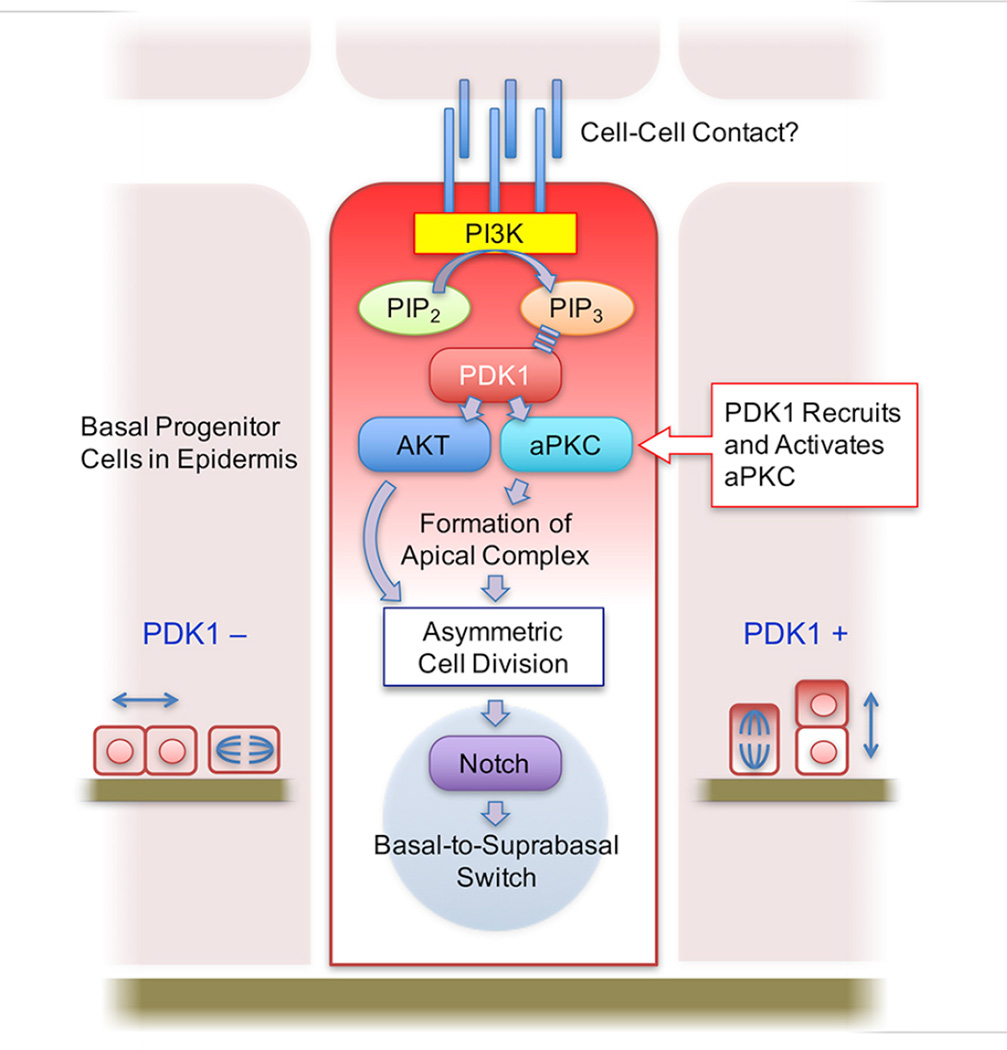
SUMMARY
Asymmetric cell division (ACD) in a perpendicular orientation promotes cell differentiation and organizes the stratified epithelium. However, the upstream cues regulating ACD have not been identified. Here we report that phosphoinositide-dependent kinase 1 (PDK1) plays a critical role in establishing ACD in the epithelium. Production of phosphatidylinositol triphosphate (PIP3) is localized to the apical side of basal cells. Asymmetric recruitment of atypical protein kinase C (aPKC), and partitioning defective (PAR) 3, are impaired in PDK1 conditional knockout (CKO) epidermis. PDK1CKO keratinocytes do not undergo calcium-induced activation of aPKC or IGF1-induced activation of AKT and fail to differentiate. PDK1CKO epidermis shows decreased expression of Notch, a downstream effector of ACD, and restoration of Notch rescues defective expression of differentiation-induced Notch targets in vitro. We therefore propose that PDK1 signaling regulates the basal-to-suprabasal switch in developing epidermis by acting as both an activator and organizer of ACD and the Notch-dependent differentiation program.
Graphical Abstract
INTRODUCTION
Generation of three-dimensional tissues with different cell types characterizes the development of all organs. This process is triggered by intrinsic or extrinsic cues, and is coupled to the generation of different cells from common progenitors through a process known as asymmetric cell division (ACD) (Knoblich, 2010). ACD drives the development and differentiation of the epidermis in mammals (Ray and Lechler, 2011; Williams et al., 2011), where a balance between symmetric and asymmetric divisions generates a tissue of the correct surface area and thickness. The differentiation of the epidermis begins with the stem cells located within the basal layer (Fuchs, 2009), and ACD in a perpendicular orientation relative to the basement membrane promotes cell differentiation mediated by several transcriptional regulators and organizes the stratified epithelium (Arnold and Watt, 2001; Hu et al., 1999; Lopez et al., 2009; Mills et al., 1999; Rangarajan et al., 2001; Takeda et al., 1999; Wang et al., 2008). However, both the molecular cues that trigger organization of the apical complex during ACD, and the signaling pathways that drive activation of apical complex components, remain to be defined.
Phosphoinositide dependent kinase 1 (PDK1) is a serine/threonine kinase of the AGC kinase group. The kinase activity of PDK1 depends on phosphatidyl inositol 3-kinase (PI3K), a key intermediate in signaling pathways including those from growth factor receptors and adhesion molecules. Substrates of PDK1, including AKT and the protein kinase C (PKC) isozymes, regulate a number of essential cell functions (Pearce et al., 2010). In particular, atypical PKC (aPKC) is involved in cell polarity and ACD (Knoblich, 2010). However, in mammalian epidermis, the role of aPKC remains unclear. There are two aPKC isozymes in mammals, PKCζ and PKCλ. Loss of PKCζ reportedly has no effect on epidermal differentiation (Leitges et al., 2001). In contrast, epidermal loss of PKCλ results in disruption of ACD, but with enhanced ACD and defective stem cell homeostasis (Niessen et al., 2013). However, in these studies, conformation of the apical complex, which is a critical cellular event at the beginning of ACD, was not affected by the absence of PKCλ as partitioning defective (PAR) 3 and other components were still recruited to the apical complex. These findings suggest either redundancy between aPKC isozymes or aPKC-independent mechanisms of apical complex assembly and ACD in epidermis.
In addition to phosphorylating PKC proteins, PDK1 may also facilitate the function of PKC proteins by acting as a scaffold molecule bridging PKC and downstream substrates. During T cell receptor signaling, which is a highly polarized signaling process that can trigger ACD (Chang et al., 2007), PDK1 facilitates signaling by acting as a structural platform that activates PCKθ and links PKCθ to downstream substrates (Lee et al., 2005; Park et al., 2009). Interestingly, a small molecule screening study suggested that activation of PDK1 enhances ES cell reprogramming (Zhu et al., 2010). Therefore, although the role of PDK1 in ACD and cell differentiation had not been previously investigated, we hypothesized that PDK1 might serve as a key organizer of the apical complex during ACD. We therefore investigated the function of PDK1 through conditional deletion of PDK1 in the epidermis.
We now report that PDK1 plays a critical role in the establishment of ACD in the epidermis. We proposed that apical signaling triggers PI-3 kinase leading to the asymmetric accumulation of the lipid effector phosphatidyl inositol triphosphate (PIP3). Enrichment of PIP3 at the apical side also leads to recruitment and activation of PDK1, thus establishing an asymmetric signaling pathway in differentiating cells. Deletion of PDK1 abolishes ACD and both activation of downstream signaling pathway components including AKT, glycogen synthase kinase (GSK)-3β, atypical protein kinase C (aPKC), and polarization of components of the apical complex. Thus, PDK1 is essential for both activation and asymmetric organization of key regulators of ACD. Consequently, loss of PDK1 leads to dramatically attenuated differentiation and stratification of the epidermis, with disrupted barrier function and perinatal lethality.
RESULTS AND DISCUSSION
PDK1 Has a Non-Redundant Role in Epidermal Differentiation and Stratification
To test whether PDK1 has any role in keratinocyte differentiation, we generated epidermis-specific PDK1 knockout mice using K14-Cre. While PDK1Δ/+ epidermis from K14-Cretg/+ PDK1Flox/+ showed a wild-type phenotype, K14-Cretg/+ PDK1Flox/Flox (PDK1CKO) mice exhibited thin and shiny epidermis (Figure 1A). They showed hypoplasia of vibrissae although they still developed rudimentary vibrissal follicles. PDK1CKO mice were unable to ingest milk, although we did not detect any cleft palate or obstruction in esophagus by dye inoculation test or histology. The PDK1CKO mice died within several hours of birth, which, based on gross appearance we suspected was due to excessive transepidermal water loss. An outside-in dye exclusion assay revealed that the barrier function of PDK1 deficient epidermis was impaired (Figure 1B). Histology of skin specimens showed that the stratification of epidermis from PDK1CKO was dramatically attenuated at embryonic day 17.5 (E17.5) although the development of wild-type and PDK1CKO epidermis was comparable at E14.5 (Figure 1C). This reduction of thickness in PDK1CKO epidermis was also quantitatively evaluated (Figure 1D). Expression of PDK1 was nearly abolished in epidermis from PDK1CKO mice (Figure 1E), suggesting that the phenotype was due to the absence of PDK1 in the epidermis.
Figure 1. Phenotype of PDK1 deficient epidermis.
A, Gross appearance of K14-Cretg/+ PDK1Flox/+ wild-type (top) and K14-Cretg/+ PDK1Flox/Flox CKO E17.5 embryos (bottom). B, Dye exclusion assay of E17.5 embryos. Blue staining shows impaired epidermal barrier function of PDK1CKO (right) compared to the wild-type (left). C, Histology of dorsal skin from E14.5 and E17.5 embryos with H&E staining, wild-type (top) and PDK1CKO (bottom) in which hair follicles appeared to be arrested in hair peg stage on E17.5; all scale bars, 50 µm. D, Thickness of epidermis from wild-type (white column, N = 30) and PDK1CKO E17.5 embryo (black, N = 45); error bars, s.d. E, PDK1 gene expression from newborn epidermis of PDK1Flox/Flox (F/F, white column), K14-Cretg/+ PDK1Flox/+ (Δ/+, gray) and K14-Cretg/+ PDK1Flox/Flox (Δ/Δ, black) mice evaluated by quantitative PCR; error bars, s.d. (N = 3). F, IF of wild-type (top) and PDK1CKO (bottom) mouse dorsal skin on E17.5, performed with anti-keratin-14, keratin-1 or loricrin (red signals, respectively) and anti-laminin antibodies (green) and DAPI staining (blue). White dashed lines indicate basement membrane; all scale bars, 50 µm. G, Gene expression profile from newborn epidermis of wild-type (white columns) and PDK1CKO (black) mice evaluated by quantitative PCR; error bars, s.d. (N = 4).
Immunofluorescence (IF) analysis revealed that the thin epidermis of PDK1CKO mice expressed normal levels of a basal cell marker keratin-14, whereas expression of a spinous cell marker, keratin-1, and a granular cell marker, loricrin, were markedly decreased (Figure 1F). The PDK1CKO epidermis expressed keratin-6, which is prominent in regeneration and pathologic states (Figure S1A). However, no clear change in cellularity of the basal cell layer was detected (Figure 1F). Furthermore, we were unable to detect a significant decrease in cell proliferation or increase in apoptosis in the inter-follicular epidermis (IFE) of PDK1CKO (Figures S1B and C), consistent with findings in PDK1-null embryos (Lawlor et al., 2002). Analysis of cell number during primary culture was comparable between wild-type and PDK1 deficient keratinocytes (Figure S1D). However the size of PDK1 deficient keratinocytes was smaller than that of wild-type cells (Figure S1E), consistent with previous observations in other tissues (Hashimoto et al., 2006; Lawlor et al., 2002).
We used quantitative PCR to analyze expression of genes implicated in epidermal differentiation. Consistent with the IF results, expression of a spinous cell marker, keratin-10, and granular cell markers, involucrin and loricrin, were markedly suppressed in PDK1 deficient mice (Figures 1G and S1F). Expression of Notch, an essential regulator of keratinocyte differentiation following ACD (Rangarajan et al., 2001; Williams et al., 2011), and its transcriptional target HES1, were also suppressed at the mRNA level (Figures 1G and S1F). In contrast, expression of ΔNp63, a dominant negative isoform of p63, that represses key genes and maintains basal cells in the progenitor state (Candi et al., 2007; Fuchs, 2009), was slightly increased in PDK1CKO epidermis (Figure 1G), likely attributable to a decrease in ΔNp63low, differentiated cells in the PDK1CKO epidermis. However levels of other transcriptional regulators of keratinocytes such as IκB kinase α (IKKα) (Hu et al., 1999; Takeda et al., 1999), AP-2 (Wang et al., 2008) or C/EBP (Lopez et al., 2009) were not affected (Figure S1F). Taken together, these results suggest that PDK1 is necessary for differentiation of basal progenitor cells into suprabasal spinous cells, whereas development of basal keratinocytes and organization of the basal layer are PDK1-independent. As a result, in the absence of PDK1 epidermal development is arrested at approximately the E14.5 developmental stage.
Basal Cell Asymmetric Division is Decreased in PDK1CKO Epidermis
To further analyze the defect in PDK1CKO epidermis, we evaluated cell division in wild-type and PDK1CKO skin specimens with hematoxylin and eosin (H&E) staining. Consistent with a previous study (Lechler and Fuchs, 2005), normal keratinocyte differentiation and stratification from basal cells occurred in an asymmetric manner, however in E17.5 PDK1 deficient epidermis, most cell divisions were symmetric - in a horizontal orientation (Figures 2A and B). Surprisingly, despite the increased rate of symmetric division of basal cells in vivo and normal proliferation of PDK1CKO keratinocytes in vitro, we were unable to detect significant changes in the cellularity of the basal layer. Given the defect in ACD in PDKCKO epidermis, we examined the distribution of PDK1 in dividing basal cells in wild-type mice. Although we could not detect significant polarization of PDK1 in metaphase or anaphase cells (Figure S2A), in telophase basal cells that were undergoing division in a perpendicular orientation, PDK1 was enriched in the apical daughter cells (Figure 2C). In cells dividing in a horizontal orientation, in contrast, PDK1 was symmetrically distributed to both daughter cells. Hence, our results suggest that PDK1 is required for ACD in a vertical orientation, perpendicular to the basement membrane, and that PDK1 is enriched in the resulting apical daughter cell.
Figure 2. Defective asymmetric cell division in PDK1 deficient epidermis.
A, Representative basal cell mitoses in dorsal epidermis from wild-type (top) and PDK1CKO (bottom) E17.5 embryo with H&E staining. Yellow lines indicate spindle orientation in anaphase (left) and telophase (middle); all scale bars, 10 µm. The spindle orientation relative to the basement membrane was observed in multiple fields of view and is presented graphically (right). B, Percentages of cell divisions in wild-type and PDK1CKO epidermis (3 each) that were either asymmetric or perpendicular to basement membrane (90° ± 30°; black bars); symmetric or parallel to basement membrane (0° ± 30°; white), or other (grey) were plotted. C, IF of actively dividing cells (circled with yellow dotted lines) in dorsal epidermis from 3 wild-type E17.5 embryo with anti-PDK1 (red signals) and γ-tubulin antibodies (green) with DAPI staining (blue). γ-tubulin signals indicate centrosomes in telophase. White dashed lines indicate basement membrane; all scale bars, 10 µm. Distribution of PDK1 signals in 113 pairs of wild-type dividing cells in telophase (91 in perpendicular, 22 in parallel) was quantified and graphed (right); blue bars, medians.
PIP3-PDK1-aPKC Signaling at the Apical Side Cues Asymmetric Cell Division
PDK1 is a key intermediate in signaling downstream of PI3K (Pearce et al., 2010). Cell-cell contact stimuli, governed by cadherin and desmoglein molecules, are one, though likely not the only, candidate for activating the PI3K pathway in keratinocytes (Calautti et al., 2005). Indeed, we found that PDK1 and E-cadherin are co-localized at sites of cell-cell contact in wild-type E17.5 dorsal epidermis (Figure S2B). E-cadherin directly activates PI3K and induces keratinocyte differentiation in a calcium-dependent manner (Calautti et al., 2005). Cell-cell contact through E-cadherin is also required for germline stem-cell spindle orientation in Drosophila (Inaba et al., 2010). While depletion of PDK1 in epidermis did not affect the expression of the E-cadherin gene (Figure 1G), phosphorylation of AKT, a substrate of PDK1, was, as expected, absent in dorsal epidermis of newborn mice (Figure 3A). Consistent with the distribution of E-cadherin and with previous studies (Calautti et al., 2005), immunofluorescence revealed that phosphorylated AKT and, also, PDK1, were localized at cell-cell contact sites in newborn wild-type suprabasal cells in vivo. Phosphorylation of AKT at cell-cell contact sites was also present in vitro (Figure S2D).
Figure 3. Absence of molecular cues for asymmetric cell division in PDK1CKO epidermis.
A, IF of wild-type (left) and PDK1CKO (right) newborn dorsal epidermis. Anti-phospho-PDK1 (top) and phospho-AKT (bottom) demonstrate red signals at cell-cell contact sites in the wild-type epidermis compared to those of isotype control (Figure S2C). Laminin (green) and DAPI staining (blue), scale bars, 20 µm. B, IF of basal keratinocytes from wild-type (left) and PDK1CKO (right) E17.5 dorsal epidermis with anti-PIP3 (red signals at apical side of basal cells, indicated by arrows) and laminin (green) antibodies with DAPI staining (blue). Note that PIP3 signals are not overlapped with laminin signals at basal side in high power views (right panels); scale bars, 20 µm (left panels), 10 µm (right). C, IF of wild-type dorsal epidermis from E14.5 embryo (top) demonstrating apical distribution of aPKC, PAR3 and PDK1 in basal cells (red signals indicated by arrows), while they are impaired in PDK1CKO epidermis (bottom). Laminin (green) and DAPI staining (blue), scale bars, 20 µm. White dashed lines indicate basement membrane.
Besides cell-cell contact stimuli, cell-matrix stimuli from the basement membrane through molecules such as laminin could also activate the integrin-PI3K pathway (Pearce et al., 2010) in basal progenitor cells. To identify the crucial cues for PDK1-dependent ACD in epidermis, we evaluated distribution of PIP3 in basal cells. We found that PIP3 is predominantly localized to the apical, but not the basolateral side, of basal cells in wild-type epidermis (Figure 3B). PIP3 was also predominantly produced at the apical side of basal cells in PDK1CKO E17.5 epidermis (Figure 3B), suggesting that apical activation of PI3-kinase and the upstream cell-cell contact stimuli are maintained in the basal cells even in the absence of PDK1. An apical distribution of PIP3 was also detected in E14.5 basal cells (Figure S2E).
We had previously found that PDK1 was essential for polarized recruitment of PKCθ to macromolecular signaling complexes during T cell activation (Lee et al., 2005). Although loss of PKCλ in keratinocytes does regulate spindle orientation, intrafollicular basal cells lacking PKCλ display increased ACD (Niessen et al., 2013). However, it is not clear if this finding is a result of compensation by PKCζ (Vorhagen and Niessen, 2014), as PKCζ is upregulated in keratinocytes lacking PKCλ (Niessen et al., 2013). Consistent with some method of compensation, no changes were observed in PAR3 localization in keratinocytes lacking PKCλ (Niessen et al., 2013). Therefore, we investigated the distribution of aPKC and other known components of the apical complex. Indeed, despite normal PIP3 distribution, localization of the PDK1 substrates aPKC and PAR3 to the apical side of E14.5 basal cells was impaired in PDK1CKO epidermis (Figures 3C and S2E). These results suggest that the epidermal differentiation and stratification in the PDK1CKO was defective at E14.5 and earlier (Fig. 1C). Notably, PDK1 exhibited a clear apical distribution in the wild-type, interphase basal cells (Figure 3C), suggesting that asymmetric PDK1 localization is not a result of, but precedes, asymmetric spindle orientation.
The distribution of aPKC is consistent with the idea that aPKC forms an apical complex early during ACD, though it is unclear whether aPKC kinase activity is required for PAR3 to enter the apical complex (Knoblich, 2010) or aPKC recruitment is mediated by PAR3 (Rodriguez-Boulan and Macara, 2014). In either case, PDK1 is required for the recruitment of both components of the apical complex. These results suggest that the molecular cues for ACD in basal progenitor cells are provided from the apical side through localized PI3K activation and PDK1 recruitment. Consequently, both downstream signaling pathways and the assembly of the apical complex are abolished in PDK1CKO epidermis.
Lack of Calcium-Induced aPKC Phosphorylation and Differentiation in PDK1CKO Keratinocytes
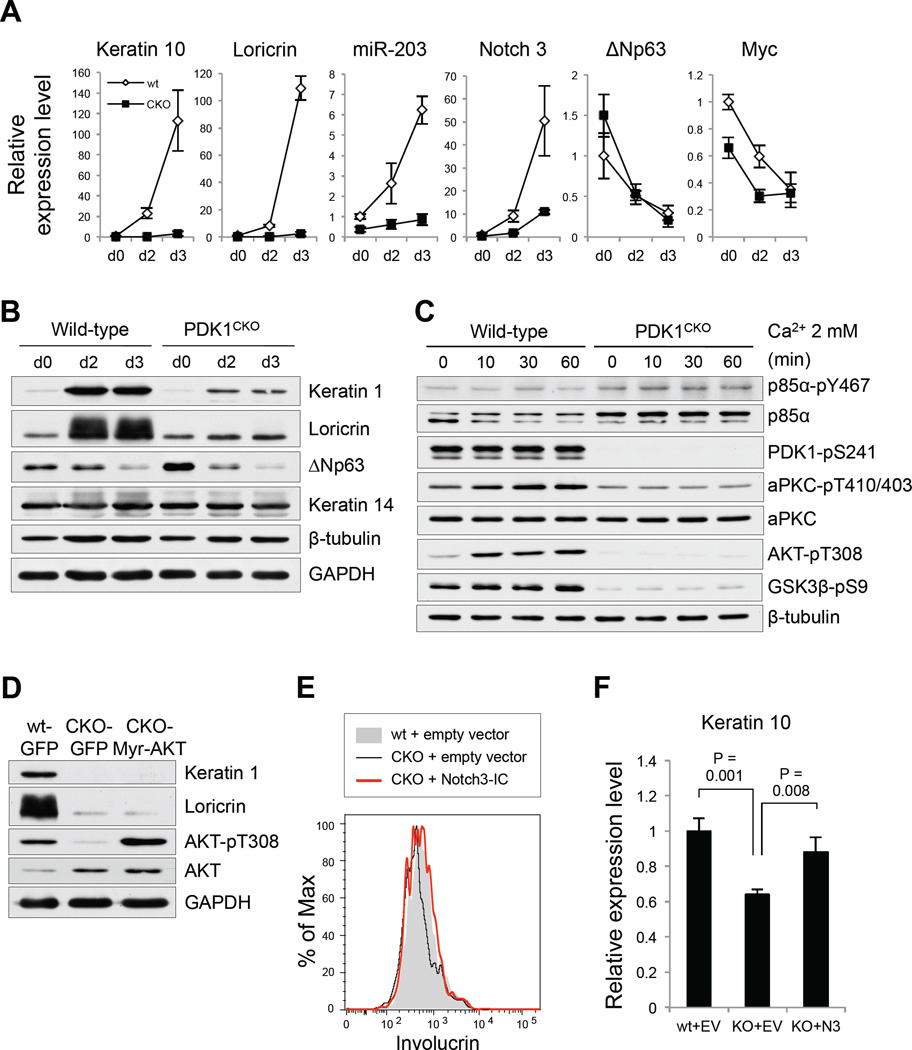
To further characterize the defect in PDK1 deficient keratinocytes, we evaluated the response of PDK1CKO keratinocytes to calcium in vitro. Switching experimentally from low to high calcium induces cell-cell contact mediated by cadherin molecules and provides a cue for cell differentiation in keratinocytes (Calautti et al., 2005; Hennings et al., 1980). Quantitative PCR showed that keratin-10 and loricrin gene expression was not induced by high-calcium in PDK1-deficient keratinocytes (Figure 4A). Induction of Notch expression was also significantly impaired in PDK1-deficient keratinocytes (Figure 4A). Induction of HES1 by calcium stimulation was not detected in wild-type or PDK1 keratinocytes in vitro (Figure S3A), suggesting that Notch-induced keratinocyte differentiation is not fully RBP-Jκ-HES1-dependent (Rangarajan et al., 2001). At the protein level, keratin-1 and loricrin expression was also not induced in PDK1-deficient keratinocytes (Figure 4B). Micro RNA (miR)-203 regulates keratinocyte differentiation by inhibiting ΔNp63 expression (Lena et al., 2008; Yi et al., 2008), which maintains stemness through its transcriptional target genes (Candi et al., 2007; Fuchs, 2009). MiR-203 expression in wild-type keratinocytes is induced by high-calcium concentration, but remarkably there was no induction of miR-203 expression in PDK1 deficient keratinocytes (Figure 4A). However, downregulation of ΔNp63 by calcium was unaffected at both the transcriptional and protein levels (Figures 4A, B and S3B), and miR-203 transfection did not rescue the PDK1 deficiency (Figure S3C). Expression of Myc (Arnold and Watt, 2001) and its target Misu was attenuated in PDK1CKO, but their levels were comparable after high-calcium culture (Figures 4A and S3A), suggesting that the decreased expression of these regulatory molecules is not responsible for the phenotype in PDK1CKO keratinocytes.
Figure 4. PDK1-dependent signaling in primary cultured mouse keratinocytes.
A, Keratinocytes from wild-type (wt) and PDK1CKO mice (CKO) grown in 0.05 mM Ca2+ were then cultured with 1.3 mM Ca2+. Gene expression was evaluated by quantitative PCR at the indicated days; error bars, s.d. (N = 3). B, Protein expression from wild-type and PDK1CKO keratinocytes was evaluated by Western blotting at the indicated days of culture with 1.3 mM Ca2+C, Wild-type and PDK1CKO keratinocytes cultured with 0.05 mM Ca2+ were stimulated with 2 mM Ca2+ after 24 h starvation of serum. Cells were lysed at the indicated timings and the protein expression and their phosphorylation levels were evaluated by Western blotting (additional results are presented in Figure S3D). D, WT keratinocytes transfected with GFP adenovirus, and CKO keratinocytes transfected with GFP or Myr-AKT adenovirus, were cultured with 1.3 mM Ca2+ for 2 days. Protein expression and phosphorylation levels were evaluated by Western blotting. E, Flow cytometry for involucrin expression. GFP+ wt and CKO keratinocytes were cultured with 1.3 mM Ca2+ for 3 days after transfection with IRES-GFP empty vector or that with Notch3 intracellular (IC) domain. F, Keratin 10 gene expression from GFP+ wt and CKO keratinocytes that were cultured with 1.3 mM Ca2+ for 3 days after transfection with IRES-GFP empty vector (EV) or that with Notch3 intracellular domain (N3); error bars, s.d. (N = 3).
As expected, PI3K phosphorylation was normal in PDK1CKO keratinocytes (Figure 4C). Although aPKC is a calcium-independent kinase, PIP3 stimulates aPKC kinase activity in the presence of calcium (Nakanishi et al., 1993), most likely through PDK1. Consistent with this model, we found that calcium rapidly induces phosphorylation of aPKC, in wild-type keratinocytes although it is not as rapid as AKT phosphorylation (Figure 4C). In PDK1 deficient keratinocytes, phosphorylation of AKT, GSK3β and aPKC was reduced or completely abolished (Figure 4C). Insulin and insulin-like growth factor (IGF)-1 signaling has been implicated ACD, and mice lacking insulin and IGF-1 receptors exhibit a phenotype similar to the PDK1CKO mice (Gunschmann et al., 2013). Consistent with the known role of PDK1, IGF-1 and epidermal growth factor (EGF) -induced phosphorylation of AKT and GSK3β was also completely abolished in PDK1 deficient keratinocytes (Figures S3E and F).
A small molecule AKT inhibitor, AKTi-1/2, inhibited calcium induced differentiation of keratinocytes in vitro similar to the reduction observed using a PDK1 inhibitor (Figures S4A and B). These results suggest that AKT could be the key target of PDK1 in keratinocyte differentiation. We, therefore, tested whether we could rescue the defect in PDK1CKO keratinocyte differentiation through activation of AKT. We transfected PDK1CKO keratinocytes with constitutively active AKT (Myr-AKT), which resulted in robust activation of AKT (Figure 4D). However, AKT activation did not rescue calcium-induced differentiation of PDK1CKO keratinocytes (Figure 4D), suggesting that the AKT pathway is not sufficient to promote differentiation in the absence of PDK1. These results are consistent with observations made in AKT1/2 knockout mice (Peng et al., 2003), which exhibit a stratified, though thin epidermis with expression of filaggrin and keratin10. In fact AKT has been shown to have an inhibitory role in keratinocyte differentiation by inducing ΔNp63 in response to EGF (Barbieri et al., 2003). These results suggest that impaired differentiation of PDK1 deficient keratinocytes is not solely due to effects on AKT, but rather likely due to combinatorial defects in the activation of AKT as well as aPKC.
Activation of Notch Rescues Differentiation Defect of PDK1CKO Keratinocytes
Unfortunately, we were unable to create a constitutively active form of aPKC that would allow us to overcome both impaired phosphorylation, and impaired localization, in the absence of PDK1. Two molecules have been suggested to be specific substrates of aPKC: lethal giant larvae (LGL) and Numb (Knoblich, 2010). The asymmetric division-null mutant, Leu-Gly-Asn repeat enriched protein (LGN) knockdown, shows impaired differentiation and stratification of epidermis, and restoration of impaired Notch signaling in the knockdown cells partially rescues the defective phenotypes, suggesting that Notch is a downstream effector of ACD (Williams et al., 2011). Inhibition of PDK1 also fully suppressed the calcium-induced expression of Notch3 mRNA in primary cultured keratinocytes in a dose-dependent manner (Figures S4C and D). However, expression of representative Notch response genes, such as Hes1, Hes5, Hey1, and Hey2, was not highly induced by calcium. Nevertheless, inhibition of PDK1 did result in statistically significant repression of expression of Hes5 and Hey2 (Figure S4E). These results were consistent with the analysis of PDK1CKO in vivo (Figure S1F) and in vitro (Figure S3A), suggesting change in Notch-dependent transcriptional programs in PDK1CKO keratinocytes.
To test whether Notch activation can rescue the impaired differentiation of PDK1CKO keratinocytes, we transfected Notch3-intracellular domain and evaluated expression of involucrin, a transcriptional target of Notch (Rangarajan et al., 2001). Impaired involucrin expression during calcium-induced differentiation in PDK1CKO keratinocytes was fully restored upon Notch3 activation at the protein level (Figure 4E). Although Keratin-1 protein levels were not as clearly rescued by this procedure (Figure S4F), we were able to observe that activation of Notch restored calcium-induced upregulation of Keratin-10 (Figure 4F). These results suggest that, in addition to being required for ACD, PDK1 is needed for Notch-dependent transcriptional programs essential for keratinocyte differentiation.
Conclusions
We propose that PDK1 plays a critical role in epithelial differentiation and stratification. Our results highlight an essential role for PDK1 in ACD, wherein PDK1 governs both the activation and localization of key signaling pathways. PDK1 regulates the activation of AKT and the activation and redistribution of aPKC and PAR3 at the apical pole of asymmetrically dividing cells. As a result, PDK1 appears to be crucial for the early signaling that is triggered at the apical side of progenitor cells leading to cell division in a perpendicular plane to the basal membrane and ultimately to the basal-to-suprabasal switch required for development of stratified epithelium.
Our studies also provide additional insight into the long-standing question of whether apical or basal stimuli provide the cue for ACD in stratified epithelia. We found that PDK1 is enriched at the apical side of dividing basal cells and that this localization correlates with enrichment of PIP3. Therefore, although PI3K can be activated at both the lateral and basal side of epithelial cells (McCaffrey and Macara, 2012), we propose that the activation and recruitment of PDK1 at the apical side is a PI3K dependent process that is crucial for establishing ACD. Despite normal PIP3 localization in cells lacking PDK1, we find that aPKC and PAR3 are no longer redistributed to the to the apical side of basal progenitor cells in the absence of PDK1. This suggests that establishing the apical complex is dependent on PI3K activation and recruitment of PDK1 to enriched PIP3 at the apical side. Thus, PDK1 acts both as a scaffold, by recruiting the apical complex to the PIP3 enriched membrane as well as a key signaling intermediate necessary for activation of aPKC, as well as AKT. These findings are consistent with the role of PDK1 in organization of signaling from the T cell receptor signaling complex (Lee et al.; Park et al.). Our data are consistent with the proposed role of aPKC as a key component of the apical complex, as we find ACD correlates with assembly of the PAR3/aPKC apical complex. However, recent analyses of ACD in the epidermis of mice lacking PKCλ do not support an essential role for aPKC in ACD (Niessen et al., 2013). Determining whether these phenotypic differences are due to compensation by other aPKC in mice lacking PKCλ, or due to the role of PDK1 in heterologous signaling pathways will be important for elucidating the true role of aPKC in ACD.
Finally, we demonstrate that PDK1 is also required for differentiation of keratinocytes. We find that PDK1 regulates Notch-dependent transcription programs that promote keratinocyte differentiation. In vitro, defective expression of differentiation markers in PDK1CKO keratinocytes induced with calcium are rescued by the introduction of Notch-ICD, demonstrating an unexpected role for PDK1 in the regulation of Notch-dependent transcriptional programs. These results demonstrate that PDK1 has multiple functions in epidermal stratification by regulating ACD and keratinocyte differentiation. As a result, PDK1CKO mice have an epidermis that exhibits a remarkable lack of stratification. Curiously, despite normal basal cellularity, we were unable to detect changes in PDKCKO keratinocyte proliferation or apoptosis either in vitro or in vivo. Nevertheless, we suspect that, although we could not detect significant changes, that there may be a subtle increase in apoptosis of PDK1CKO cells that are masked by either rapid clearance or sloughing of dying cells as has been observed at other epithelial surfaces (Bondow et al., 2012). In summary, PDK1, triggered PI3K-PIP3 signaling, is indispensable for asymmetric signaling events that promote ACD and the development of stratified epithelia.
EXPERIMENTAL PROCEDURES
Animals
PDK1Flox/Flox mice (Hashimoto et al., 2006) were bred with K14-Cre transgenic mice (Dassule et al., 2000) to generate K14-Cretg/+ PDK1Flox/+ and K14-Cretg/+ PDK1Flox/Flox mice. All mice were kept in specific pathogen-free conditions in the animal care facility at Columbia University (New York, NY). All mouse experiments were approved by the Columbia University IACUC. Immunostaining was visualized using a Carl Zeiss Axio Imager 2 (Carl Zeiss, Thornwood, NY) and BioRevo BZ-9000 (KEYENCE, Osaka, Japan).
Quantitative PCR
For quantitative RT-PCR, total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) or RNeasy Mini (Qiagen, Valencia, CA) and reverse transcribed by Superscript III (Invitrogen) or miRCURY LNA™ Universal RT microRNA PCR cDNA synthesis kit (EXIQON, Woburn, MA). cDNAs were used for PCR with SYBR Green reagents (Quanta Biosciences, Gaithersburg, MD) on MX3000 bioanalyzer (Stratagene, La Jolla, CA) and CFX Connect real-time PCR detection system (Bio Rad, Hercules, CA). The data was normalized to β-actin expression, or 5S rRNA expression for miR-203 expression. Product sizes were confirmed by gel electrophoresis. Primer sets for miR-203 and 5S rRNA were purchased from EXIQON. Additional primer sequences are provided in Table S1.
Cell culture
Primary keratinocytes were isolated from newborn mice, cultured in low calcium medium (0.05mM Ca2+), and induced to differentiate by raising calcium to 1.3mM as described previously (Lichti et al., 2008). For cell signaling experiments, cells were serum-starved for 24 h prior to stimulation.
Supplementary Material
Acknowledgments
We thank Drs. A.M. Christiano, D.M. Owens and S. Raghavan from the Columbia University Skin Disease Research Center (SDRC) for technical assistance, reagents and advice. We thank Drs. K. Yasutomo and Y. Maekawa for plasmids; Dr. F. Toyoshima for antibodies and critical advice; Drs. C. Schindler, Y. Ben-Neriah and S.L. Reiner and members of the Ghosh laboratory for their help. This work is partly supported by SDRC Pilot & Feasibility Studies Program (P30AR44535) (SG), The Uehara Memorial Foundation Research Fellowship (TD), JSID's Fellowship SHISEIDO Award (TD), JSPS KAKENHI (Grant Number 26670529) and NIH grant R37-AI33443 (SG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
Supplemental Information includes eight figures, one table and Supplementary Experimental Procedures can be found with this article online at:
AUTHOR CONTRIBUTIONS
T.D. designed all experiments, performed most of the experiments and wrote the paper, M.S.H. analyzed data, performed some experiments and helped in writing the paper, S-G.P. generated the knockout mice, H.O., J.S.S., Y.G-B. and K.M.B performed some experiments, M.Y., K.K and T.H. analyzed data and S.G. conceived and guided the study and wrote the paper.
REFERENCES
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Barbieri CE, Barton CE, Pietenpol JA. Delta Np63 alpha expression is regulated by the phosphoinositide 3-kinase pathway. J Biol Chem. 2003;278:51408–51414. doi: 10.1074/jbc.M309943200. [DOI] [PubMed] [Google Scholar]
- Bondow BJ, Faber ML, Wojta KJ, Walker EM, Battle MA. E-cadherin is required for intestinal morphogenesis in the mouse. Developmental biology. 2012;371:1–12. doi: 10.1016/j.ydbio.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005;280:32856–32865. doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- Candi E, Dinsdale D, Rufini A, Salomoni P, Knight RA, Mueller M, Krammer PH, Melino G. TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle. 2007;6:274–285. doi: 10.4161/cc.6.3.3797. [DOI] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Finding one's niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunschmann C, Stachelscheid H, Akyuz MD, Schmitz A, Missero C, Bruning JC, Niessen CM. Insulin/IGF-1 controls epidermal morphogenesis via regulation of FoxO-mediated p63 inhibition. Developmental cell. 2013;26:176–187. doi: 10.1016/j.devcel.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Kido Y, Uchida T, Asahara S, Shigeyama Y, Matsuda T, Takeda A, Tsuchihashi D, Nishizawa A, Ogawa W, et al. Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat Genet. 2006;38:589–593. doi: 10.1038/ng1774. [DOI] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Inaba M, Yuan H, Salzmann V, Fuller MT, Yamashita YM. E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS One. 2010;5:e12473. doi: 10.1371/journal.pone.0012473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor MA, Mora A, Ashby PR, Williams MR, Murray-Tait V, Malone L, Prescott AR, Lucocq JM, Alessi DR. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, D'Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- Lena AM, Shalom-Feuerstein R, Rivetti di Val Cervo P, Aberdam D, Knight RA, Melino G, Candi E. miR-203 represses 'stemness' by repressing DeltaNp63. Cell Death Differ. 2008;15:1187–1195. doi: 10.1038/cdd.2008.69. [DOI] [PubMed] [Google Scholar]
- Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RG, Garcia-Silva S, Moore SJ, Bereshchenko O, Martinez-Cruz AB, Ermakova O, Kurz E, Paramio JM, Nerlov C. C/EBPalpha and beta couple interfollicular keratinocyte proliferation arrest to commitment and terminal differentiation. Nat Cell Biol. 2009;11:1181–1190. doi: 10.1038/ncb1960. [DOI] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. Signaling pathways in cell polarity. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Brewer KA, Exton JH. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- Niessen MT, Scott J, Zielinski JG, Vorhagen S, Sotiropoulou PA, Blanpain C, Leitges M, Niessen CM. aPKClambda controls epidermal homeostasis and stem cell fate through regulation of division orientation. J Cell Biol. 2013;202:887–900. doi: 10.1083/jcb.201307001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol. 2009;10:158–166. doi: 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Lechler T. Regulation of asymmetric cell division in the epidermis. Cell Div. 2011;6:12. doi: 10.1186/1747-1028-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Vorhagen S, Niessen CM. Mammalian aPKC/Par polarity complex mediated regulation of epithelial division orientation and cell fate. Experimental cell research. 2014;328:296–302. doi: 10.1016/j.yexcr.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Wang X, Pasolli HA, Williams T, Fuchs E. AP-2 factors act in concert with Notch to orchestrate terminal differentiation in skin epidermis. J Cell Biol. 2008;183:37–48. doi: 10.1083/jcb.200804030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing 'stemness'. Nature. 2008;452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.