Abstract
The AB5 toxin Shiga toxin 2 (Stx2) has been implicated as a major virulence factor of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains in the progression of intestinal disease to more severe systemic complications. Here, we demonstrate that supernatant from a normal E. coli isolate, FI-29, neutralizes the effect of Stx2, but not the related Stx1, on Vero cells. Biochemical characterization of the neutralizing activity identified the lipopolysaccharide (LPS) of FI-29, a serogroup O107/O117 strain, as the toxin-neutralizing component. LPSs from FI-29 as well as from type strains E. coli O107 and E. coli O117 were able bind Stx2 but not Stx1, indicating that the mechanism of toxin neutralization may involve inhibition of the interaction between Stx2 and the Gb3 receptor on Vero cells.
Escherichia coli O157:H7, a food-borne pathogen, causes approximately 73,000 cases of illness per year in the United States (21). Disease manifestations associated with this pathogen include hemorrhagic colitis (33) and hemolytic-uremic syndrome (HUS) (11), a potentially fatal complication that usually affects children under 10 years of age. Like enteropathogenic E. coli, E. coli O157:H7 possesses the locus of enterocyte effacement (LEE) pathogenicity island which encodes colonization factors, including the ability to promote the formation of attaching and effacing lesions on intestinal epithelial microvilli (32, 36, 42). In addition, E. coli O157:H7, and some other E. coli strains that are like enteropathogenic E. coli, produce the potent virulence factor Shiga toxin (Stx).
Stx is a classic AB5 toxin, with one active (A) subunit bound to a ring of five identical binding (B) subunits. The B pentamer targets the A subunit to host cells that express the glycosphingolipid globotriaosylceramide (Gb3), which has the general structure of Galα(1→4)Galβ(1→4)Glc-ceramide (18), where Gal represents galactose and Glc represents glucose. Specifically, the terminal digalactose moiety of the Gb3 receptor binds the Stx B subunit, resulting in the internalization of the holotoxin, retrograde transport to the endoplasmic reticulum, and subsequent translocation of the A subunit to the cytosol (34). Once inside the cell, the A subunit cleaves out a single adenine residue from the host cell rRNA, halting protein synthesis and leading to cell death (5).
Two major antigenic forms of Stx, Stx1 and Stx2, have been described. DNA and amino acid sequence homology between Stx1 and Stx2 is approximately 55%. Stx-producing E. coli (STEC) strains, including serotype O157:H7, can produce Stx1, Stx2, or both (20). While both Stx1 and Stx2 can cause cellular damage, the progression of E. coli O157:H7 infection to severe disease has been epidemiologically associated with strains capable of producing Stx2 (3, 28), suggesting that Stx2 plays a critical role in the development of HUS. This has been confirmed by use of a baboon model; animals injected with Stx2 developed HUS, while animals that received a similar dose of Stx1 did not develop HUS (35). While the enzymatic function of the A subunit from both Stx1 and Stx2 is identical, the association of Stx2 with severe systemic disease implicates differences in the B pentamer and receptor binding in mediating systemic disease manifestations. The B pentamers of Stx1 and Stx2 both bind to Gb3; however, the binding affinities for the receptor differ. The Kd of Stx1 is 4.6 × 10−8 M, whereas the Kd of Stx2 is 3.7 × 10−7 M (8). Furthermore, a hybrid toxin, in which the A subunit of Stx1 was coupled with the B pentamer of Stx2, displayed toxicities and specificities of the Stx2 B pentamer on Vero and MRC-5 cells, whereas a hybrid toxin containing the Stx2 A subunit with the Stx1 B pentamer displayed the toxicities and specificities of Stx1 (8). It has been suggested that the lower binding affinity of Stx2 compared to Stx1 may allow increased amounts of Stx2 to leave the intestinal tract and gain access to the circulation system and distal organs, resulting in the increased renal and neurological sequelae observed in Stx2-producing STEC infections compared to Stx1 (10).
The genes for the Stx1 and Stx2 A and B subunits are located in the late gene region of a lambdoid bacteriophage lysogenized in the E. coli O157:H7 chromosome (22, 31). Stx is not synthesized during lysogeny; however, entry of the toxin-encoding phage to the lytic cycle results in the production and release of new phage and toxin (23, 39). Conditions that stress the bacteria, including antibiotic therapy, can induce the phage to enter the lytic cycle and, concomitantly, to produce Stx (13). Zhang et al. (43) demonstrated that the administration of the antibiotic ciprofloxacin to mice infected with E. coli O157:H7 resulted in the production of elevated levels of Stx2. A prospective study of children with E. coli O157:H7-related diarrheal disease concluded that antibiotic treatment increased the risk of developing HUS (41). Therefore, the use of antibiotics for the treatment of E. coli O157:H7 infection is questionable, and there is a need to develop therapeutics for controlling E. coli O157:H7-related disease.
Previous studies that examined the interactions of intestinal E. coli and Shiga toxin-encoding phage from E. coli O157:H7 resulted in the discovery of one human isolate, FI-29, that appeared to neutralize the cytotoxic effects of Shiga toxin (7). In this study, we demonstrate that this toxin-neutralizing activity is specific to Stx2 and is a function of the lipopolysaccharide (LPS) type of FI-29.
MATERIALS AND METHODS
Bacterial strains and reagents.
E. coli strains FI-29 and FI-4, isolated from healthy human volunteers, were described previously (7). Serotyping was performed by the Gastroenteric Disease Center at Pennsylvania State University (http://ecoli.cas.psu.edu/). E. coli strains C600, C600::933W (E. coli strain C600 lysogenized with Stx2-converting phage 933W), and C600::H19B (E. coli strain C600 lysogenized with Stx1-converting phage H19B) were obtained from Alison O'Brien (26). E. coli type strains O107 and O117 were obtained from the Gastroenteric Disease Center at Pennsylvania State University. The ECOR E. coli reference strain collection was obtained from the STEC Center (http://foodsafe.msu.edu/whittam/ecor/obtain.html). Typing sera were obtained from Statens Serum Institut (Copenhagen, Denmark). All strains were grown at 37°C on Luria-Bertani (LB) agar or in LB broth with shaking.
Purified Stx1 and Stx2 were obtained from Toxin Technology (Sarasota, Fla.). For binding studies, Stx1 and Stx2 were obtained from filter-sterilized culture supernatants of C600::933W (Stx2) or C600::H19B (Stx1) induced with 30 ng of ciprofloxacin/ml as previously described (7). The amount of toxin in the induced culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA; Premier EHEC ELISA; Meridian Diagnostics, Cincinnati, Ohio).
Determination of LPS core type.
The genetic identity of the genes encoding the core region of LPS was determined by PCR using the primers described by Amor et al. (1) for core types R1, R2, R3, and K-12 and primer set 5′-AAATATGAACATGTACGACGA and R4K14 (1) for core type R4. For R1, R2, R4, and K-12 PCRs, after an initial denaturation step at 94°C for 1 min, 31 cycles of 94°C for 20 s, 55°C for 30 s, and 72°C for 2 min 15 s were done to amplify DNA, followed by a final extension at 72°C for 2 min. For the R3 PCR, the annealing temperature during the 31 cycles was 50°C. PCR products were separated on a 1% agarose gel (SeaKem GTG; BioWhittaker Molecular Applications, Rockland, Maine) and stained with ethidium bromide.
Vero cell assay for detection of Shiga toxin and toxin neutralization.
To examine the neutralization of toxicity to Vero cells, E. coli was grown in LB broth overnight at 37°C with shaking. The cultures were centrifuged (5,000 × g, 10 min), and the filter-sterilized supernatants were incubated with 100 or 250 ng of purified Stx1 or Stx2 per ml at 37°C for 30 min. The Vero cell assay was performed as previously described (7). Briefly, serial twofold dilutions of toxin were made in 25 μl of phosphate-buffered saline (PBS) in a 96-well microtiter plate and overlaid with 100 μl of 105 Vero cells. Plates were incubated at 37°C with 5% CO2 for 3 days. The cells were stained with Giemsa stain, and the 50% cytotoxic dose was determined as the reciprocal of the dilution at which 50% of the Vero cells were dead. The activity of toxin incubated with the inhibitor was compared to untreated toxin. Three independent assays were performed, and the data were analyzed by using Student's t test.
Purification of LPS.
E. coli was grown in 500 ml of LB broth at 37°C overnight with shaking. Cultures were centrifuged (7,500 × g, 20 min), and the hot phenol-water protocol of Westphal and Jann (40) was used to extract LPS from the pellets. Briefly, bacteria were suspended in 60 ml of deionized water, homogenized (Kinematica homogenizer, model PT 10/35, setting 4; Brinkmann Instruments, Inc., Westbury, N.Y.), and centrifuged (7,500 × g, 20 min). The pellet was suspended in 16 ml of water, homogenized, and heated to 65°C in a water bath. An equal volume of phenol at 65°C was added to the suspension and homogenized, and the mixture was incubated on ice for 30 min, followed by centrifugation (7,500 × g, 1 h). The aqueous phase was collected, solid sodium acetate (to 1 mg/ml) and acetone (3 volumes) were added, and the sample was incubated at 4°C overnight. The sample was centrifuged (7,500 × g, 20 min), and the pellet was suspended in 25 ml of 80% acetone, followed by centrifugation (10,000 × g, 15 min). The pellet was suspended in 20 ml of deionized water and centrifuged at 100,000 × g for 16 h. The final pellet of purified LPS was suspended in 1 ml of water and stored at −20°C.
LPS recovery was determined from dried samples, and the amount of LPS in each preparation was also quantified by using the Purpald assay to detect unsubstituted terminal vicinal glycols on 2-keto-3-deoxyoctonate (KDO) and heptose in the LPS inner core (15). The protein content of samples was determined by Coomassie staining or by the Bradford assay (Bio-Rad Laboratories, Inc., Hercules, Calif.).
SDS-PAGE, Stx affinity blots, and Western blots.
Purified LPS (18 μg) was loaded onto a 15% acrylamide gel for Stx2 affinity blots or an 8 to 16% Tris-glycine precast gel (Cambrex Bioscience Rockland Inc., Rockland, Maine) for Stx1 affinity blots and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE of culture supernatants was done by using 8 to 16% Tris-glycine precast gels (Cambrex Bioscience Rockland, Inc.). Gels were run in Tris-glycine buffer (25 mM Tris, 190 mM glycine, 0.1% SDS) by using the Mini-Protean II system (Bio-Rad Laboratories). Gels were silver stained by using a silver stain kit (Bio-Rad Laboratories) to visualize LPS bands.
For affinity blots, bands were transferred onto polyvinylidene difluoride (PVDF) membranes by using a semidry blotting apparatus (Fisher Scientific, Pittsburgh, Pa.). Membranes were blocked in 5% nonfat dry milk for 1 h and washed in buffer (125 mM Na2HPO4, 25 mM NaH2PO4, 100 mM NaCl, 0.5% Tween 20, 0.25% nonfat dry milk). The membranes were incubated for 30 min at room temperature with 5 ml of Stx1 (400 μg/ml) or Stx2 (55 μg/ml) from C600::H19B or C600::933W supernatant, respectively. Membranes were washed and probed with either Stx1 monoclonal antibody 13C4 (American Type Culture Collection, Manassas, Va.) or Stx2 monoclonal antibody 11E10 (American Type Culture Collection), washed, and probed with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Cappel, Aurora, Ohio) at a 1:75,000 dilution.
To detect binding of the O-antigen typing sera, the membranes containing LPS were blocked and incubated with rabbit antibody to O107 and O117 diluted 1:100. The membranes were washed and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Cappel) at a 1:80,000 dilution. All membranes were developed by using a Western Lightning chemiluminescence kit (Perkin-Elmer Life Sciences, Boston, Mass.).
Coprecipitation of LPS and Stx2.
Equal amounts of purified LPS units (36 μM KDO) from FI-29 and FI-4 were incubated at 37°C for 30 min with 10 ml of LB broth or with 10 ml of Stx2 (55 μg/ml) from filter-sterilized 933W supernatant. Ten milliliters of Stx2 incubated without LPS served as a negative control. Samples were centrifuged at 150,000 × g at 4°C for 3 h by using a Sorvall Ti880 fixed-angle rotor, the supernatants were decanted, and pellets were gently washed in 1 ml of PBS and suspended in 50 μl of PBS. If no pellet was observed, the centrifuge tube walls were gently washed with 1 ml of PBS, the wash buffer was discarded, and 50 μl of fresh PBS was added to the tube. Fifteen microliters of sample was loaded per well onto a 12% Tris-glycine precast gel (Cambrex Bioscience Rockland, Inc.), and the presence of Stx2 in the pellet was determined by Western analysis. The relative intensities of the signals were determined by ImageQuant software (version 5.1; Molecular Dynamics, Amersham Biosciences, Piscataway, N.J.).
LPS ELISAs.
ELISAs for LPS were done by using the methods of Chart et al. (4). Purified LPS from FI-29, FI-4, O107, and O117 were diluted in coating buffer (1.59 g of Na2CO3 and 2.93 g of NaHCO3 per liter, pH 9.6) and added to microtiter plates (Pro-Bind; BD Biosciences, Bedford, Mass.) for a final LPS KDO concentration of 38 nM/well and incubated at 37°C for 2 h. Wells were washed in triplicate with PBS containing 0.5% Tween 20 (PBS-Tween) by using an ELX405 microplate washer (Bio-Tek Instruments, Inc., Winooski, Vt.), blocked with 200 μl of 1% bovine serum albumin, and washed again with PBS-Tween. Duplicate wells for each LPS sample were incubated with either wash buffer (negative control) or Stx2 from 933W culture supernatant (15 μg/well) for 30 min at room temperature. Wells were washed with PBS-Tween, incubated with anti-Stx2 monoclonal antibody 11E10 at room temperature for 1 h, washed, and incubated with alkaline phosphatase-conjugated secondary antibody (1:13,000) at room temperature for 1 h. After the wells were washed, p-nitrophenyl phosphate (Fast tablets; Sigma, St. Louis, Mo.) was added and plates were incubated for 15 min in the dark at room temperature. The absorbance (405 nm) was read by using an ELX800 microplate reader (Bio-Tek Instruments, Inc.). For blocking the LPS-Stx2 interactions with specific antisera, the microtiter wells coated with LPS were incubated with antiserum (1:100) for 1 h at room temperature. FI-4 LPS was incubated with O25 antiserum, FI-29 and E. coli O107 were incubated with O107 antiserum, and E. coli O117 was incubated with O117 antiserum. Wells were washed with PBS-Tween, followed by incubation with Stx2 and detection of bound toxin as described above.
RESULTS
Neutralization of Shiga toxin activity on Vero cells.
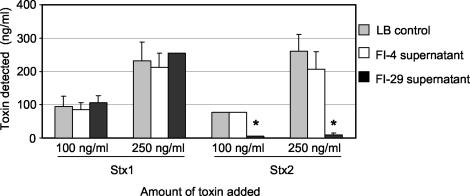
Previous studies suggested that FI-29 could neutralize Shiga toxin (7). To determine if the neutralizing activity was released from the bacteria or if it was present on the bacterial cell, the filter-sterilized supernatant of an overnight culture of FI-29 in LB broth was mixed with 100 or 250 ng of purified Stx1 or Stx2 per ml and incubated at 37°C for 30 min. The amount of active toxin was determined by using the Vero cell assay (Fig. 1). LB broth alone and supernatant from FI-4, an isolate that does not neutralize Shiga toxin (7), were used as negative controls. Supernatant from FI-29 significantly reduced (P < 0.05) the toxicity due to Stx2 compared to the LB medium control for both concentrations (Fig. 1). However, no neutralization of Stx1 was observed at either concentration (Fig. 1). LB and FI-4 supernatant were not able to neutralize the effects of either Stx1 or Stx2 on Vero cells.
FIG. 1.
Effect of E. coli culture supernatants on Stx toxicity to Vero cells. Purified Stx1 or Stx2 was added to culture supernatants to a final concentration of 100 or 250 ng per ml and incubated for 30 min. Vero cells were added and incubated for 3 days. Results are the averages of three trials. An asterisk denotes statistical significance (P < 0.05) compared to the LB medium control.
Biochemical characterization of toxin-neutralizing activity.
Biochemical purification of the neutralizing activity from the culture supernatant of FI-29 was undertaken. Ammonium sulfate was used to precipitate the toxin-neutralizing factor, which precipitated in the 45 to 55% fraction (data not shown). One milliliter of this fraction was loaded onto a fluid-phase liquid chromatography (FPLC) Sephadex 200 column, and 1-ml fractions were collected. The neutralizing activity eluted in the void volume (data not shown), indicating that the size of the factor was >1,000 kDa. Bradford assays did not detect protein in the FPLC fractions with neutralizing activity, suggesting that the activity was not from a protein. Gram-negative bacteria are known to shed vesicles of outer membrane (12). The large size of the neutralizing factor as determined by FPLC suggested that the neutralizing factor was a component of membrane vesicles.
Membrane vesicles can be pelleted at high speeds. FI-29 culture supernatant was centrifuged at 150,000 × g for 2 h. The pellet was suspended in PBS, mixed with 100 ng of Stx2/ml, and incubated with Vero cells. The pellet was able to neutralize Stx2 (data not shown), confirming that the bacterial factor was a component of membrane vesicles. The lack of protein in the neutralizing fractions suggested that the neutralizing activity was not due to enzymatic degradation of Stx2. Rather, we hypothesized that a component of the membrane vesicles was binding Stx2, thereby preventing the toxin from binding to the Gb3 receptor on Vero cells. The most likely candidate for the Stx2 binding component of the membrane vesicles is LPS, a chain of repeating saccharide units attached to a core and anchored to the bacterial outer membrane by lipid A.
Coprecipitation of Stx2 with FI-29 LPS.
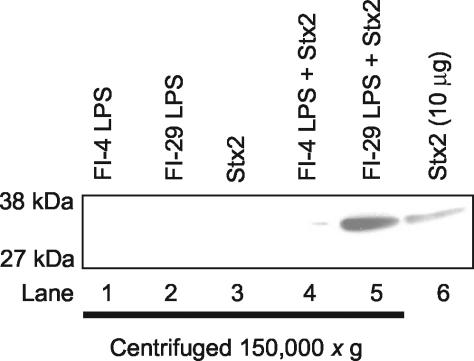
Purified LPS lacking protein was prepared from various bacterial isolates. Stx2 (55 μg/ml) was incubated with FI-29 LPS or FI-4 LPS (negative control) for 30 min at 37°C and centrifuged at 150,000 × g for 3 h to determine if Stx2 could interact with purified LPS. Pellets were suspended in PBS, and Stx2 was detected by Western analysis.
Stx2 did not pellet in the absence of exogenous LPS (Fig. 2, lane 3). Stx2 incubated with LPS from FI-29 was pelleted, as evidenced by a band that comigrated with the Stx2 control (Fig. 2, lane 5). However, very little Stx2 was detected in the pellet of FI-4, the isolate that does not neutralize Stx2 (Fig. 2, lane 4), and the intensity of the signal from Stx2 mixed with FI-4 LPS was less than 2% of the signal detected for Stx2 mixed with FI-29 LPS as determined by ImageQuant analysis. The coprecipitation results suggest that toxin neutralization is due to a direct interaction between the LPS of FI-29 and Stx2 and not degradation of the toxin.
FIG. 2.
Stx2 coprecipitates with FI-29 LPS. LPS from FI-4 and FI-29 alone, Stx2 from an induced culture of C600::933W, or Stx2 from the induced culture with LPS from FI-4 or FI-29 was centrifuged at 150,000 × g. The presence of Stx2 in the pellets was detected by Western analysis with anti-Stx2 monoclonal antibody 11E10. Stx2 (10 μg) was loaded onto the gel as a positive control for Stx2 detection.
Stx2 binding to LPS O-polysaccharide.
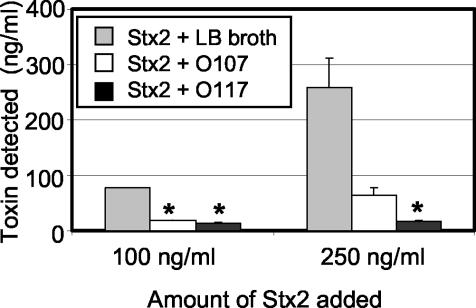
We wanted to determine which region of the LPS of FI-29 binds to Stx2. LPS consists of lipid A, core, and O antigen. The structure of lipid A is conserved in E. coli. E. coli expresses about five core types; however, O antigen is highly variable. The O antigen is formed by the repeated addition of a polysaccharide consisting of up to eight sugars. Presently, approximately 160 LPS types have been identified in E. coli by serology based on the composition of the O polysaccharide (6). The LPSs from FI-4 and FI-29 were serotyped by the Pennsylvania State University Gastroenteric Disease Center. FI-4 expresses type O25, and FI-29 expresses the cross-reacting types O107 and O117. The E. coli type strains for serogroup O107 and O117 were obtained. Culture supernatant from both E. coli O107 and O117 were able to neutralize the effect of 100 ng of Stx2/ml on Vero cells (Fig. 3). However, while both O107 and O117 supernatants were able to reduce the cytotoxic effect of 250 ng of Stx2/ml on Vero cells, only neutralization of this amount of Stx2 by O117 supernatant was statistically significant.
FIG. 3.
Neutralization of Stx2 toxicity to Vero cells by O107 and O117 culture supernatants. Purified Stx2 was added to E. coli O107 or O117 culture supernatants to a final concentration of 100 or 250 ng/ml and incubated for 30 min before incubation with Vero cells for 3 days. Results are the averages of three trials. An asterisk denotes statistical significance (P < 0.05) compared to the LB medium control.
To determine the core types, the strains were typed by PCR. Strains FI-29, O107, and O117 all possessed the genes for the K-12 core type (data not shown). FI-4, which does not neutralize Shiga toxin, possessed the genes for the R1 core type.
The ECOR collection consists of 72 isolates of E. coli from human and animal sources that are thought to be representative of the genetic diversity of the species (27). These strains have been extensively characterized and represent all five core types and more than 30 different O serogroups, but not serogroup O107 and O117 (1). None of the ECOR strains neutralized the cytotoxic effect of Stx1 or Stx2 on Vero cells (data not shown). These results suggest that Stx2 neutralization is not a widespread property of E. coli LPS.
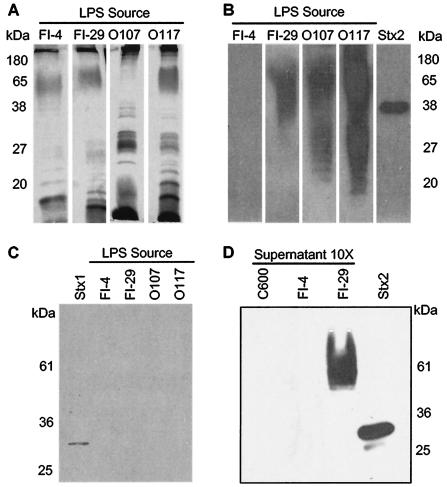
Affinity blot analyses were performed to determine if Stx2 binds to the LPS of the different serogroups that neutralized Stx2 on Vero cells. Purified LPS from FI-4, FI-29, O107, and O117 were separated by SDS-PAGE (Fig. 4A), transferred to a PVDF membrane, and incubated with Stx2. The LPSs from FI-29, O107, and O117 were able to bind Stx2, whereas the LPS from FI-4 did not bind Stx2 (Fig. 4B). The broad band of reactivity with the LPS is consistent with the heterogeneous size of LPS molecules due to the presence of a variable number of O subunits. None of the LPS types were able to bind Stx1 (Fig. 4C), confirming the results from the Vero cell assay and demonstrating that toxin neutralization is specific to Stx2.
FIG. 4.
Characterization of toxin binding to LPS. A silver stain (A) and toxin affinity blots (B, C, and D) of E. coli LPS are shown. (A) Purified LPS was separated by SDS-PAGE, and the bands were silver stained. (B) LPS bands were transferred to PVDF membranes and probed with Stx2. Bound Stx2 was detected by using anti-Stx2 monoclonal antibody 11E10. Stx2 (10 μg) was loaded and used as a positive control. (C) LPS bands were transferred to PVDF membrane and probed with Stx1. Bound Stx1 was detected by using anti-Stx1 monoclonal antibody 13C4. Stx1 (80 μg) was loaded as a positive control. (D) Concentrated (10×) supernatants of C600, FI-4 (negative control), and FI-29 (positive control) were separated by SDS-PAGE, transferred to PVDF membrane, and probed with Stx2. Bound Stx2 was detected with Stx2 monoclonal antibody 11E10.
The toxin-neutralizing strains all expressed the K-12 core type; to exclude core as the toxin binding region, we characterized the laboratory strain C600, which expresses the K-12 core but does not express the O107 or O117 serogroup. C600 does not neutralize Stx2 in the Vero cell assay (data not shown). Affinity blot analyses of culture supernatants were performed (Fig. 4D). FI-29 bound Stx2, whereas C600 did not bind Stx2. These results suggest that Stx2 binding is mediated by the O antigen from the related serogroups O107 and O117 and not the core or lipid A regions of LPS.
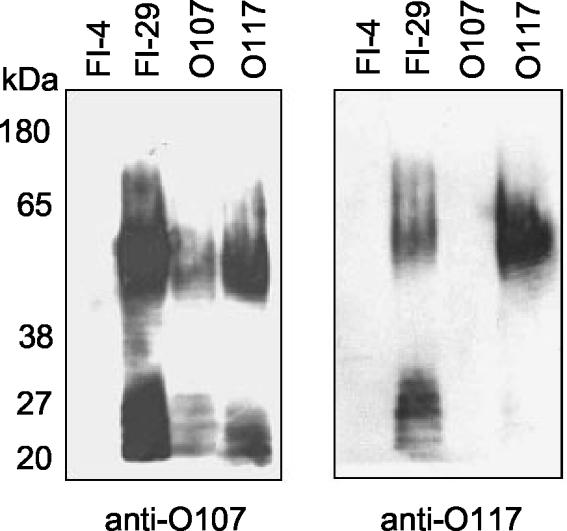
To further characterize the relationship between the LPSs of FI-29 and the O107 and O117 serogroups, O-specific sera were obtained from Statens Serum Institut. Western analysis showed that FI-29 LPS binds both O107 and O117 antisera (Fig. 5). The O107 antiserum bound both E. coli O107 and E. coli O117 LPS, demonstrating the cross-reactivity of these LPS types. In contrast, the O117 antiserum bound the O117 LPS but not the O107 LPS. Initially the antiserum to O117 also bound E. coli O107 LPS; however, the cross-reacting antibodies to O107 had been removed by adsorption by the manufacturer.
FIG. 5.
FI-29 LPS cross-reacts with antibody to serogroups O107 and O117. Purified LPS was separated by SDS-PAGE, and bands were transferred to PVDF membranes. The membranes were probed with anti-O107 or anti-O117 rabbit polyclonal antibody.
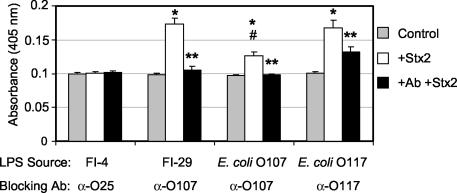
LPS ELISAs were used to further evaluate Stx2 binding to LPS. ELISAs were performed by using hydrophobic microtiter plates to favor the orientation of the LPS so that the lipid A portion would bind the plate and the O polysaccharide would be exposed. The wells were coated with LPS from FI-4, FI-29, O107, and O117 and incubated with Stx2. Stx2 binding was detected by using anti-Stx2 monoclonal antibodies. The LPSs from FI-29, O107, and O117 were able to bind Stx2, as evidenced by a significant (P < 0.005) increase in absorbance compared to that of wells to which no Stx2 was added (Fig. 6, compare gray and white bars). While E. coli O107 LPS did bind Stx2, the binding was significantly (P < 0.02) less than the binding of Stx2 to FI-29 or E. coli O117 LPS (Fig. 6). No increase in absorbance was observed for FI-4, which does not neutralize toxin.
FIG. 6.
Antibodies specific to O107 and O117 block Stx2 binding to LPS. Microtiter wells coated with purified LPS (38 nM KDO/well) were incubated with wash buffer (control, no Stx2), Stx2 (15 μg/well), or type O-specific blocking antibody (Ab, as indicated) followed by Stx2. Stx2 binding was determined by using anti-Stx2 monoclonal antibody 11E10 and alkaline phosphatase-conjugated anti-mouse secondary antibody. Results are the average of at least three trials. For each LPS source, an asterisk denotes a statistically higher (P < 0.005) level of Stx2 detected than that for control samples, and a double asterisk denotes a statistically lower (P < 0.05) level of Stx2 detected compared to wells with LPS and Stx2. Between LPS sources, a pound sign denotes a statistically lower (P < 0.02) level of Stx2 binding by E. coli O107 LPS compared to Stx2 binding by FI-29 and O117 LPS types.
The ability of the serogroup antibody to block toxin binding was assessed by incubating the LPS-coated wells with anti-LPS antibody prior to the addition of Stx2. The increase in absorbance due to Stx2 binding to LPS was inhibited significantly (P < 0.05) for all three LPS types when the LPS was preincubated with the typing antiserum (Fig. 6). For FI-29 and O107 LPSs, the absorbance was reduced to background levels. Stx2 binding to O117 LPS was also significantly reduced; however, the antibody did not completely inhibit toxin binding (Fig. 6). The adsorption of the O117 antiserum with the O107 strain by the manufacturer may have removed some of the blocking activity.
DISCUSSION
We have shown, for the first time, that LPS from a normal intestinal E. coli isolate can bind and neutralize the cytotoxic effects of Stx2 on Vero cells. The O antigen from this isolate, FI-29, was identified by serology as the cross-reacting O107 and O117 types. FI-29 appears to be distinct from O107 based on differences in binding to the O107 and O117 typing antisera (Fig. 5), the increased binding of Stx2 to LPS from FI-29 and O117 compared to LPS of O107 (Fig. 6), and the decreased ability of LPS from O107 to neutralize Stx2 on Vero cells (Fig. 3). This suggests that the toxin-binding properties of O107 and O117 LPS types may be distinct and that the LPS from FI-29 is more similar to that of O117 than of O107.
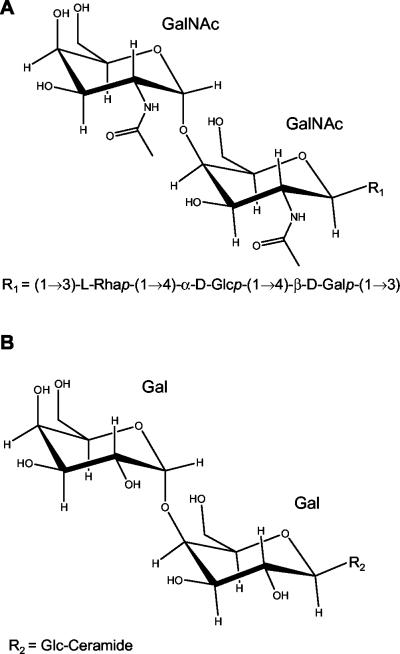
The structure of the O polysaccharide of the E. coli O117 serogroup has previously been determined (16) to be composed of repeating, linear pentasaccharide units (Fig. 7A). Interestingly, this structure has a terminal digalactose moiety, where the galactose residues each possess an N-acetyl substitution. The known Shiga toxin cellular receptor Gb3 (Fig. 7B) also has a terminal digalactose, and the modified digalactose moiety in the O117 LPS may in part be responsible for the ability of this serogroup to bind Stx2. In contrast to Gb3, which binds both Stx1 and Stx2, the FI-29, O107, and O117 LPSs were not able to bind Stx1 or neutralize its effects on Vero cells. Molecular modeling studies (24) and structural analyses of Stx1 with Gb3 (19, 30) and of an Stx2 variant with Gb3 (17) indicate that the two toxins bind the terminal digalactose moiety of the Gb3 differently. Nyholm et al. (25) have shown that Stx1 and Stx2 display different binding preferences for Gb3 analogs lacking different hydroxyl groups. However, both Stx1 and Stx2 bound to analogs lacking the hydroxyl at the 2 position in either sugar of the digalactose, the site that is acetylated in the LPS of serogroup O117. These results suggest that a direct interaction with the hydroxyls at position 2 in the digalactose is not essential for binding of either Stx1 or Stx2 and that the ability of Stx2 but not Stx1 to bind the LPS may be due to the ability of Stx2 to accommodate the larger acetylated sugars. Studies to elucidate the structures of O107 and FI-29 LPS, and how these molecules bind to Stx2, are ongoing.
FIG. 7.
Structures of the O117 O-polysaccharide (A) and Gb3 (B).
Stx binding agents comprised of the Gb3 trisaccharide coupled to inert carrier molecules (2, 14, 37) have been used to treat E. coli O157:H7-related disease. While this type of synthetic agent has shown promise in in vitro studies, thus far, success has not been proven in vivo. In clinical trials, the oral Shiga toxin-binding agent Synsorb-Pk was unable to decrease the severity of disease (9, 38). It is believed that the toxin binders that have been clinically tested thus far have failed because they were not administered early enough after the onset of disease. It is likely that once a patient displays symptoms, it is too late to neutralize the toxin.
In contrast to the synthetic, inert receptor analogs, Paton et al. (29) developed a recombinant E. coli strain that expresses a Neisseria lipooligosaccharide identical to the trisaccharide moiety of Gb3. This bacterium was able to neutralize Stx1 and Stx2 cytotoxicity on Vero cells and, importantly, protected mice from fatal disease. Stx2-binding strains could serve as probiotics. For example, FI-29 was isolated from a healthy infant. If this child became infected with E. coli O157:H7, toxin produced throughout the infection might be neutralized. This is especially important since antibiotic therapy has been linked to the progression of E. coli O157:H7 infection to life-threatening disease (41), leaving few treatment options. While the strains we have identified bind only Stx2 and not Stx1, Stx2 is the toxin type that has been linked to the development of life-threatening disease complications (3, 28). Stx2-binding strains should be able to bind intestinal Stx2, similar to the recombinant toxin-binding strain of E. coli (29). Studies to examine the Stx2-neutralizing activity in disease models, and the nature of the LPS-Stx2 interaction, are under way.
Acknowledgments
We thank John Monaco and Lance Barton for assistance with the FPLC analysis and Cojean Wang for technical assistance.
This work was supported by grant R21-AI-02-008 to A.A.W. and by a University Research Council Summer Fellowship to S.D.G.
REFERENCES
- 1.Amor, K., D. E. Heinrichs, E. Frirdich, K. Ziebell, R. P. Johnson, and C. Whitfield. 2000. Distribution of core oligosaccharide types in lipopolysaccharides from Escherichia coli. Infect. Immun. 68:1116-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, G. D., P. C. Rowe, P. Goodyer, E. Orrbine, T. P. Klassen, G. Wells, A. MacKenzie, H. Lior, C. Blanchard, F. Auclair, et al. 1995. A phase I study of chemically synthesized verotoxin (Shiga-like toxin) Pk-trisaccharide receptors attached to chromosorb for preventing hemolytic-uremic syndrome. J. Infect. Dis. 171:1042-1045. [DOI] [PubMed] [Google Scholar]
- 3.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chart, H., S. M. Scotland, H. R. Smith, and B. Rowe. 1989. Antibodies to Escherichia coli O157 in patients with haemorrhagic colitis and haemolytic uraemic syndrome. J. Clin. Pathol. 42:973-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endo, Y., K. Tsurugi, T. Yutsudo, Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171:45-50. [DOI] [PubMed] [Google Scholar]
- 6.Erridge, C., E. Bennett-Guerrero, and I. R. Poxton. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837-851. [DOI] [PubMed] [Google Scholar]
- 7.Gamage, S. D., J. E. Strasser, C. L. Chalk, and A. A. Weiss. 2003. Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin. Infect. Immun. 71:3107-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Head, S. C., M. A. Karmali, and C. A. Lingwood. 1991. Preparation of VT1 and VT2 hybrid toxins from their purified dissociated subunits. Evidence for B subunit modulation of a subunit function. J. Biol. Chem. 266:3617-3621. [PubMed] [Google Scholar]
- 9.Ito, H., T. Takeda, M. Honda, T. Igarashi, K. Joh, T. Hashizume, and K. Yamaoka. 2002. Preventive effect of TAK-751S on complications of hemorrhagic colitis (results of clinical study of TAK-751S). Jpn. J. Antibiot. 55:203-227. [PubMed] [Google Scholar]
- 10.Jacewicz, M. S., D. W. Acheson, D. G. Binion, G. A. West, L. L. Lincicome, C. Fiocchi, and G. T. Keusch. 1999. Responses of human intestinal microvascular endothelial cells to Shiga toxins 1 and 2 and pathogenesis of hemorrhagic colitis. Infect. Immun. 67:1439-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karmali, M. A., B. T. Steele, M. Petric, and C. Lim. 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet i:619-620. [DOI] [PubMed] [Google Scholar]
- 12.Kesty, N. C., and M. J. Kuehn. 2004. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 279:2069-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimmitt, P. T., C. R. Harwood, and M. R. Barer. 2000. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6:458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitov, P. I., J. M. Sadowska, G. Mulvey, G. D. Armstrong, H. Ling, N. S. Pannu, R. J. Read, and D. R. Bundle. 2000. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 403:669-672. [DOI] [PubMed] [Google Scholar]
- 15.Lee, C. H., and C. M. Tsai. 1999. Quantification of bacterial lipopolysaccharides by the Purpald assay: measuring formaldehyde generated from 2-keto-3-deoxyoctonate and heptose at the inner core by periodate oxidation. Anal. Biochem. 267:161-168. [DOI] [PubMed] [Google Scholar]
- 16.Leslie, M. R., H. Parolis, and L. A. Parolis. 2000. The structure of the O-specific polysaccharide of Escherichia coli O117:K98:H4. Carbohydr. Res. 323:103-110. [DOI] [PubMed] [Google Scholar]
- 17.Ling, H., N. S. Pannu, A. Boodhoo, G. D. Armstrong, C. G. Clark, J. L. Brunton, and R. J. Read. 2000. A mutant Shiga-like toxin IIe bound to its receptor Gb(3): structure of a group II Shiga-like toxin with altered binding specificity. Structure Fold. Des. 8:253-264. [DOI] [PubMed] [Google Scholar]
- 18.Lingwood, C. A. 1999. Glycolipid receptors for verotoxin and Helicobacter pylori: role in pathology. Biochim. Biophys. Acta 1455:375-386. [DOI] [PubMed] [Google Scholar]
- 19.Lingwood, C. A. 2003. Shiga toxin receptor glycolipid binding. Pathology and utility. Methods Mol. Med. 73:165-186. [DOI] [PubMed] [Google Scholar]
- 20.Mead, P. S., and P. M. Griffin. 1998. Escherichia coli O157:H7. Lancet 352:1207-1212. [DOI] [PubMed] [Google Scholar]
- 21.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neely, M. N., and D. I. Friedman. 1998. Arrangement and functional identification of genes in the regulatory region of lambdoid phage H-19B, a carrier of a Shiga-like toxin. Gene 223:105-113. [DOI] [PubMed] [Google Scholar]
- 23.Neely, M. N., and D. I. Friedman. 1998. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of Shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol. Microbiol. 28:1255-1267. [DOI] [PubMed] [Google Scholar]
- 24.Nyholm, P. G., J. L. Brunton, and C. A. Lingwood. 1995. Modelling of the interaction of verotoxin-1 (VT1) with its glycolipid receptor, globotriaosylceramide (Gb3). Int. J. Biol. Macromol. 17:199-204. [DOI] [PubMed] [Google Scholar]
- 25.Nyholm, P. G., G. Magnusson, Z. Zheng, R. Norel, B. Binnington-Boyd, and C. A. Lingwood. 1996. Two distinct binding sites for globotriaosyl ceramide on verotoxins: identification by molecular modelling and confirmation using deoxy analogues and a new glycolipid receptor for all verotoxins. Chem. Biol. 3:263-275. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien, A. D., J. W. Newland, S. F. Miller, R. K. Holmes, H. W. Smith, and S. B. Formal. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694-696. [DOI] [PubMed] [Google Scholar]
- 27.Ochman, H., and R. K. Selander. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostroff, S. M., P. I. Tarr, M. A. Neill, J. H. Lewis, N. Hargrett-Bean, and J. M. Kobayashi. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 160:994-998. [DOI] [PubMed] [Google Scholar]
- 29.Paton, A. W., R. Morona, and J. C. Paton. 2000. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat. Med. 6:265-270. [DOI] [PubMed] [Google Scholar]
- 30.Picking, W. D., J. A. McCann, A. Nutikka, and C. A. Lingwood. 1999. Localization of the binding site for modified Gb3 on verotoxin 1 using fluorescence analysis. Biochemistry 38:7177-7184. [DOI] [PubMed] [Google Scholar]
- 31.Plunkett, G., III, D. J. Rose, T. J. Durfee, and F. R. Blattner. 1999. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J. Bacteriol. 181:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posfai, G., M. D. Koob, H. A. Kirkpatrick, and F. R. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 34.Sandvig, K., and B. van Deurs. 1996. Endocytosis, intracellular transport, and cytotoxic action of Shiga toxin and ricin. Physiol. Rev. 76:949-966. [DOI] [PubMed] [Google Scholar]
- 35.Siegler, R. L., T. G. Obrig, T. J. Pysher, V. L. Tesh, N. D. Denkers, and F. B. Taylor. 2003. Response to Shiga toxin 1 and 2 in a baboon model of hemolytic uremic syndrome. Pediatr. Nephrol. 18:92-96. [DOI] [PubMed] [Google Scholar]
- 36.Sperandio, V., J. B. Kaper, M. R. Bortolini, B. C. Neves, R. Keller, and L. R. Trabulsi. 1998. Characterization of the locus of enterocyte effacement (LEE) in different enteropathogenic Escherichia coli (EPEC) and Shiga-toxin producing Escherichia coli (STEC) serotypes. FEMS Microbiol. Lett. 164:133-139. [DOI] [PubMed] [Google Scholar]
- 37.Takeda, T., K. Yoshino, E. Adachi, Y. Sato, and K. Yamagata. 1999. In vitro assessment of a chemically synthesized Shiga toxin receptor analog attached to chromosorb P (Synsorb Pk) as a specific absorbing agent of Shiga toxin 1 and 2. Microbiol. Immunol. 43:331-337. [DOI] [PubMed] [Google Scholar]
- 38.Trachtman, H., A. Cnaan, E. Christen, K. Gibbs, S. Zhao, D. W. Acheson, R. Weiss, F. J. Kaskel, A. Spitzer, and G. H. Hirschman. 2003. Effect of an oral Shiga toxin-binding agent on diarrhea-associated hemolytic uremic syndrome in children: a randomized controlled trial. JAMA 290:1337-1344. [DOI] [PubMed] [Google Scholar]
- 39.Wagner, P. L., M. N. Neely, X. Zhang, D. W. Acheson, M. K. Waldor, and D. I. Friedman. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 183:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure, p. 83-91. In R. L. Whistler (ed.), Methods in carbohydrate chemistry, vol. 5. Academic Press, Inc., New York, N.Y.
- 41.Wong, C. S., S. Jelacic, R. L. Habeeb, S. L. Watkins, and P. I. Tarr. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu, J., and J. B. Kaper. 1992. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 6:411-417. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, X., A. D. McDaniel, L. E. Wolf, G. T. Keusch, M. K. Waldor, and D. W. Acheson. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181:664-670. [DOI] [PubMed] [Google Scholar]