Abstract
Background and Aim
Liver biopsy (LB) has lost popularity to stage liver fibrosis in the era of highly effective anti-hepatitis C virus (HCV) therapy, yet diagnosis of persistent cirrhosis may have important implications following HCV eradication. As performance of serological non-invasive tests (NITs) to predict residual fibrosis in non-viremic HCV patients is unknown, we investigated accuracy of NITs to predict residual fibrosis in cirrhotics after a sustained virological response (SVR) to interferon (IFN).
Methods
Thirty-eight patients with a pre-treatment histological diagnosis of cirrhosis and a 48–104 months post-SVR LB were tested with APRI, CDS, FIB-4, FibroQ, Forns Score, GUCI Index, King Score, Lok Index, PLF, ELF. In 23 (61%) patients, cirrhosis had histologically regressed.
Results
All NITs values declined after SVR without any significant difference between regressors and non-regressors (AUROC 0.52–0.75). Using viremic cut-offs, PPV ranged from 34% to 100%, with lower NPV (63% - 68%). NITs performance did not improve using derived cut-offs (PPV: 40% - 80%; NPV: 66% - 100%). PLF, which combines several NITs with transient elastography, had the best diagnostic performance (AUROC 0.75, Sn 61%, Sp 90%, PPV 80%, NPV 78%). After treatment, none of the NITs resulted significantly associated with any of the histological features (activity grade, fibrosis stage, area of fibrosis).
Conclusions
The diagnostic estimates obtained using both viremic and derived cut-off values of NITs were suboptimal, indicating that none of these tests helps predicting residual fibrosis and that LB remains the gold standard for this purpose.
Introduction
The long-standing dogma that cirrhosis is an irreversible condition has been repeatedly challenged in last decades. Indeed many studies in hepatitis C (HCV) patients with a sustained virological response (SVR) to Interferon (IFN)-based regimens showed cirrhosis regression in 30% to 60% patients 3 to 5 years after SVR [1–16]; this histological outcome was associated with reduced risk of developing hepatocellular carcinoma (HCC), hepatic decompensation and variceal bleeding, while, on the contrary, a clinical benefit was not observed in SVR patients with residual cirrhosis [9]. These findings rose the argument about need for assessing residual cirrhosis in HCV patients with an SVR, in order to deliver accurate prognosis and optimize clinical management. Indeed, all SVR cirrhotic patients are currently recommended to keep lifelong surveillance for HCC with abdominal ultrasound (US) every 6 months [17], as these patients still carry a 0.6%-1.2% yearly risk of HCC [13,16,18,19]. The demonstration of cirrhosis regression would allow for tailored surveillance intervals, as the reduced risk of HCC in cirrhosis regressors would make US surveillance every 6 months non cost/effective.
Liver biopsy (LB) currently represents the gold standard for accurately assessing liver fibrosis stage and consequently identifying cirrhosis regression, however it has limited application because it is an invasive procedure, poorly accepted by patients and ultimately carries a risk of complications [20,21]. Moreover, LB accuracy is biased by heterogeneous deposition of fibrosis in the liver, sampling error and inter-observer variability [22,23]. These drawbacks highlight the need for alternative non-invasive methods to assess fibrosis stage following an SVR. In this setting, we previously demonstrated that transient elastography (TE) has low diagnostic accuracy in identifying residual cirrhotic patients after an SVR, mainly as a consequence of fibrosis remodeling [24]. The aim of this study was to investigate the diagnostic performance of most popular serological markers of hepatic fibrosis in a unique albeit small group of patients with histologically documented cirrhosis who underwent a second liver biopsy 5 years after achieving an SVR to IFN-based therapies.
Material and Methods
Patient population
This study is a post-hoc analysis of a cohort study of 38 HCV cirrhotic patients, who underwent a LB following an SVR to assess histological modifications associated with viral clearance [12]. The first LB was performed within 12 months before anti-HCV treatment, while the second at least 4 years after the SVR [12]. Exclusion criteria were HBV or HIV co-infection, >40 g/day alcohol intake, malignancies, contraindication to LB. At the time of post-SVR LB, all patients had liver stage concomitantly assessed through TE and the following serological non-invasive tests (NITs): APRI, CDS, FIB-4, FibroQ, Forns Score, GUCI Index, King Score, Lok Index, PLF and ELF (see Material and Methods Section). All patients gave their written informed consent to use of their medical records and perform the post-treatment LB for the study, which was approved by the Institutional Review Board of the Department of Internal Medicine.
Histological assessment
All pre-treatment LBs were performed with a cutting needle (Tru-cut 16 G, TSK Laboratories, Tokyo, Japan), whilst a Menghini-like semiautomatic biopsy device (Biomol 16 G, HS Hospital Service, Latina, Italy) was used for post-treatment samples. Liver biopsies were considered adequate for histological analysis if longer than 10 mm and/or carrying at least 12 portal tracts. All biopsies were read independently by two expert Pathologists blinded to any clinical information. Fibrosis stage was assessed according to the METAVIR scoring system [25], while hepatic inflammation was evaluated according to both METAVIR and Ishak grading systems [25,26]. Cirrhosis regression was defined as decrease from F4 to F3 or less when comparing pre- and post-treatment LBs [12]. Area of fibrosis was calculated by using the HISTOLAB® software (ALPHELYS, Plaisir, France), and was defined as ratio of fibrous tissue area stained with picrosirius red (%) upon the total tissue surface, as previously described [12].
Non-invasive measurements
Transient elastography
Transient elastography was performed as already described [27]; liver stiffness measurement (LSM) was expressed in kiloPascal (kPa) as the median value of the successful measurements. As previously published [28] TE threshold of ≥ 12 kPa was used for the diagnosis of cirrhosis.
Serological non-invasive tests
The following serological indirect and direct markers of liver fibrosis [29] were tested according to their formula:
-
Indirect serological markers
APRI (AST to platelet ratio): AST levels (× ULN)/platelets count (103/l) × 100
CDS (Cirrhosis Discriminate Score): calculated by summing the scores awarded for the following laboratory results (Table 1)
FIB-4: [age (yr) × AST (U/l)]/[(PLT (109/l)] × [ALT (U/l)1/2]
FibroQ: [(10 x age (yr)) x AST (U/l) x PT INR)/[PLT (109/l) x ALT (U/l)]
Forns Score: 7.811–3.131 × ln [PLT (109/l)] × 0.781 ln [GGT (U/l)] + 3.467 × ln [age (yr)] - 0.014 [cholesterol (mg/dl)]
GUCI (Goteborg University Cirrhosis Index): (AST/ULN) × INR × 100/PLT (109/l)
King Score: age (yr) × AST (U/l) × INR/PLT (109/l)
Lok Index:– 5.56–0.0089 × PLT (103/mm) + 1.26 × AST/ALT ratio + 5.27 × INR
PLF (Predicted Liver Fibrosis): 0.956 + 0.084 × TE –0.004 × King Score + 0.124 × Forns Score + 0.202 × APRI Score.
Reference cut-offs for cirrhosis, obtained from literature, were APRI >1.5, CDS ≥8, Fib4 >3.25, FibroQ >2.6, Forns Score ≥6.9, GUCI >0.26, King Score ≥16.7, Lok Index >0.5 and PLF ≥2.98 [30–39].
Direct serological markers. Enhanced Liver Fibrosis (ELF) test combines semiquantitative serum measurements of tissue inhibitor of metallo-proteinases-1 (TIMP-1), amino-terminal propeptide of type III collagen (PIIINP) and hyaluronic acid (HA). ADVIA Centaur CP immunochemical analyzer was used according to manufacturer’s instructions and the ELF score was calculated by the following equation: -7.412 + [ln(HA)*0.681] + [ln(PIIINP)*0.775] + [ln(TIMP-1)*0.494] + 10 [40–43]. Four cut-offs (9.3, 9.8, 10.3, 11.3) were used for the diagnosis of cirrhosis (F4), since a univocal standard threshold for F4 has not been validated [41–44].
Table 1. Cirrhosis Discriminate Score (CDS) calculation.
| Parameters | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| INR | <1.1 | 1.1–1.4 | >1.4 | ||||
| ALT/AST | >1.7 | 1.7–1.2 | 1.19–0.6 | <0.6 | |||
| PLT /mm3 | >340 | 340–280 | 279–220 | 219–160 | 159–100 | 99–40 | <40 |
Statistical analysis
Comparisons between groups were made by using the Mann–Whitney U or the Student’s-t tests for continuous variables, and the χ2 or Fisher test for categorical data. A probability value of p<0.05 was considered statistically significant. The diagnostic accuracy of non-invasive markers in SVR patients was expressed as sensitivity, specificity, positive and negative likelihood ratios (LR+ and LR-), positive and negative predictive values (PPV and NPV), using receiver operating characteristic (ROC) curves analysis. The accuracy of NIT and TE to identify cirrhosis has been assessed. A receiver operating characteristic (ROC) curve analysis was performed and the area under the ROC curve (AUC) has been reported.
Categorical data were reported as counts (percentages) and continuous variables as mean (standard deviation) or median (interquartile range, IQR), as appropriate.
Uni- and multivariate linear regression analyses were performed to assess the ability of the NITs (APRI, CDS, FIB-4, FIBRO-Q, FORNS score, GUCI index, King score, Lok index, ELF, PLF) in predicting the histological features (i.e. activity grade, fibrosis stage, area of fibrosis). Also TE was included in the analysis. Only those parameters that showed a statistically significant result at the univariate analysis were entered in the multivariate analysis. The performance of the linear models was evaluated by the determination coefficient (R2). Two analyses were performed considering, in separate, pre- and post-treatment (SVR) data. P values lower than 0.05, two sided, were considered statistically significant. All statistical analyses were performed with SAS statistical software (release 9.4; SAS Institute Inc, Cary, NC).
Results
Patient population
Clinical data were available at baseline and at the time of second LB, 61 (48–104) months after the SVR. Thirty-three (89%) patients had also a post-SVR LSM, while none had a baseline TE assessment. At post-SVR LB, 35 (92%) patients showed normal transaminases whereas 36 (95%) had a platelet count higher than 100,000/mm3. Post-SVR liver biopsies showed cirrhosis regression in 23 (61%) patients: F3 in 14 (61%), F2 in 7 (30%), and F1 in 2 (9%). Cirrhosis persisted in the remaining 15 (39%). No significant clinical differences were observed between patients staged F4 or <F4, after treatment (Table 2).
Table 2. Patients' characteristics at the time of post-SVR liver biopsy.
| Feature | Overall | Regressors | Non-Regressors | p-value§ |
|---|---|---|---|---|
| (n = 38) | (n = 23) | (n = 15) | ||
| Males* | 24 (63%) | 15 (65%) | 9 (60%) | 1.0 |
| Age, years** | 66 (46–75) | 64 (46–75) | 64 (56–72) | 0.98 |
| Body weight, Kg** | 75 (50–98) | 73 (52–90) | 73 (55–93) | 0.53 |
| BMI > 25 kg/m2 * | 16 (42%) | 12 (52%) | 6 (40%) | 0.60 |
| pnALT±* | 35 (92%) | 21 (91%) | 14 (93%) | 1.0 |
| PLT > 100x103/mm3* | 36 (95%) | 22 (96%) | 15 (100%) | 1.0 |
| INR < 1.2 | 38 (100%) | 23 (100%) | 15 (100%) | 1.0 |
| Liver core size, mm** | 30 (10–45) | 30 (15–50) | 30 (10–45) | 0.22 |
| Fibrosis stage | 0.31 | |||
| F0 | 0 (0%) | 0 (0%) | 0 (0%) | |
| F1 | 2 (5%) | 2 (9%) | 0 (0%) | |
| F2 | 7 (18%) | 7 (30%) | 0 (0%) | |
| F3 | 14 (37%) | 14 (61%) | 0 (0%) | |
| F4 | 15 (40%) | 0 (0%) | 15 (100%) |
§ p-value: regressors vs. non-regressors
*number (%)
** median (range)
± pn: persistent normal
Indirect markers of fibrosis
NITs values at post-SVR LB and their reference cut-offs for the diagnosis of cirrhosis are shown in Table 3. Forns Score and PLF were calculated in 33 (87%) patients with available cholesterol and TE values.
Table 3. Median# NIT values at post-SVR liver biopsy and reference cut-offs for the diagnosis of cirrhosis.
| Test | Reference | Overall | Regressors | Non-Regressors | p-value |
|---|---|---|---|---|---|
| Cut-off for F4 | (n = 38) | (n = 23) | (n = 15) | ||
| APRI | ≥ 1.5 | 0.3 (0.2–0.9) | 0.3 (0.2–0.9) | 0.3 (0.2–0.8) | 0.48 |
| CDS | > 8 | 5 (2–7) | 5 (2–6) | 5 (3–7) | 0.49 |
| FIB-4 | > 3.25 | 1.7 (0.8–3.7) | 1.7 (0.8–2.7) | 1.7 (1.1–3.7) | 0.38 |
| FibroQ | > 2.6 | 3.9 (1.5–7.9) | 3.9 (1.5–6.3) | 4.0 (1.7–7.9) | 0.26 |
| Forns Score* | > 6.9 | 5.1 (3.1–8.5) | 5.1 (3.1–8.5) | 5.3 (4.0–8.0) | 0.83 |
| GUCI Index | > 0.26 | 0.4 (0.2–1.0) | 0.4 (0.18–0.95) | 0.4 (0.21–0.90) | 0.56 |
| King Score | > 16.7 | 7.7 (3.8–20.6) | 7.7 (3.8–16.9) | 7.7 (4.7–20.6) | 0.48 |
| Lok Index | > 0.5 | 0.4 (0.1–0.7) | 0.4 (0.1–0.6) | 0.4 (0.1–0.7) | 0.24 |
| PLF* | > 2.98 | 2.5 (1.7–5.0) | 2.5 (1.7–5.0) | 2.5 (2.2–4.7) | 0.01 |
| ELF** | >9.3, >9.8 | 8.6 (6.8–10.0) | 8.6 (7.0–10.0) | 8.4 (6.8–9.9) | 0.70 |
| >10.3, >11.3 |
# Results are reported as median (range) values
* Calculated in 33 patients for whom valid TE assessments and/or cholesterol values were available
** Calculated in 29 patients
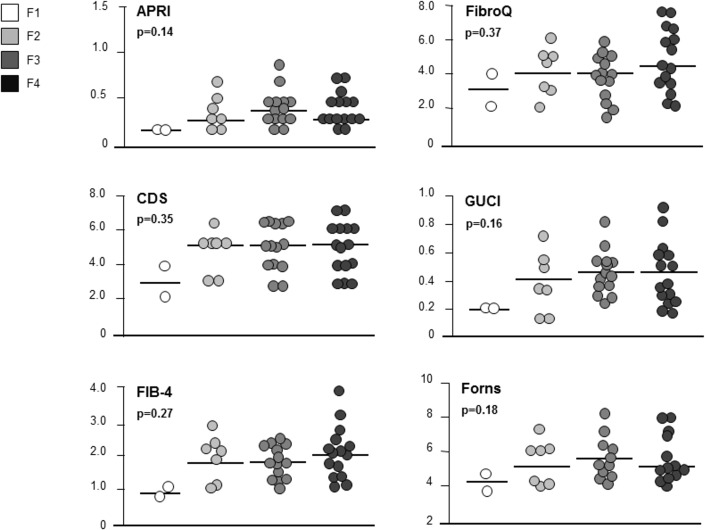
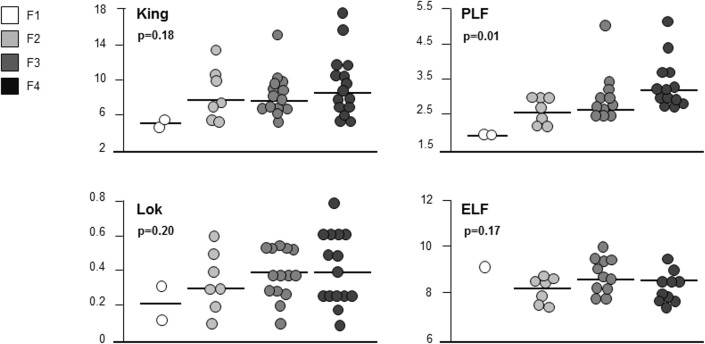
Apart from PLF, NITs values did not significantly differ in regressed and non-regressed patients (Table 3), and also when stratifying data according to post-SVR fibrosis stage [(F1 vs. F2 vs. F3 vs. F4): APRI p = 0.14, CDS p = 0.35, FIB-4 p = 0.27, FibroQ p = 0.37, Forns Score p = 0.18, GUCI p = 0.16, King Score p = 0.18, Lok p = 0.20], due to an extensive overlap of NITs values across all stages of fibrosis (Figs 1 and 2). PLF was the only NIT to show significant differences when comparing fibrosis stages at post-SVR LB (F4 vs. <F4: p = 0.01; F4 vs. F3 vs. F2 vs. F1: p = 0.01) (Table 3, Fig 2).
Fig 1. Non-invasive Tests (NITs) values stratification according to post-SVR fibrosis stage.
Fig 2. Non-invasive Tests (NITs) values stratification according to post-SVR fibrosis stage.
When analyzing diagnostic performances according to ROC curves generated on reference cut-offs, all NITs demonstrated low diagnostic accuracy in identifying patients with residual cirrhosis after the SVR. Indeed, due to low sensitivity and specificity, AUROC ranged from 0.51 (CDS) to 0.75 (PLF) (Table 4). NITs accuracy remained low also when using the derived thresholds, obtained by combining the best sensitivity/specificity, with PPV values ranging between 40% (CDS) and 80% (FIB-4 and PLF), and NPV between 66% (APRI and Lok Index) and 100% (CDS) (Table 4). Therefore, 5% to 40% of SVR patients were misclassified as regressors (<F4) by NITs, despite cirrhosis persistence at post-SVR LB.
Table 4. Non-invasive tests (NITs) diagnostic accuracy for the identification of patients with residual cirrhosis.
| Test | Cut-off | Patients | Sens | Spec | LR+ | LR- | PPV | NPV | AUROC |
|---|---|---|---|---|---|---|---|---|---|
| (n) | (%) | (%) | (%) | (%) | (95%CI) | ||||
| APRI | > 0.5 | 9 | 91 | 27 | 3.0 | 0.8 | 47 | 66 | 0.58 (0.39–0.75) |
| >1.5 | 0 | - | - | - | - | - | - | ||
| CDS | > 2 | 37 | 100 | 4.3 | 1.03 | 0.001 | 40 | 100 | 0.51 (0.35–0.68) |
| >8 | 0 | - | - | - | - | - | - | ||
| FIB-4 | > 2.3 | 7 | 27 | 96 | 6.1 | 0.7 | 80 | 67 | 0.59 (0.41–0.74) |
| > 3.25 | 2 | 6 | 100 | - | 0.9 | 100 | 62 | ||
| FibroQ | > 5.2 | 10 | 40 | 87 | 3.0 | 0.60 | 67 | 69 | 0.58 (0.41–0.74) |
| > 2.6 | 29 | 80 | 26 | 1.1 | 0.7 | 41 | 67 | ||
| Forns Score | > 6.4 | 7 | 38 | 90 | 3.8 | 0.6 | 71 | 69 | 0.56 (0.37–0.73) |
| > 6.9 | 6 | 23 | 90 | 2.3 | 0.8 | 60 | 64 | ||
| Guci Index | > 0.52 | 9 | 33 | 87 | 2.5 | 0.7 | 62 | 68 | 0.56 (0.39–0.72) |
| > 0.26 | 29 | 80 | 26 | 1.1 | 0.7 | 41 | 68 | ||
| King Score | > 10.1 | 9 | 46 | 87 | 3.1 | 0.6 | 68 | 69 | 0.59 (0.40–0.75) |
| > 16.7 | 3 | 13 | 100 | - | 0.8 | 100 | 63 | ||
| Lok Index | > 0.5 | 9 | 27 | 91 | 3.0 | 0.8 | 67 | 66 | 0.57 (0.40–0.73) |
| PLF | > 2.6 | 14 | 61 | 90 | 5.8 | 0.4 | 80 | 78 | 0.75 (0.57–0.89) |
| > 2.98 | 4 | 31 | 95 | 6.1 | 0.7 | 80 | 68 | ||
| ELF Score | > 8.1 | 18 | 60 | 74 | 2.3 | 0.5 | 54 | 78 | 0.63 (0.43–0.80) |
| > 9.8 | 2 | 90 | 10 | 1.1 | 0.9 | 34 | 67 |
Sn: Sensitivity; Sp: Specificity; LR+: positive likelihood ratio; LR-: negative; likelihood ratio; PPV: positive predictive value, NPV: negative predictive value.
In bold are reported the diagnostic estimates according to the cut-off maximizing sensitivity and specificity derived from present data. In plain text the results according to the cut off reported in the literature.
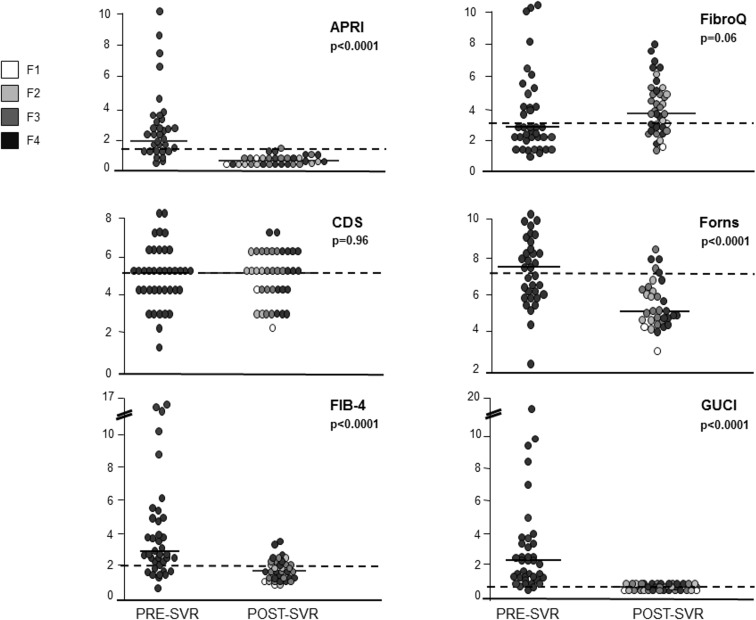
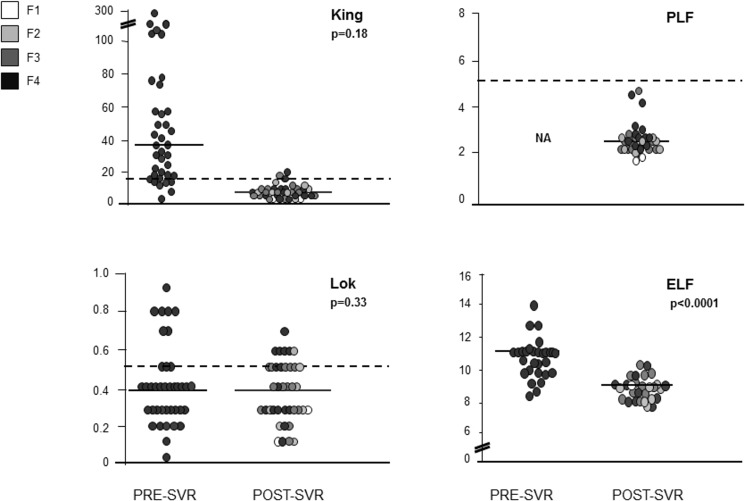
NITs values at baseline are reported in Table 5. Most NITs showed a significant post-treatment decrease (Table 5; Figs 3 and 4), except for CDS, FibroQ and Lok Index.
Table 5. Non-invasive tests (NITs) values before and after treatment.
| Test | Baseline | SVR | p-value |
|---|---|---|---|
| APRI | 2.0 (0.2–16.4) | 0.3 (0.2–0.9) | <0.0001 |
| CDS | 5 (1–8) | 5 (2–7) | 0.96 |
| FIB-4 | 2.9 (0.4–17.3) | 1.7 (0.8–3.7) | <0.0001 |
| FibroQ | 2.8 (0.6–13.6) | 3.9 (1.5–7.9) | 0.06 |
| Forns Score* | 7.4 (2.3–10.0) | 5.1 (3.1–8.5) | <0.0001 |
| GUCI Index | 2.1 (0.2–20.2) | 0.4 (0.2–1) | <0.0001 |
| King Score | 38.3 (3.4–37.4) | 7.7 (3.8–20.6) | <0.0001 |
| Lok Index | 0.4 (0–0.9) | 0.4 (0.1–0.7) | 0.33 |
| PLF | na | 2.5 (1.7–5.0) | na |
| ELF** | 10.7 (7.9–14) | 8.6 (6.8–10) | <0.0001 |
* Calculated in 33 patients for whom cholesterol values were available
** Calculated in 29 patients
Fig 3. Non-invasive tests (NITs) values distribution at baseline and after an SVR.
Fig 4. Non-invasive tests (NITs) values distribution at baseline and after an SVR.
Before treatment, none of the NITs showed any correlation with the most important histological features (i.e. fibrosis, activity, area of fibrosis). After treatment, some of the NITs (CDS, FIB-4, FibroQ, PLF) showed a correlation with the morphometric area of fibrosis, only; however, this correlation was lost at multivariate analysis (Table 6).
Table 6. Correlation between NITs and TE and histological features at univariate linear regression analysis.
| Pre-treatment | Post-treatment | Pre-Treatment | Post-Treatment | Pre-treatment | Post-Treatment | |||
|---|---|---|---|---|---|---|---|---|
| Tests | p-value | p-value | p-value | p-value | r2 | p-value | r2 | p-value |
| APRI | 0.69 | 0.38 | - | 0.56 | 0.00001 | 0.99 | 0.08 | 0.08 |
| CDS | 0.56 | 0.32 | - | 0.57 | 0.002 | 0.77 | 0.17 | 0.04 |
| FIB-4 | 0.59 | 0.18 | - | 0.20 | 0.003 | 0.91 | 0.21 | 0.003 |
| FibroQ | 0.82 | 0.15 | - | 0.14 | 0.011 | 0.52 | 0.14 | 0.01 |
| Forns Score | 0.25 | 0.36 | - | 0.72 | 0.0003 | 0.90 | 0.10 | 0.06 |
| GUCI Index | 0.65 | 0.62 | - | 0.54 | 0.0007 | 0.95 | 0.08 | 0.07 |
| King Score | 0.58 | 0.40 | - | 0.32 | 0.14 | 0.14 | 0.14 | 0.01 |
| Lok Index | 0.42 | 0.06 | - | 0.23 | 0.03 | 0.25 | 0.08 | 0.07 |
| PLF | 0.17 | 0.53 | - | 0.09 | 0.0003 | 0.97 | 0.30 | 0.0009 |
| ELF | 0.17 | 0.83 | - | 0.69 | 0.11 | 0.06 | 0.08 | 0.11 |
| TE | - | 0.58 | - | 0.07 | - | - | 0.34 | 0.0004* |
* Only TE maintained its statistical significance at multivariate analysis
TE: Transient Elastography
Direct marker of fibrosis: ELF test
ELF test was assessed in 29 (76%) patients at baseline and after the SVR. Median post-treatment value was 8.6 (6.8–10.0) (Table 3), without differences between regressors and non-regressors (8.6 vs. 8.4, p = 0.70) and across all stages of fibrosis (F1 vs. F2 vs. F3 vs. F4: p = 0.17) (Fig 2). After the SVR, most patients had ELF test values below the 4 cut-offs suggested for the diagnosis of cirrhosis: 25 (86%) patients were < 9.3, 26 (90%) < 9.8, 29 (100%) < 10.3 and 29 (100%) <11.3; therefore ELF diagnostic accuracy could not be assessed according to the reference cut-offs. When trying to derive a new cut-off with the best sensitivity [60% (18.7–81.3)] and specificity [74% (43.4–87.4)], that resulted 8.1 in our cohort, it still carried a suboptimal performance (AUROC 0.63) for identifying residual cirrhosis (Table 4). According to this cut-off, 5/18 (28%) patients were correctly classified as cirrhotics.
Before treatment, 25 (86%), 20 (69%) 17 (59%) and 5 (17%) patients were correctly classified as cirrhotics, according to the 9.3, 9.8, 10.3 and 11.3 cut-offs. Pre-treatment median ELF value was 10.7 (7.9–14), similar in regressors and non-regressors (10.6 vs. 10.8; p = 0.63). At the time of post-SVR LB, median values declined both in patients with and without cirrhosis regression (10.7 vs. 8.4, p<0.0001; 10.6 vs. 8.6, p = 0.0015) (Table 5; Fig 4).
At linear regression analysis, ELF values did not correlate with any of the histological parameters as assessed at LB (activity, fibrosis, area of fibrosis), both at baseline and after the achievement of an SVR (Table 6).
Discussion
In this proof of concept study about diagnostic accuracy of serological assays for liver fibrosis, all tests failed to separate SVR patients with residual cirrhosis from minor levels of fibrosis. Cumulatively, the diagnostic accuracy of these tests by AUROC ranged from 0.51 and 0.75, whereas, individually, none had a cut-off value that could distinguish between different stages of fibrosis according to METAVIR.
In HCV patients, serological markers of liver fibrosis are becoming increasingly popular, as they have shown satisfactory accuracy for liver fibrosis staging, with AUROC ranging between 0.78 and 0.89 according to different studies [29, 45]. However, the performance of serological tests in HCV patients is lower than TE [28,29,45]. We previously demonstrated that TE failed to identify residual cirrhosis after the SVR using the classical viremic TE cut-off of 11.9 kPa [24], speculating that the reduced TE accuracy in non-viremic patients could be the consequence of fibrosis remodeling occurring after the SVR. The present study focuses on this same subgroup of patients who were evaluated with both direct and indirect serological markers of fibrosis. Nine indirect tests failed to identify patients with residual cirrhosis, showing lower diagnostic accuracy in comparison with their performance reported in viremic patients [29]. This poor diagnostic accuracy was in line with the reduced TE performance in this non-viremic population (AUROC serum assays vs. TE: 0.51–0.75 vs. 0.75) [24]. The loss in diagnostic accuracy was probably driven by the normalization of biochemical parameters that are considered in each test assessed, thus confirming a previous report by Sebastiani et al, who showed poor performance of APRI, AAR, Forns’ Index, Fibrotest and Fibroindex in 80 viremic patients with persistently normal transaminase values [46]. On the other hand, a recent study in 115 patients tested with APRI, FIB-4 and Forns Score 6 years after the SVR found increased values in parallel with histological fibrosis stage (p<0.0001), however with low specificity and PPV [47]. These findings discrepant from our study could be mainly explained by differences in patient demography, stage of fibrosis and cumulative analysis of residual F3 and F4 stages.
To overcome the pitfalls of a single-test approach in a relatively small study, we tested whether the combination of different serological tests and TE resulted in improved diagnostic accuracy. This notwithstanding, PLF, which combines four indirect markers of fibrosis and TE, showed poor diagnostic accuracy, although it carried the best performance among all tests. Differences observed in PLF values between regressors and non-regressors (p = 0.01) and across all stages of fibrosis (p = 0.01) were likely consequence of the inclusion of TE values in the test formula, since TE values significantly separated F4 from <F4 patients (p = 0.01) and correlated with post-SVR METAVIR stage (r = 0.56, p = 0.001). Not surprisingly, PLF diagnostic performance was close to that reported for TE in the same cohort in the previous study (AUROC PLF vs. TE: 0.75 vs. 0.77) [24].
Since indirect serological tests are mostly based upon biochemical parameters of liver inflammation, which do not directly reflect extracellular matrix (ECM) remodeling, we also tested the diagnostic accuracy of ELF test, a direct marker of liver fibrosis that could be an ideal candidate for our study purposes. Indeed, ELF formula includes specific compounds of the ECM (TIMP-1, PIIINP, HA), which could be more sensitive to hepatic fibrosis rearrangement occurring after HCV eradication. Although we were not able to calculate ELF values for all the patients included in this study, we observed that these values were significantly reduced in post-SVR assessment compared to pre-treatment sampling. ELF diagnostic performance for identifying residual cirrhosis was sub-optimal in line with other indirect tests. Even the 8.1 derived cut-off did not further improve ELF diagnostic accuracy (AUROC 0.63). Another study in 81 SVR patients (22% with cirrhosis) found lower ELF values at follow-up than at baseline, while median ELF values in SVR patients were lower than in non-responder patients [48]. However, this study was flawed by the lack of a histological reference standard.
While a reduction of serum tests for fibrosis following an SVR has already been reported in HCV patients [47,49], the present study has the merit to correlate serological tests with post-treatment stages of liver fibrosis. Indeed, identification of residual cirrhotics in SVR patients bears clinical interest, since cirrhotic patients who achieve an SVR following antiviral treatments are currently maintained under surveillance due to their residual risk of HCC [17]. A few years ago, Mallet and colleagues demonstrated that patients who achieved cirrhosis regression after an SVR remained free from liver-related complications in the following 5 years [9] whilst HCC developed in patients with persistent cirrhosis, only. Thus, assessment of post-SVR fibrosis stage might help individualizing the surveillance algorithm in patients with a pre-treatment diagnosis of cirrhosis who achieved viral eradication, although this individual approach is not recommended by international societies [17]. A point which needs clarifications is also whether serum assays of liver fibrosis predict long-term outcome and prognosis of SVR patients as they do with non-viremic patients [41,50]. By the same token, it could be of interest to identify post-SVR cut-offs of fibrosis tests predicting clinical events during surveillance. In our small study, we recorded no liver-related events over a 5-year follow-up, thus preventing the possibility to correlate post-SVR fibrosis stage with clinical outcomes, or to identify those patients at risk of complications according to their post-treatment values of serum tests. Due to the lack of robust studies assessing the accuracy of non-invasive tests in staging post-SVR fibrosis and/or predicting clinical events after HCV eradication, international guidelines do not recommend their use in tailoring the management of post-treatment follow-up [45].
We acknowledge weaknesses of our study that may ultimately affect the strength of our conclusions. No doubts that sample size is one such weakness, but it was to some extent the result of our stringent criteria of patient selection in terms of well-compensated patients fitting IFN-based therapy, who gave their informed consent to perform a second liver biopsy and the long term surveillance of 5 year on average post-SVR. In addition, for some of these patients Forns Score, PLF and ELF tests were not available, thus probably limiting the accuracy of their predictive value among this cohort. Finally, the retrospective design of the study prevented the dynamic study of serum tests of fibrosis. On the other hand, we think that these cons are counterbalanced by several strengths including the single center design in which liver biopsies were collected before treatment and 5 years following an SVR, and read by two expert pathologists blinded to any clinical features.
In conclusion, this study demonstrates that serological biomarkers of liver fibrosis failed to stratify SVR patients by degree of residual fibrosis and, most important, did not allow identification of patients with residual cirrhosis following an SVR.
Abbreviations
- HCV
Hepatitis C Virus
- SVR
Sustained Virological response
- IFN
Interferon
- HCC
Hepatocellular Carcinoma
- LB
Liver Biopsy
- TE
Transient Elastography
- NITs
Non Invasive Tests
- APRI
AST to platelet ratio
- CDS
Cirrhosis Discriminate Score
- GUCI
Goteborg University Cirrhosis Index
- PLF
Predicted Liver Fibrosis
- ELF
Enhanced Liver Fibrosis
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 2010;52:886–93 10.1002/hep.23785 [DOI] [PubMed] [Google Scholar]
- 2.Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu ss, et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol 2011;9:274–6 10.1016/j.cgh.2010.11.040 [DOI] [PubMed] [Google Scholar]
- 3.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381:468–75 10.1016/S0140-6736(12)61425-1 [DOI] [PubMed] [Google Scholar]
- 4.Reichard O, Glaumann H, Frydén A, Norkrans G, Wejstål R, Weiland O. Long-term follow-up of chronic hepatitis C patients with sustained virological response to alpha-interferon. J Hepatol 1999;30:783–7 [DOI] [PubMed] [Google Scholar]
- 5.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 2000;132:517–24 [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, McHutchison J, Manns M, Trepo C, Linsday K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 2002;122:1303–13 [DOI] [PubMed] [Google Scholar]
- 7.Arif A, Levine RA, Sanderson SO, Bank L, Velu RP, Shah A, et al. Regression of fibrosis in chronic hepatitis C after therapy with interferon and ribavirin. Dig Dis Sci 2003;48:1425–30 [DOI] [PubMed] [Google Scholar]
- 8.Pol S, Carnot F, Nalpas B, Lagneau JL, Fontaine H, Serpaggi J, et al. Reversibility of hepatitis C virus-related cirrhosis. Hum Pathol 2004;35:107–12 [DOI] [PubMed] [Google Scholar]
- 9.Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med 2008;149:399–403 [DOI] [PubMed] [Google Scholar]
- 10.Everson GT, Balart L, Lee SS, Reindollar RW, Shiffman ML, Minuk GY, et al. Histological benefits of virological response to peginterferon alfa-2a monotherapy in patients with hepatitis C and advanced fibrosis or compensated cirrhosis. Aliment Pharmacol Ther. 2008;27:542–51 10.1111/j.1365-2036.2008.03620.x [DOI] [PubMed] [Google Scholar]
- 11.George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology 2009;49:729–38 10.1002/hep.22694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 2012;56:532–43 10.1002/hep.25606 [DOI] [PubMed] [Google Scholar]
- 13.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology 2007;45:579–87 [DOI] [PubMed] [Google Scholar]
- 14.Bruno S, Crosignani A, Facciotto C, Rossi S, Roffi L, Redaelli A, et al. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology 2010;51:2069–76 10.1002/hep.23528 [DOI] [PubMed] [Google Scholar]
- 15.D'Ambrosio R, Aghemo A, Rumi MG, Primignani M, Dell’Era A, Lampertico P, Donato MF, et al. The course of esophageal varices in patients with hepatitis C cirrhosis responding to interferon/ribavirin therapy. Antivir Ther 2011;16:677–84 10.3851/IMP1807 [DOI] [PubMed] [Google Scholar]
- 16.Van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584–93 10.1001/jama.2012.144878 [DOI] [PubMed] [Google Scholar]
- 17.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–943 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 18.Aleman S, Rahbin N, Weiland O, Davidsdottir L, Hedenstierna M, Rose N, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis 2013;57:230–236 10.1093/cid/cit234 [DOI] [PubMed] [Google Scholar]
- 19.Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol 2010;52:652–657 10.1016/j.jhep.2009.12.028 [DOI] [PubMed] [Google Scholar]
- 20.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495–500 [DOI] [PubMed] [Google Scholar]
- 21.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, et al. Sampling variability and its influence on the diagnostic yeld of percutaneous needle biopsy of the liver. Lancet 1986;1:523–525 [DOI] [PubMed] [Google Scholar]
- 22.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614–2618 [DOI] [PubMed] [Google Scholar]
- 23.Rousselet MC, Michalak S, Dupré F, Croué A, Bedossa P, Saint-André JP, et al. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology 2005;41:257–264 [DOI] [PubMed] [Google Scholar]
- 24.D’Ambrosio R, Aghemo A, Fraquelli M, Rumi MG; Donato MF, Paradis V, et al. The diagnostic accuracy of Fibroscan for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol 2013;59:251–256 10.1016/j.jhep.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 25.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996;24:289–293 [DOI] [PubMed] [Google Scholar]
- 26.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–699 [DOI] [PubMed] [Google Scholar]
- 27.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fornier C, Mal F, et al. Transient elastography: a new non-invasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705–1713 [DOI] [PubMed] [Google Scholar]
- 28.Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007;56:968–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castera L. Non-invasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012;142:1293–1302 10.1053/j.gastro.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 30.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–526 [DOI] [PubMed] [Google Scholar]
- 31.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1997;92:1302–1304 [PubMed] [Google Scholar]
- 32.Colli A, Colucci A, Paggi S, Fraquelli M, Massironi S, Andreoletti M, et al. Accuracy of a predictive model for severe hepatic fibrosis or cirrhosis in chronic hepatitis C. Word J Gastroenterol 2005;11:7318–7322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparisons with liver biopsy and Fibrotest. Hepatology 2007;46:32–36 [DOI] [PubMed] [Google Scholar]
- 34.Hsieh YY, Tung SY, Lee K, Wu CS, Wei KL, Shen CH, et al. Routine blood tests to predict liver fibrosis in chronic hepatitis C. World J Gastroenterol 2012;18:746–753 10.3748/wjg.v18.i8.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002;36:986–992 [DOI] [PubMed] [Google Scholar]
- 36.Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005;40:867–872 [DOI] [PubMed] [Google Scholar]
- 37.Cross TJS, Rizzi P, Berry PA, Bruce M, Portmann B, Harrison PM. King’s Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol 2009;21:730–738 10.1097/MEG.0b013e32830dfcb3 [DOI] [PubMed] [Google Scholar]
- 38.Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology 2005;42:282–292 [DOI] [PubMed] [Google Scholar]
- 39.Bota S, Sirli R, Sporea I, Focsa M, Popescu A, Danila M, et al. A new scoring system for prediction of fibrosis in chronic hepatitis C. Hepat Mon 2011;11:548–555 [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127:1704–1713 [DOI] [PubMed] [Google Scholar]
- 41.Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 2006;44:462–474 [DOI] [PubMed] [Google Scholar]
- 42.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The enhanced liver fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol 2013;59:236–242. 10.1016/j.jhep.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 43.Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterology 2010;10:103 10.1186/1471-230X-10-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guéchot J, Trocmé C, Renversez JC, Sturm N, Zarski JP, and the ANRS HC EP 23 Fibrostar Study Group. Independent validation of the enhanced liver fibrosis (ELF) score in the ANRS HC EP 23 Fibrostar cohort of patients with chronic hepatitis C. Clin Chem Lab Med 2012;50:693–699. 10.1515/cclm-2011-0858 [DOI] [PubMed] [Google Scholar]
- 45.EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–264. 10.1016/j.jhep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 46.Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2008;15:212–218. 10.1111/j.1365-2893.2007.00932.x [DOI] [PubMed] [Google Scholar]
- 47.Tachi Y, Hirai T, Toyoda H, Tada T, Hayashi K, Honda T, et al. Predictive ability of laboratory indices for liver fibrosis in patients with chronic hepatitis C after the eradication of hepatitis C virus. Plos One 2015;10: e0133515 10.1371/journal.pone.0133515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontana RJ, Bonkovsky HL, Naishadham D, Dienstag JL, Sterling RK, Lok AS, et al. ; HALT-C Trial Group. Serum fibrosis markers levels decrease after successful antiviral treatment in chronic hepatitis C patients with advanced fibrosis. Clin Gastroenterol Hepatol 2009;7:219–226. 10.1016/j.cgh.2008.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez SM, Fernandez-Varo G, Gonzalez P, Sampson E, Bruquera M, Navasa M, et al. Assessment of liver fibrosis before and after antiviral therapy by different serum marker panels in patients with chronic hepatitis C. Aliment Pharmacol Ther 2011;33:138–148 10.1111/j.1365-2036.2010.04500.x [DOI] [PubMed] [Google Scholar]
- 50.Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011;140:1970–1979. 10.1053/j.gastro.2011.02.058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.