fibrin or factor Va, 250–500 nM thrombin or FPR-thrombin was incubated with 0–5 μM inhibitor and injected into the flow cells. Because of the fast on- and off-rates, all SPR experiments were performed at a flow rate of 25 μl/min [28]. Association times were 120 s and dissociation was monitored by washing the flow cells with HBS-Tw for 600 s. Between runs, flow cells were regenerated with a 45 s wash with HBS-Tw containing 0.5 M CaCl2.
The Kd values for one-site binding of thrombin to γA/γA-fibrin were determined from plots of RU values at equilibrium (Req) versus thrombin or FPR-thrombin concentration using Biacore T200 Evaluation software v 1.0. The rapid approach to equilibrium at the low flow rate nullified any potential mass transport concerns. Nonspecific binding was accounted for by subtracting the control RU values obtained from the unmodified flow cell. To calculate two-site binding of thrombin to γA/γ′-fibrin or factor Va, plots were subjected to kinetic analysis using a two-site binding model. EC50 values for the effect of dabigatran, argatroban, or DAPA on thrombin binding were determined by plotting Req values against the concentration of active site inhibitor and using non-linear regression to fit these to a rectangular hyperbola.
To examine the effect of the active site inhibitors on exosite 2-mediated binding, 1 mg of GpIbα269-286ppp in HBS was biotinylated at its NH2-terminus by incubation with a 10-fold molar excess of Hook-Sulfo-NHS-LC biotin (G-Biosciences, St. Louis, MO) for 1 h at 23°C. After separation from unincorporated reagent by chromatography on Sephadex G15, fractions containing biotinylated peptide were identified by monitoring absorbance at 204 nm. Streptavidin (Sigma) was attached to flow cells of a CM5 chip to about 12000 RU and biotin (b)-GpIbα269-286ppp was then adsorbed to about 150 RU above the streptavidin background. Aliquots of thrombin or RA-thrombin up to 4 μM in HBS-Tw were injected into the flow cell at 25 μl/min for 80 s, followed by HBS-Tw for 200 s. Between runs, flow cells were regenerated with 1 M NaCl for 30 s. To measure the effect of dabigatran, argatroban, or DAPA on this interaction, 250 nM thrombin was injected in the presence of 0–5000 nM dabigatran, argatroban, or DAPA. EC50 values were determined as above. To quantify the effects of inhibitors on binding affinity, 0–2 μM thrombin was injected in the presence of 2.5 μM active site inhibitor at 25 μl/min for 60 s. Binding affinities were calculated as described above.
Effect of dabigatran, argatroban and DAPA on 125I-thrombin binding to fibrin clots
To a series of 1.5-ml micro-centrifuge tubes containing 0–1 μM dabigatran or argatroban, 0–4 μM DAPA, or 0–0.1 μM hirudin were added 10 nM 125I-thrombin, 3 μM γA/γA-fibrinogen or 1 μM γA/γ′-fibrinogen, 2 mM CaCl2 and 1 unit/mL batroxobin in TBS-Tw in a total volume of 100 μl [24,28,32]. Batroxobin was used to induce clotting because its activity is unaffected by the inhibitors (not shown). After incubation for 1 h at 23°C, clots were pelleted by centrifugation at 14,000 × g for 4 min and 50-μl aliquots of supernatant were removed and counted for radioactivity using a gamma counter (Wizard2 2470, Perkin-Elmer) to determine the concentration of unbound 125I-thrombin. EC50 values for the effect of the inhibitors were calculated by fitting plots of bound 125I-thrombin versus the inhibitor concentration by non-linear regression analysis to an equation for a rectangular hyperbola. Ki values were then determined using the Cheng-Prusoff equation and the dissociation constant of 125I-thrombin for fibrinogen as determined in a separate experiment.
Dissociation of 125I-thrombin from fibrin clots
Dissociation of 125I-thrombin from preformed fibrin clots was performed as described [28]. Briefly, clots were formed around plastic inoculation loops by clotting a 130 μl solution containing 3 μM γA/γA-fibrinogen and 30 nM factor XIII in TBS-Tw containing 2 mM CaCl2 with 10 nM 125I-thrombin. After incubation for 1 h, clots were immersed in 50 ml plastic tubes containing 10 ml of 100 nM dabigatran, argatroban or DAPA, or 2 M NaCl in TBS-Tw at 23°C. At defined intervals, aliquots were removed, counted for radioactivity, and returned to the tubes. Plots of residual radioactivity versus time were analyzed using a 2-phase exponential decay equation [28].
Statistical analysis–All experiments were performed at least three times. Results are presented as the mean ± standard deviation (SD). Paired data were compared using Student t-tests; whereas group means were compared using one-way ANOVA followed by Tukey's test for multiple comparisons. For all analyses, p values less than 0.05 were considered statistically significant.
Results
SPR analysis of the interaction of thrombin with immobilized fibrin or factor Va
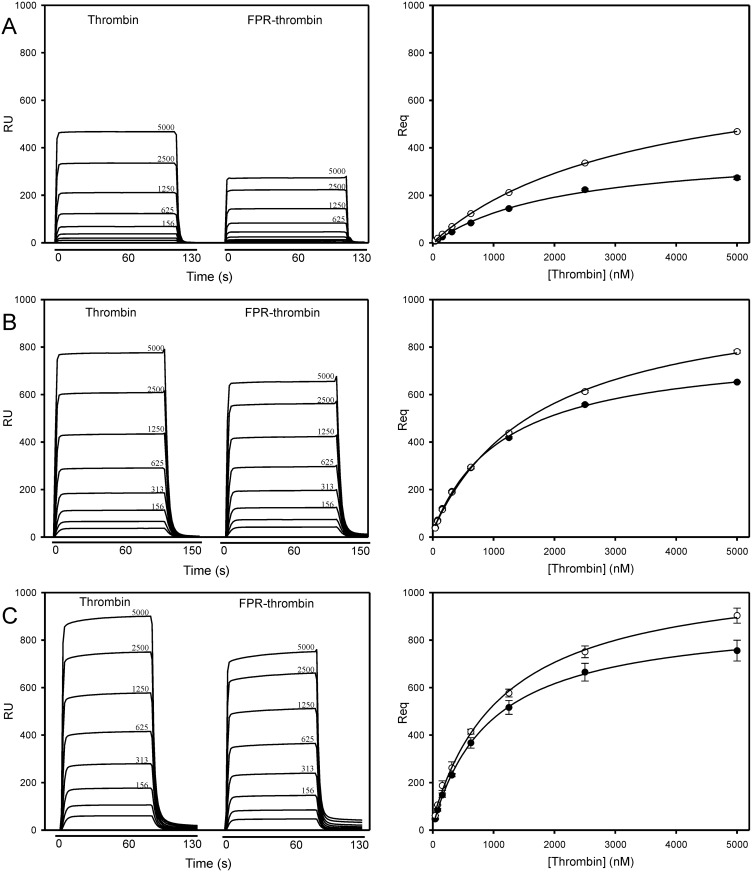
Increasing concentrations of thrombin or FPR-thrombin were injected into flow cells containing immobilized γA/γA-fibrin, γA/γ′-fibrin, or factor Va [24,28]. Because sensorgram profiles rapidly reached equilibrium, Req values were determined and plotted versus the analyte concentration to determine Kd (Fig 1). Non-specific binding is not a major concern because prothrombin exhibited only weak affinity for immobilized fibrin, and thrombin did not bind to immobilized ovalbumin (not shown). As expected, more thrombin and FPR-thrombin bound to γA/γ′-fibrin than to γA/γA-fibrin. Binding to γA/γA-fibrin was via a single lower affinity site, whereas binding to γA/γ′-fibrin occurred through higher and lower affinity interactions, demonstrating involvement of both exosites (Table 1) [28,32]. Affinities are similar to those reported previously using SPR and fibrin clots [7,24]. More thrombin and FPR-thrombin also bound to factor Va than to γA/γA-fibrin. The higher affinities for factor Va than for γA/γA-fibrin are consistent with interaction mediated by both exosites [8–10]. The affinities of FPR-thrombin for fibrin and factor Va were ~50% higher than those of active thrombin (p < 0.001).
Fig 1. Binding of thrombin or FPR-thrombin to immobilized γA/γA-fibrin, γA/γ′-fibrin, or factor Va.
Increasing concentrations of thrombin or FPR-thrombin up to 5000 nM were injected into individual flow cells of a CM5 biosensor chip containing immobilized (A) γA/γA-fibrin, (B) γA/γ′-fibrin, or (C) factor Va. Samples were injected for 120 s at 25 μl/min, followed by a 600 s wash to monitor dissociation. Left panels; sensorgram tracings of representative runs for thrombin and FPR-thrombin are shown in succession. Analyte concentrations are 39, 78, 156, 313, 625, 1250, 2500, 5000 nM, or as indicated. Right panels; from each sensorgram, the amount of thrombin (open circles) or FPR-thrombin (closed circles) bound at equilibrium after background correction (Req) was determined and plotted against the thrombin concentration. Data points represent the mean ± S.D. of 3 experiments, and the lines represent nonlinear regression analysis.
Table 1. Dissociation constants (Kd) for the binding of thrombin to fibrin, factor Va and GpIbα269-286ppp.
The binding of thrombin or FPR-thrombin to immobilized γA/γA-fibrin, γA/γ′-fibrin, factor Va or GpIbα269-286ppp was quantified using SPR. Kd values for thrombin binding to γA/γA-fibrin and GpIbα269-286ppp were determined by steady state analysis, whereas bivalent binding of thrombin to γA/γ′-fibrin and factor Va was determined by kinetic analysis for a two-site model using BIAevaluation software. Kd values are shown as mean ± S.D. for three separate experiments.
| Thrombin | FPR-thrombin | |||
|---|---|---|---|---|
| Kd1 | Kd2 | Kd1 | Kd2 | |
| Target | (nM) | |||
| γA/γA-fibrin | 3887 ± 36 | - | 2110 ± 140 | - |
| γA/γ′-fibrin | 2631 ± 18 | 211.6 ± 0.9 | 1434.2 ± 56.8 | 110.4 ± 14.0 |
| Factor Va | 1471 ± 73 | 219.8 ± 189.4 | 831.2 ± 13.7 | 130.4 ± 17.7 |
| GpIbα269-286ppp | 306 ± 11 | - | 211 ± 1 | - |
Effects of dabigatran, argatroban or DAPA on thrombin binding to immobilized fibrin or factor Va
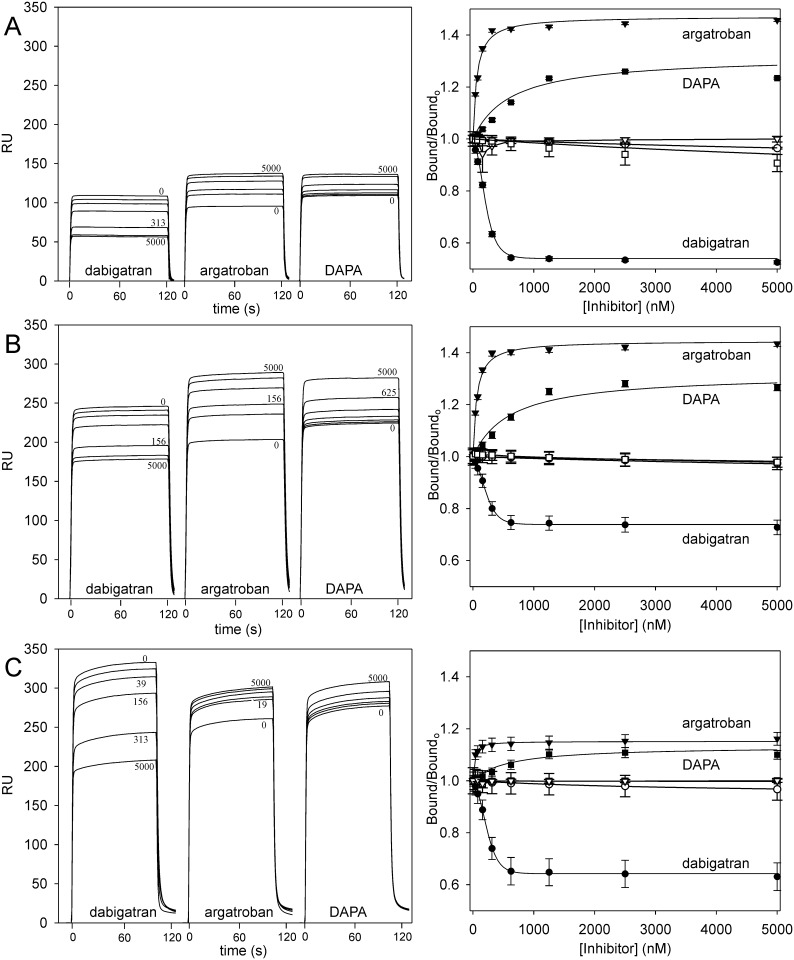
Binding of 250–500 nM thrombin or FPR-thrombin to immobilized γA/γA-fibrin, γA/γ′-fibrin, or factor Va was assessed by SPR in the presence of increasing concentrations of dabigatran, argatroban, or DAPA. Dabigatran reduced thrombin bound to γA/γA-fibrin, γA/γ′-fibrin, and factor Va in a concentration-dependent manner (Fig 2) with EC50 values of 184.6 ± 4.3 nM, 182.4 ± 15.0 nM, and 204.2 ± 17.0 nM, respectively. At saturation, dabigatran reduced thrombin binding to γA/γA-fibrin, γA/γ′-fibrin, and factor Va by 47.6 ± 0.4%, 28.4 ± 1.6%, and 37.9 ± 3.2%, respectively (Table 2). As a control, experiments using FPR-thrombin in place of thrombin demonstrated no effect of dabigatran on binding, confirming that dabigatran binds specifically to the active site (Fig 2).
Fig 2. Effect of dabigatran, argatroban, or DAPA on thrombin binding to immobilized γA/γA-fibrin, γA/γ′-fibrin, or factor Va.
Increasing concentrations of dabigatran (closed circles), argatroban (closed triangles), or DAPA (closed squares) up to 5 μM were pre-incubated with thrombin prior to injection into individual flow cells containing immobilized (A) γA/γA-fibrin, (B) γA/γ′-fibrin, or (C) factor Va. Sample injection was followed by a 600 s wash with buffer to monitor dissociation. Left panels; representative sensorgrams of the effects of increasing concentrations of dabigatran, argatroban or DAPA on thrombin binding are shown in succession. Right panels; the ratios of Req values of thrombin bound in the absence (Boundo) and presence (Bound) of dabigatran, argatroban or DAPA are plotted against the input inhibitor concentrations. Open symbols represent the effects of the inhibitors on FPR-thrombin binding to γA/γA-fibrin, γA/γ′-fibrin, or factor Va. Data points represent the mean ± S.D. of 3 experiments, and the lines represent nonlinear regression analysis.
Table 2. Effect of dabigatran, argatroban, or DAPA on thrombin binding to γA/γA-fibrin, γA/γ′-fibrin, factor Va, or GpIbα269-286ppp.
The effect of 0–5000 nM dabigatran, argatroban, or DAPA on the binding of thrombin to immobilized γA/γA-fibrin, γA/γ′-fibrin, factor Va or GpIbα269-286ppp was quantified using SPR. EC50 values were determined by plotting the Req values against the inhibitor concentration and fitting by nonlinear regression analysis using BIAevaluation software. Change is the calculated difference between thrombin binding in the absence of inhibitors and that determined at saturating inhibitor concentration. Values are shown as mean ± S.D. for three separate experiments.
| Dabigatran | Argatroban | DAPA | ||||
|---|---|---|---|---|---|---|
| Target | EC50 (nM) | Change (%) | EC50 (nM) | Change (%) | EC50 (nM) | Change (%) |
| γA/γA-fibrin | 184.6 ± 4.3 | - 47.6 ± 0.4 | 62.4 ± 4.8 | + 47.2 ± 1.0 | 514.1 ± 24.0 | + 25.1 ± 0.5 |
| γA/γ′-fibrin | 182.4 ± 15.0 | - 28.4 ± 1.6 | 59.4 ± 5.1 | + 44.5 ± 2.3 | 515.9 ± 31.0 | + 27.7 ± 1.5 |
| Factor Va | 204.2 ± 17.0 | - 37.9 ± 3.2 | 23.4 ± 8.8 | + 15.1 ± 3.9 | 565.1 ± 200 | + 13.2 ± 3.2 |
| GpIbα269-286ppp | — | 0 | 161.0 ± 2.6 | +17.3 ± 0.6 | 1288.3 ± 32.3 | +25.4 ± 0.0 |
In contrast to the results with dabigatran, argatroban and DAPA increased thrombin binding to γA/γA-fibrin, γA/γ′-fibrin, and factor Va in a concentration-dependent manner with EC50 values for argatroban of 62.4 ± 4.8 nM, 59.4 ± 5.1 nM, and 23.4 ± 8.8 nM, respectively, and for DAPA of 514.1 ± 24.0 nM, 515.9 ± 31.0 nM, and 565.1 ± 200 nM, respectively (Fig 2). Although argatroban and DAPA are reported to bind thrombin with similar affinities, the EC50 values for argatroban were more than 10-fold lower than those for DAPA. As observed with dabigatran, neither argatroban nor DAPA had any effect on FPR-thrombin binding to γA/γA-fibrin, γA/γ′-fibrin, or factor Va (Fig 2). Therefore, reversible engagement of the active site by small molecules allosterically modulates the exosite-mediated interactions of thrombin with fibrin and factor Va.
Effects of dabigatran, argatroban and DAPA on thrombin binding to GpIbα269-286ppp
Thrombin bound to the immobilized GpIbα269-286ppp peptide with a Kd of 306 ± 11 nM (Fig 3A; Table 1); an affinity intermediate between that reported for a similar peptide and for glycocalicin [30,34]. In contrast, RA-thrombin, an exosite 2 variant, did not bind (not shown), consistent with the reported specificity of this interaction for exosite 2 [30]. Thrombin binding to the GpIbα269-286ppp peptide was then quantified in the presence of increasing concentrations of inhibitors. Whereas dabigatran had no effect on thrombin binding, argatroban and DAPA increased the amount of thrombin bound by 17% and 25%, respectively (Fig 3B; Table 2). Binding to GpIbα269-286ppp was then quantified in the presence of 2.5 μM dabigatran, argatroban or DAPA. Whereas thrombin bound with similar affinity in the presence of dabigatran (Kd 306 ± 11 nM; not shown) as in its absence, the affinity of thrombin for GpIbα269-286ppp significantly (p < 0.001 by ANOVA) increased by 25–35% in the presence of argatroban or DAPA (Kd values of 229 ± 7 nM and 197 ± 5 nM, respectively). These findings demonstrate that occupation of the active site by some, but not all, small molecules increases the affinity of ligands for exosite 2, similar to the observation for exosite 1-dependent interactions.
Fig 3. Binding of thrombin to immobilized GpIbα269-286ppp.
A. Increasing concentrations of thrombin up 4 μM were injected at a rate of 25 μl/min into flow cells of a streptavidin-coated CM5 biosensor chip containing adsorbed biotinlylated GpIbα269-286ppp. Response units (RU) are plotted versus time for the indicated thrombin concentrations (in nM) in triplicate (inset). RU values at equilibrium (Req) were determined and plotted versus the input thrombin concentration. The data were analyzed by nonlinear regression (line) to a rectangular hyperbola to determine Kd. Symbols represent mean ± SD for from 3 runs. B. Thrombin (250 nM) was injected into flow cells containing immobilized biotinlylated GpIbα269-286ppp in the presence of 0–5000 nM dabigatran (circles), argatroban (triangles), or DAPA (squares). Req values were determined, converted to a fraction of that measured in the absence of inhibitor (Bound/Bound0), and plotted versus the inhibitor concentration. Data points indicate mean ± SD for 3 determinations, and the lines represent nonlinear regression analyses.
Effects of dabigatran, argatroban and DAPA on 125I-thrombin binding to fibrin clots
Although the affinities of thrombin and FPR-thrombin for γA/γA- and γA/γ′-fibrin determined by SPR are comparable with those obtained with fibrin clots [28], it was important to confirm that the allosteric response observed using SPR also occurred in a functional assay with three-dimensional fibrin clots. 125I-thrombin bound γA/γA- and γA/γ′-fibrin clots with Kd values of 3.6 ± 0.3 μM and 1.2 ± 0.2 μM (not shown), respectively; values comparable with those obtained using SPR and with those published previously [32]. Up to 70% of the 125I-thrombin bound to fibrin, demonstrating that labeling had little effect on the capacity of thrombin to bind to fibrin.
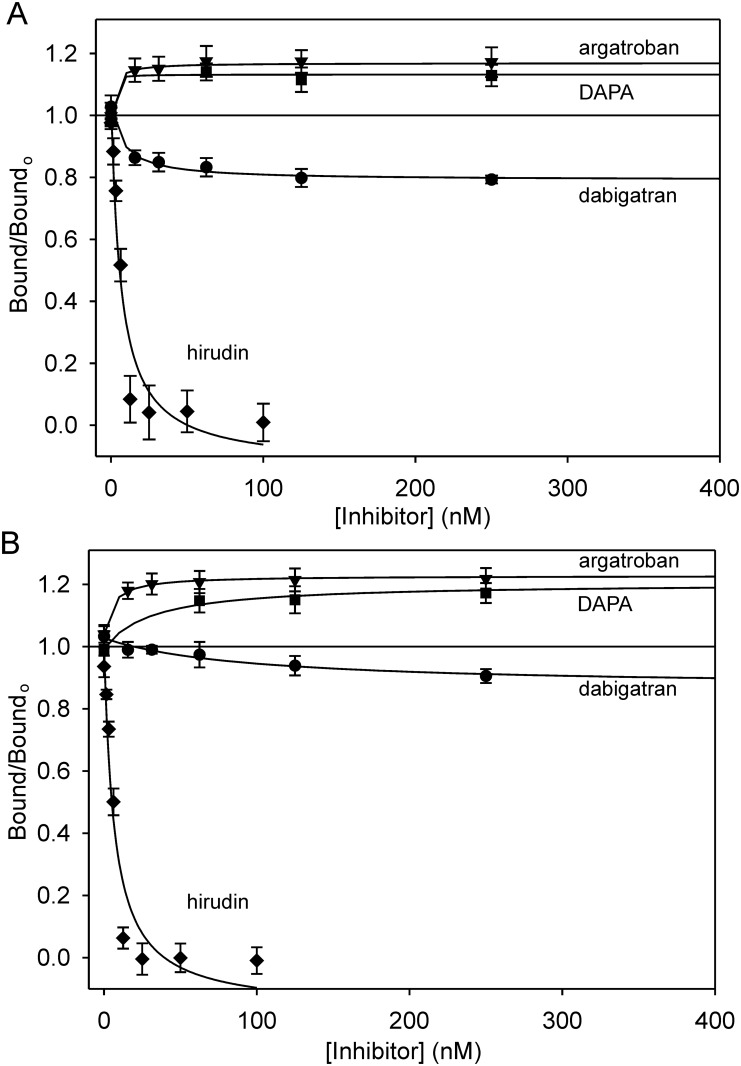
Dabigatran reduced 125I-thrombin binding to γA/γA- and γA/γ′-fibrin clots in a concentration-dependent manner with Ki values of 3.8 ± 1.5 nM and 26.0 ± 4.0 nM, respectively (Fig 4). The Ki value for the thrombin interaction with γA/γA-fibrin is comparable with the value of 4.5 nM obtained by chromogenic assay [35]. The Ki value is higher with γA/γ′-fibrin than γA/γA-fibrin, likely reflecting the higher affinity, bivalent interaction mediated by both exosites [7]. As observed using SPR, dabigatran produced a 2-fold greater reduction in thrombin binding to γA/γA-fibrin than to γA/γ′-fibrin (p < 0.05); a difference that likely reflects the higher affinity of thrombin for γA/γ′-fibrin. To verify the role of exosite 1 in binding, the effect of hirudin also was examined. As expected, hirudin fully inhibited 125I-thrombin binding to γA/γA- and γA/γ′-fibrin. In agreement with the SPR observations, argatroban and DAPA enhanced the binding of 125I-thrombin to γA/γA- and γA/γ′-fibrin clots to a similar extent (~15–25%) (Fig 4, Table 3). Collectively, therefore, the data obtained with fibrin clots support the SPR results and suggest that active site engagement induces allosteric changes in the thrombin exosites, with the direction and magnitude determined by the ligand.
Fig 4. Effect of dabigatran, argatroban, DAPA, or hirudin on the binding of 125I-thrombin to fibrin clots.
Increasing concentrations of dabigatran (circles), argatroban (triangles), DAPA (squares), or hirudin (diamonds) were added to samples containing 10 nM 125I-thrombin, 2 mM CaCl2, 1 U/mL batroxobin and (A) 3 μM γA/γA-fibrinogen, or (B) 1 μM γA/γ′-fibrinogen. After incubation for 1 h, clots were pelleted and aliquots of supernatant were counted for radioactivity. The ratios of 125I-thrombin bound in the absence or presence of inhibitor are plotted against the inhibitor concentrations. Data points represent the mean ± S.D. of 2–3 experiments, each performed in duplicate, and the lines represent nonlinear regression analysis.
Table 3. Effect of dabigatran, argatroban, DAPA or hirudin on 125I-thrombin binding to fibrin clots.
Binding of 125I-thrombin to γA/γA- or γA/γ′-fibrin clots in the absence or presence of dabigatran, argatroban, DAPA, or hirudin was determined by measuring the amount of 125I-thrombin in the supernatants of compacted clots prepared by incubating γA/γA- or γA/γ′-fibrinogen with a catalytic amount of batroxobin. EC50 values were determined by nonlinear regression analysis and converted to Ki values by correction for Kd of 125I-thrombin for fibrin. Change denotes the maximal change in the percentage of bound 125I-thrombin compared with that in the absence of inhibitor.
| Dabigatran | Argatroban | DAPA | Hirudin | |||||
|---|---|---|---|---|---|---|---|---|
| Ligand | Ki (nM) | Change (%) | Ki (nM) | Change (%) | Ki (nM) | Change (%) | Ki (nM) | Change (%) |
| γA/γA-fibrin | 3.8 ± 1.5 | -21.4 ± 3.3 | 2.4 ± 1.9 | +18.3 ± 5.0 | 2.1 ± 0.9 | +17.0 ± 2.7 | 3.4 ± 0.3 | -100 |
| γA/γ′-fibrin | 26.0 ± 4.0 | -9.4 ± 1.4 | 7.0 ± 5.6 | +26.1 ± 0.4 | 19.6 ± 19.1 | +20.1 ± 3.6 | 3.4 ± 0.1 | -100 |
Effects of dabigatran, argatroban and DAPA on 125I-thrombin dissociation from fibrin clots
To determine whether the alteration in the affinity of thrombin for fibrin in the presence of active site inhibitors influences the amount of thrombin associated with fibrin, dissociation from fibrin clots was examined [28]. Clots formed from γA/γA-fibrinogen in the presence of 125I-thrombin were incubated in buffer in the absence or presence of active site inhibitors and the amount of 125I-thrombin released from the clot over time was quantified. Biphasic analysis was used to evaluate the fast non-adsorbent and slow bound phases of dissociation of thrombin from fibrin. In the control, the bound fraction dissociated with a half-life of 20.5 ± 3.1 h, whereas in the presence of 2 M NaCl where ionic interactions are abrogated, the rate of dissociation was significantly faster (p = 0.02) at 14.2 ± 1.2 h (Fig 5). The half-life values in the presence of 2.5 μM dabigatran, argatroban, and DAPA were 17.7 ± 2.3 h, 25.5 ± 2.9 h, and 23.7 ± 2.9 h, respectively. Although these values were not significantly different from the control, there was a trend for more rapid dissociation in the presence of dabigatran. However, the half-lives in the presence of argatroban and DAPA were significantly longer than that in the presence of dabigatran (p = 0.01 and 0.02, respectively). These data are consistent with the effects of the inhibitors on the affinity of thrombin for fibrin, and confirm the differences in response between dabigatran and argatroban or DAPA.
Fig 5. Effect of dabigatran, argatroban, DAPA, or hirudin on the dissociation of 125I-thrombin from fibrin clots.
Clots were formed around plastic loops by incubating 3 μM γA/γA-fibrinogen and 30 nM factor XIII with 10 nM 125I-thrombin in the presence of 2 mM CaCl2 for 1 h. Clots were immersed in 10 ml TSTwCa buffer alone, or buffer containing 100 nM dabigatran, argatroban, or DAPA, or 2 M NaCl. At intervals, aliquots were removed and counted for radioactivity. Residual counts were normalized with respect to time zero and plotted versus time. Data were analyzed by two phase exponential decay (lines) to determine the half-lives of dissociation. Symbols represent the mean ± SD of 4 experiments.
Discussion
The purpose of this study was to determine whether occupation of the active site of thrombin by small molecules induces functional changes at its exosites. We chose fibrin, factor Va and GpIbα269-286ppp as targets for three reasons. First, their use enabled studies with unmodified, active thrombin. Second, the mode of interaction of thrombin with these ligands has been well characterized; thus, binding of thrombin to γA/γA-fibrin and GpIbα269-286ppp is solely dependent on exosite 1 and exosite 2, respectively, whereas thrombin binding to γA/γ′-fibrin and factor Va requires both exosites. Third, the use of native ligands such as fibrin and factor Va would complement previous results obtained with peptide ligands, and the use of fibrin would enable confirmation in functional assays. Dabigatran and argatroban were chosen because they are highly specific for thrombin and their interactions with the active site of thrombin have been extensively investigated and because both agents are in clinical use. DAPA was chosen to complement the results with argatroban because both incorporate an Arg group that is essential for specificity [36,37].
Dabigatran saturably reduces thrombin binding to γA/γA-fibrin and factor Va by 28–48%, but has no effect on the interaction of thrombin with Gp1bα269-286ppp. Surprisingly, argatroban and DAPA increase thrombin binding to γA/γA-fibrin, γA/γ′-fibrin, factor Va and GpIbα269-286ppp by 13–47%. The observations with fibrin were confirmed using three-dimensional fibrin clots and were not dependent on the affinity of the inhibitors for thrombin because dabigatran, argatroban and DAPA inhibit thrombin with similar nanomolar Ki values. DAPA and argatroban also slow the dissociation of thrombin from fibrin clots in a manner consistent with their enhancement of the affinity of thrombin for fibrin. Thus, these studies demonstrate allosteric effects of active site binding on thrombin, and provide evidence that responses are ligand specific.
Investigation into the allosteric regulation of thrombin has shown that binding of a variety of effectors to the exosites modulates the active site. This phenomenon has been attributed to a complex allosteric network within thrombin that is subject to modulation through surface residues [13,16]. Within the active site, residues in the S1-4 subsites and the 60- and autolysis-loops contribute to this allosteric network [16,17,38,39]. These regions within the active site make unique contact with individual substrates and are perturbed by exosite ligands, thereby rendering them central to allosteric regulation. It is interesting to compare the interaction of dabigatran, argatroban and DAPA with the active site of thrombin because dabigatran induces effects that differ from those of argatroban and DAPA. Although dabigatran and argatroban engage the S1, S2, and S4 subsites in the active site, they do so in distinct ways [35–37,40]. Thus, the differences in exosite response elicited by the various inhibitors likely reflect unique points of contact within the allosteric network. In an analogous fashion, different exosite 1 ligands evoke unique changes in thrombin activity [14,24,39,41,42], suggesting that the exosites are composed of distinct subdomains [2,43–46]. Consistent with the results observed here with fibrin and factor Va, argatroban has previously been shown to increase the affinity of hirudin peptide for exosite 1 on thrombin [47]. These observations were not limited to exosite 1 because argatroban and DAPA in the current study also increased the affinity of thrombin for GpIbα peptide; an interaction mediated solely by exosite 2. The structural explanations for how different small molecules can bind to active site of thrombin and evoke distinct responses at the exosites remain to be determined.
The observation that binding of dabigatran, argatroban or DAPA to the active site of thrombin induces changes at the exosites is consistent with the hypothesis that changes transmitted over the allosteric network are bidirectional. Most studies have focused on the effect of exosite ligands on active site function. However, calorimetry and fluorescence studies using peptide ligands demonstrate that structural changes that result from ligand binding at the active site or exosite 1 can run in both directions [21,47,48]. This property suggests that covalent occupation of the active site by FPR should alter exosite function; a concept supported by some studies [38,49–52], but not by others [21,53–55]. Results presented here suggest that noncovalent adducts also can modify exosite function, and represent another way by which thrombin can convert between different functional and conformational states [23]. Although extensive investigations of thrombin structure have not provided a unifying model of thrombin allostery, they demonstrate that thrombin is a highly dynamic molecule with numerous conformations and networks of internal communication.
The capacity of dabigatran to attenuate thrombin binding to fibrin and factor Va reveals a unique mechanism by which this inhibitor might function as an anticoagulant. Thus, in addition to inhibiting the catalytic activity of thrombin, dabigatran may displace thrombin from fibrin or platelets. This would reduce the protection of thrombin from inhibition afforded by such binding, and could further attenuate the thrombogenic potential of the thrombus [56,57]. Such displacement would be similar to that produced by exosite-directed ligands, which also displace thrombin from fibrin or platelets [3,58]. Because argatroban promotes thrombin binding to fibrin, it is likely that not all direct thrombin inhibitors benefit from this secondary effect. This raises the possibility that in addition to simple target potency, future efforts at structure-based drug design should consider the secondary effects of inhibitors; effects that may be altered by refining contacts within the active site.
In summary, our results suggest that active site inhibitors modulate thrombin function mediated by the exosites. This reverse direction of allosteric signaling provides greater insight into the dynamic nature of thrombin. Furthermore, different active site-directed agents evoke unique responses, demonstrating that their inhibitory function can be refined. Thus, exploitation of its allosteric network endows thrombin with additional mechanism of regulation without compromising its repertoire of substrates. This demonstrates that the versatility of thrombin is a consequence of the intricate connections between the active site and the exosites.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was funded in part by grants from the Canadian Institutes of Health Research (MOP-136820 and FRN-3992) and the Heart and Stroke Foundation of Canada (T6357). C.H.Y. is supported by a Doctoral Scholarship from the Canadian Institutes of Health Research. J.I.W. holds the Canada Research Chair (Tier I) in Thrombosis and the Heart and Stroke Foundation J. Fraser Mustard Chair in Cardiovascular Research at McMaster University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Krishnaswamy S. The transition of prothrombin to thrombin. J Thromb Haemost. 2013; 11 Suppl 1: 265–276. 10.1111/jth.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock PE, Panizzi P, Verhamme IM. Exosites in the substrate specificity of blood coagulation reactions. J Thromb Haemost. 2007; 5 Suppl 1: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J Biol Chem. 2001; 276: 4692–4698. [DOI] [PubMed] [Google Scholar]
- 4.Zarpellon A, Celikel R, Roberts JR, McClintock RA, Mendolicchio GL, Moore KL et al. Binding of alpha-thrombin to surface-anchored platelet glycoprotein Ib(alpha) sulfotyrosines through a two-site mechanism involving exosite I. Proc Nat Acad Sci USA. 2011; 108: 8628–8633. 10.1073/pnas.1017042108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfenstein-Todel C, Mosesson MW. Carboxy-terminal amino acid sequence of a human fibrinogen gamma-chain variant (gamma'). Biochemistry. 1981; 20: 6146–6149. [DOI] [PubMed] [Google Scholar]
- 6.Meh DA, Siebenlist KR, Mosesson MW. Identification and characterization of the thrombin binding sites on fibrin. J Biol Chem. 1996; 271: 23121–23125. [DOI] [PubMed] [Google Scholar]
- 7.Pospisil CH, Stafford AR, Fredenburgh JC, Weitz JI. Evidence that both exosites on thrombin participate in its high affinity interaction with fibrin. J Biol Chem. 2003; 278: 21584–21591. [DOI] [PubMed] [Google Scholar]
- 8.Esmon CT, Lollar P. Involvement of thrombin anion-binding exosites 1 and 2 in the activation of factor V and factor VIII. J Biol Chem. 1996; 271: 13882–13887. [DOI] [PubMed] [Google Scholar]
- 9.Segers K, Dahlback B, Bock PE, Tans G, Rosing J, Nicolaes GA. The role of thrombin exosites I and II in the activation of human coagulation factor V. J Biol Chem. 2007; 282: 33915–33924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dharmawardana KR, Olson ST, Bock PE. Role of regulatory exosite I in binding of thrombin to human factor V, factor Va, factor Va subunits, and activation fragments. J Biol Chem. 1999; 274: 18635–18643. [DOI] [PubMed] [Google Scholar]
- 11.Pozzi N, Chen R, Chen Z, Bah A, Di Cera E. Rigidification of the autolysis loop enhances Na(+) binding to thrombin. Biophys Chem. 2011; 159: 6–13. 10.1016/j.bpc.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gohara DW, Di Cera E. Allostery in trypsin-like proteases suggests new therapeutic strategies. Trends Biotechnol. 2011; 29: 577–585. 10.1016/j.tibtech.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechtenberg BC, Johnson DJ, Freund SM, Huntington JA. NMR resonance assignments of thrombin reveal the conformational and dynamic effects of ligation. Proc Nat Acad Sci USA. 2010; 107: 14087–14092. 10.1073/pnas.1005255107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredenburgh JC, Stafford AR, Weitz JI. Evidence for allosteric linkage between exosites 1 and 2 of thrombin. J Biol Chem. 1997; 272: 25493–25499. [DOI] [PubMed] [Google Scholar]
- 15.Verhamme IM, Olson ST, Tollefsen DM, Bock PE. Binding of exosite ligands to human thrombin. Re-evaluation of allosteric linkage between thrombin exosites I and II. J Biol Chem. 2002; 277: 6788–6798. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi PS, Chen Z, Mathews FS, Di Cera E. Structural identification of the pathway of long-range communication in an allosteric enzyme. Proc Nat Acad Sci USA. 2008; 105: 1832–1837. 10.1073/pnas.0710894105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams TE, Li W, Huntington JA. Molecular basis of thrombomodulin activation of slow thrombin. J Thromb Haemost. 2009; 7: 1688–1695. 10.1111/j.1538-7836.2009.03563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasper PM, Fuglestad B, Komives EA, Markwick PR, McCammon JA. Allosteric networks in thrombin distinguish procoagulant vs. anticoagulant activities. Proc Nat Acad Sci USA. 2012; 109: 21216–21222. 10.1073/pnas.1218414109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li CQ, Vindigni A, Sadler JE, Wardell MR. Platelet glycoprotein Ib alpha binds to thrombin anion-binding exosite II inducing allosteric changes in the activity of thrombin. J Biol Chem. 2001; 276: 6161–6168. [DOI] [PubMed] [Google Scholar]
- 20.Malovichko MV, Sabo TM, Maurer MC. Ligand binding to anion-binding exosites regulates conformational properties of thrombin. J Biol Chem. 2013; 288: 8667–8678. 10.1074/jbc.M112.410829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treuheit NA, Beach MA, Komives EA. Thermodynamic compensation upon binding to exosite 1 and the active site of thrombin. Biochemistry. 2011; 50: 4590–4596. 10.1021/bi2004069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabo TM, Farrell DH, Maurer MC. Conformational analysis of gamma' peptide (410–427) interactions with thrombin anion binding exosite II. Biochemistry. 2006; 45: 7434–7445. [DOI] [PubMed] [Google Scholar]
- 23.Kamath P, Huntington JA, Krishnaswamy S. Ligand binding shuttles thrombin along a continuum of zymogen- and proteinase-like states. J Biol Chem. 2010; 285: 28651–28658. 10.1074/jbc.M110.154914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrera NS, Stafford AR, Leslie BA, Kretz CA, Fredenburgh JC, Weitz JI. Long range communication between exosites 1 and 2 modulates thrombin function. J Biol Chem. 2009; 284: 25620–25629. 10.1074/jbc.M109.000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007; 98: 155–162. [PubMed] [Google Scholar]
- 26.Fitzgerald D, Murphy N. Argatroban: a synthetic thrombin inhibitor of low relative molecular mass. Coron Artery Dis. 1996; 7: 455–458. [PubMed] [Google Scholar]
- 27.Nesheim ME, Prendergast FG, Mann KG. Interactions of a fluorescent active-site-directed inhibitor of thrombin: Dansylarginine N-(3-Ethyl-1,5-pentanediyl)amide. Biochemistry. 1979; 18: 996–1003. [DOI] [PubMed] [Google Scholar]
- 28.Fredenburgh JC, Stafford AR, Leslie BA, Weitz JI. Bivalent binding to gamma A/gamma'-fibrin engages both exosites of thrombin and protects it from inhibition by the antithrombin-heparin complex. J Biol Chem. 2008; 283: 2470–2477. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer AV, Leslie BA, Rischke JA, Stafford AR, Fredenburgh JC, Weitz JI. Incorporation of fragment X into fibrin clots renders them more susceptible to lysis by plasmin. Biochemistry. 2006; 45: 4257–4265. [DOI] [PubMed] [Google Scholar]
- 30.Lechtenberg BC, Freund SM, Huntington JA. GpIbalpha interacts exclusively with exosite II of thrombin. J Mol Biol. 2014; 426: 881–893. 10.1016/j.jmb.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabo TM, Maurer MC. Biophysical investigation of GpIbalpha binding to thrombin anion binding exosite II. Biochemistry. 2009; 48: 7110–7122. 10.1021/bi900745b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vu TT, Stafford AR, Leslie BA, Kim PY, Fredenburgh JC, Weitz JI. Batroxobin binds fibrin with higher affinity and promotes clot expansion to a greater extent than thrombin. J Biol Chem. 2013; 288: 16862–16871. 10.1074/jbc.M113.464750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim PY, Tieu LD, Stafford AR, Fredenburgh JC, Weitz JI. A high affinity interaction of plasminogen with fibrin is not essential for efficient activation by tissue-type plasminogen activator. J Biol Chem. 2012; 287: 4652–4661. 10.1074/jbc.M111.317719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Cristofaro R, De Candia E, Rutella S, Weitz JI. The Asp(272)-Glu(282) region of platelet glycoprotein Ibalpha interacts with the heparin-binding site of alpha-thrombin and protects the enzyme from the heparin-catalyzed inhibition by antithrombin III. J Biol Chem. 2000; 275: 3887–3895. [DOI] [PubMed] [Google Scholar]
- 35.Hauel NH, Nar H, Priepke H, Ries U, Stassen JM, Wienen W. Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem. 2002; 45: 1757–1766. [DOI] [PubMed] [Google Scholar]
- 36.Brandstetter H, Turk D, Hoeffken HW, Grosse D, Sturzebecher J, Martin PD et al. Refined 2.3 A X-ray crystal structure of bovine thrombin complexes formed with the benzmidine and arginine-based thrombin inhibitors NAPAP, 4-TAPAP and MQPA. A starting point for improving antithrombotics. J Mol Biol. 1992; 226: 1085–1099. [DOI] [PubMed] [Google Scholar]
- 37.Mathews II, Tulinsky A. Active-site mimetic inhibition of thrombin. Acta Crystallogr D Biol Crystallogr. 1995; 51: 550–559. [DOI] [PubMed] [Google Scholar]
- 38.Croy CH, Koeppe JR, Bergqvist S, Komives EA. Allosteric changes in solvent accessibility observed in thrombin upon active site occupation. Biochemistry. 2004; 43: 5246–5255. [DOI] [PubMed] [Google Scholar]
- 39.Ng NM, Quinsey NS, Matthews AY, Kaiserman D, Wijeyewickrema LC, Bird et al. The effects of exosite occupancy on the substrate specificity of thrombin. Arch Biochem Biophys. 2009; 489: 48–54. 10.1016/j.abb.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 40.Bode W, Turk D, Karshikov A. The refined 1.9 A X-ray crystal structure of D-Phe-Pro-Arg chloromethylketone-inhibited human a-thrombin: Structure analysis, overall structure, electrostatic properties, detailed active site geometry, and structure-function relationships. Protein Sci. 1992; 1: 426–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye J, Esmon NL, Esmon CT, Johnson AE. The active site of thrombin is altered upon binding to thrombomodulin. Two distinct structural changes are detected by fluorescence, but only one correlates with protein C activation. J Biol Chem. 1991; 266: 23016–23021. [PubMed] [Google Scholar]
- 42.Liu LW, Vu TKH, Esmon CT, Coughlin SR. The region of the thrombin receptor resembling hirudin binds to thrombin and alters enzyme specificity. J Biol Chem. 1991; 266: 16977–16980. [PubMed] [Google Scholar]
- 43.Kretz CA, Stafford AR, Fredenburgh JC, Weitz JI. HD1, a thrombin-directed aptamer, binds exosite 1 on prothrombin with high affinity and inhibits its activation by prothrombinase. J Biol Chem. 2006; 281: 37477–37485. [DOI] [PubMed] [Google Scholar]
- 44.Abdel Aziz MH, Sidhu PS, Liang A, Kim JY, Mosier PD, Zhou Q et al. Designing allosteric regulators of thrombin. Monosulfated benzofuran dimers selectively interact with Arg173 of exosite 2 to induce inhibition. J Med Chem. 2012; 55: 6888–6897. 10.1021/jm300670q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bode W. Structure and interaction modes of thrombin. Blood Cells Mol Dis. 2006; 36: 122–130. [DOI] [PubMed] [Google Scholar]
- 46.Page MJ, MacGillivray RT, Di Cera E. Determinants of specificity in coagulation proteases. J Thromb Haemost. 2005; 3: 2401–2408. [DOI] [PubMed] [Google Scholar]
- 47.Parry MAA, Stone SR, Hofsteenge J, Jackman MP. Evidence for common structural changes in thrombin induced by active-site or exosite binding. Biochem J. 1993; 290: 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Cristofaro R, De Candia E, Picozzi M, Landolfi R. Conformational transitions linked to active site ligation in human thrombin: Effect on the interaction with fibrinogen and the cleavable platelet receptor. J Mol Biol. 1995; 245: 447–458. [DOI] [PubMed] [Google Scholar]
- 49.De Cristofaro R, Landolfi R. Thermodynamics of substrates and reversible inhibitors binding to the active site cleft of human α-thrombin. J Mol Biol. 1994; 239: 569–577. [DOI] [PubMed] [Google Scholar]
- 50.Bock PE, Olson ST, Bjork I. Inactivation of thrombin by antithrombin is accompanied by inactivation of regulatory exosite I. J Biol Chem. 1997; 272: 19837–19845. [DOI] [PubMed] [Google Scholar]
- 51.Fredenburgh JC, Stafford AR, Weitz JI. Conformational changes in thrombin when complexed by serpins. J Biol Chem. 2001; 276: 44828–44834. [DOI] [PubMed] [Google Scholar]
- 52.Li W, Johnson DJ, Adams TE, Pozzi N, De F V, Huntington JA. Thrombin inhibition by serpins disrupts exosite II. J Biol Chem. 2010; 285: 38621–38629. 10.1074/jbc.M110.144964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroh HK, Tans G, Nicolaes GA, Rosing J, Bock PE. Expression of allosteric linkage between the sodium ion binding site and exosite I of thrombin during prothrombin activation. J Biol Chem. 2007; 282: 16095–16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Figueiredo AC, Clement CC, Zakia S, Gingold J, Philipp M, Pereira PJ. Rational design and characterization of D-Phe-Pro-D-Arg-derived direct thrombin inhibitors. PLoS ONE. 2012; 7: e34354 10.1371/journal.pone.0034354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pozzi N, Acquasaliente L, Frasson R, Cristiani A, Moro S, Banzato A et al. beta2 -Glycoprotein I binds to thrombin and selectively inhibits the enzyme procoagulant functions. J Thromb Haemost. 2013; 11: 1093–1102. 10.1111/jth.12238 [DOI] [PubMed] [Google Scholar]
- 56.Weitz JI, Hudoba M, Massel D, Maraganore J, Hirsh J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990; 86: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becker DL, Fredenburgh JC, Stafford AR, Weitz JI. Exosites 1 and 2 are essential for protection of fibrin-bound thrombin from heparin-catalyzed inhibition by antithrombin and heparin cofactor II. J Biol Chem. 1999; 274: 6226–6233. [DOI] [PubMed] [Google Scholar]
- 58.Naski MC, Fenton JWI, Maraganore JM, Olson ST, Shafer JA. The COOH-terminal domain of hirudin. An exosite-directed competitive inhibitor of the action of alpha-thrombin on fibrinogen. J Biol Chem. 1990; 265: 13484–13489. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.