Abstract
Traumatic brain injury (TBI) often has long-term debilitating sequelae in cognitive and behavioral domains. Understanding how TBI impacts functional integrity of brain networks that underlie these domains is key to guiding future approaches to TBI rehabilitation. In the current study, we investigated the differences in inter-hemispheric functional connectivity (FC) of resting state networks (RSNs) between chronic mild-to-severe TBI patients and normal comparisons (NC), focusing on two externally oriented networks (i.e., the fronto-parietal network [FPN] and the executive control network [ECN]), one internally oriented network (i.e., the default mode network [DMN]), and one somato-motor network (SMN). Seed voxel correlation analysis revealed that TBI patients displayed significantly less FC between lateralized seeds and both homologous and non-homologous regions in the opposite hemisphere for externally oriented networks but not for DMN or SMN; conversely, TBI patients showed increased FC within regions of the DMN, especially precuneus and parahippocampal gyrus. Region of interest correlation analyses confirmed the presence of significantly higher inter-hemispheric FC in NC for the FPN (p < 0.01), and ECN (p < 0.05), but not for the DMN (p > 0.05) or SMN (p > 0.05). Further analysis revealed that performance on a neuropsychological test measuring organizational skills and visuo-spatial abilities administered to the TBI group, the Rey-Osterrieth Complex Figure Test, positively correlated with FC between the right FPN and homologous regions. Our findings suggest that distinct RSNs display specific patterns of aberrant FC following TBI; this represents a step forward in the search for biomarkers useful for early diagnosis and treatment of TBI-related cognitive impairment.
Key words: : externally oriented networks, inter-hemispheric functional connectivity, internally oriented networks, resting state fMRI, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a global public health issue with the incidence of TBI rising worldwide.1 In the United States alone, more than 1.7 million cases are reported annually. Although outcomes following TBI are highly variable, in about one out of five cases TBI marks the onset of long-term disabilities, evolving into persistent impairments in domains such as attention, memory, movement, sensation, executive functions and social conduct.2,3 In an effort to design ad hoc effective rehabilitation protocols and to guarantee TBI patients a higher quality of life, interdisciplinary research that combines neuropsychology and neuroimaging has endeavored to obtain a better understanding of TBI outcomes on the brain and behavior. In particular, cognitive neuropsychology and brain imaging methods have been recruited with the aim of gaining insight into how specific profiles of behavioral impairment may correspond to specific patterns of functional impairment and/or anatomical brain damage.4
Individuals with TBI usually manifest a variety of co-existing complaints and complex cognitive and behavioral clinical outcomes; this observation hints at the fact that long-term impairment following brain injury parallel abnormalities not (or not entirely) due to damage in focal brain regions, but in the interactions between areas that participate in networks of functionally and/or anatomically connected neural systems.5 Indeed, although TBI, especially in severe cases, may be accompanied by focal lesions caused by cerebral contusion, it most frequently presents white matter microstructure changes and is characterized by diffuse axonal injury, a result of axon shearing due to the force of the angular and linear acceleration of the soft brain tissue inside the skull at the moment of impact.6
Resting state functional magnetic resonance imaging (rs-fMRI) is a widely used approach to explore the functional integrity of neural systems.7 Resting state fMRI scans are collected as participants lie in the scanner without engaging in a specific task; the resulting patterns of synchronous activity at rest resemble functional networks typically observed during performance of cognitive tasks.8,9 In this way, rs-fMRI offers a tool for a task-independent measure of functional integrity of large-scale cognitive systems. Analyses of rs-fMRI data often measure the level of synchrony, or functional connectivity (FC), by computing correlations between blood oxygen–level dependent (BOLD) signal fluctuations of brain regions of interest (ROIs) proposed to work together as parts of functional networks. The methodological advantages of measuring FC with this cognitive task-free data collection method, in contrast to task-related fMRI, include greater generalizability for predicting behavior, lack of practice or retest effects, and perhaps most importantly when dealing with a TBI population, the elimination of possible confounds due to task difficulty or floor/ceiling effects.10
Several studies have begun to characterize what aberrant patterns of FC at rest are associated with TBIs of different severities. In particular, research has focused on characterizing the consequences of TBI on FC between specific ROIs in three resting state networks (RSNs) that have been found to support some of the cognitive functions most commonly impaired in TBI populations: the default mode network (DMN), the fronto-parietal network (FPN), and the executive control network (ECN).11–14 At a broader level it is useful to conceptualize the DMN as an internally oriented network (ION) and the FPN and ECN as externally oriented networks (EONs) based on the endogenous or exogenous quality of the information they manipulate, respectively.15
The DMN comprises anterior medial prefrontal cortex, posterior cingulate cortex (PCC), medial temporal lobe (MTL), and lateral parietal areas, and it is known to have a role in internally-directed cognition, such as autobiographical memory, theory of mind and future oriented thought.16 FC in the DMN has been consistently found to be abnormal in both mild and severe TBI populations, albeit with seemingly contradictory findings. For instance, two studies noticed greater FC within the DMN for TBI, compared with healthy controls, particularly with the precuneus and posterior cingulate cortex17,18; however, others reported less FC within the DMN for TBI, compared with controls, especially between more posterior regions of the DMN.19–21 These differences may be due to the heterogeneity of analytical frameworks employed, time elapsed since onset of injury, TBI severity, and extent of gray and white matter damage.
The FPN comprises the dorsolateral frontal and parietal cortices, and has traditionally been considered part of a broader “task positive” network due to its association with orienting attention to the environment rather than the self. It has been postulated that the FPN is involved in executive functions and externally-directed cognitive abilities, such as goal directed behavior—all higher level functions that consistently have been found to be impaired in TBI populations of all severity levels.12,15,22–24 Similarly, the ECN includes mainly frontal regions involved in executive processes (goal oriented action and inhibition), emotion, and perception.9 Not surprisingly, several studies on TBI samples have discovered deficits in FC between regions in the FPN, some additionally reporting associations between neuroimaging findings and behavioral performance.18,25,26
Interestingly, some recent task-related and rs-fMRI studies have demonstrated less inter-hemispheric FC in their TBI samples, compared with healthy controls, with reports of less FC between motor areas in a sample including mild, moderate, and severe TBI patients, between left and right hippocampi and anterior cingulate cortices in acute severe TBI patients, and between homologous prefrontal, parietal and hippocampal ROIs in acute mild TBI individuals.27–32 In particular, Sours and colleagues29 observed positive correlations between cognitive performance on subtests of a computerized cognitive assessment battery and inter-hemispheric FC in acute and sub-acute stages of TBI, while Marquez de la Plata and colleagues28 found that bilateral hippocampal connectivity was associated with memory skills. These findings are perhaps not surprising, given that inter-hemispheric FC has been associated with, albeit not always in a linear fashion, white matter integrity, which often shows axonal degeneration following a TBI episode.27–32
To date, although some effort has been expended trying to identify the precise patterns of aberrant FC following TBI, no work has compared inter-hemispheric functional disconnection across distinct RSNs within a sample. Thus, the main purpose of the current study was to examine the differences in inter-hemispheric FC across large-scale brain networks. In particular, we explored the possible clinical significance of abnormal inter-hemispheric FC in EONs (FPN and ECN), an ION (DMN), and a fourth network (somato-motor network [SMN]) specifically selected to investigate the behavior of sensory-motor network FC following TBI damage. We obtained rs-fMRI data on a sample of 21 TBI individuals in the chronic stage ranging from mild to severe and 21 healthy comparison participants using ROIs from RSNs. We hypothesized that TBI individuals would show differences in FC between homologous areas of EON's, an ION, and a sensory network when compared with apparently healthy individuals matched for sex, education, age, and handedness.
Methods
Experiment 1
Participants
Individuals with TBI were recruited through the Traumatic Brain Injury Registry at the University of Iowa. All TBI patients were right handed and in the chronic stage of their injury (more than 6 months since injury onset, as in TBI most of the behavioral recovery is believed to occur during the first 6 months following injury; Table 1).33,34 The Glasgow Coma Scale (GCS), in combination with available information on loss of consciousness (LOC), post-traumatic anterograde amnesia (PTA) and acute computed tomography (CT) findings, were employed to assess TBI severity in correspondence with the criteria described in the Mayo Classification System.35,36 The Mayo Classification System was selected because of the opportunity it offers to maximally capitalize on the positive findings available for each individual TBI patient and their trauma-related history in order to retrospectively determine TBI severity.
Table 1.
Demographic and Injury Information for Patients with Traumatic Brain Injury
| Subject | Age | Sex | Education (years) | Etiology | GCS | Retrograde amnesia | PTA | LOC | Acute CT findings | Focal lesions (chronic MRI) | Severity | Chronicity (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TBI1 | 71 | F | 12 | Fall | 3 | 2 h | 22 days | 2 weeks | SAH (required hemicraniotomy) | Left temporal lobe, Right Frontal lobe | Moderate-severe | 8.6 |
| TBI2 | 24 | M | 15 | Fall | 8 | 15 min | 2 days | N/A | Bifrontal contusions (required craniotomy) | Bilateral frontal lobe, right temporal pole, left cerebellum | Moderate-severe | 33.4 |
| TBI3 | 25 | M | 15 | Fall | N/A | A few minutes | 1 day | ∼2 days | Basilar skull fracture | Bilateral frontal pole and orbital regions | Moderate-severe | 55.4 |
| TBI4 | 64 | F | 12 | Fall | N/A | A few minutes | A few minutes | N/A | SAH | No | Moderate-severe | 11.2 |
| TBI5 | 70 | M | 20 | Fall | 15 | A few minutes | A few minutes | N/A | Small SDH | No | Moderate-severe | 145.7 |
| TBI6* | 50 | M | 12 | Fall/MVA | N/A / 15 | A few seconds / A few seconds | N/A / N/A | 1 day/No | N/A / N/A | No | Moderate-severe | 277.8/48.2 |
| TBI7 | 48 | F | 16 | MVA, unhelmeted | 6 | 1 year | Duration unclear | Duration unclear | SAH | Left superior frontal gyrus | Moderate-severe | 15.2 |
| TBI8 | 41 | F | 13 | Fall | N/A | No | No | A few minutes | SAH | No | Moderate-severe | 22.5 |
| TBI9 | 59 | M | 12 | Fall | N/A | A few minutes | 1 month | A few seconds | Negative | No | Mild | 18.6 |
| TBI10 | 53 | F | 13 | Fall | 15 | A few minutes | A few minutes | A few minutes | Bifrontal hemorrhagic contusions | Bilateral frontal pole | Moderate-severe | 44.5 |
| TBI11 | 60 | F | 14 | Fall | N/A | A few minutes | A few hours | N/A | SAH | No | Moderate-severe | 12.0 |
| TBI12 | 21 | M | 13 | Fall | 15 | None | N/A | Duration unclear | EDH, right temporal bone fracture (required craniotomy) | No | Moderate-severe | 12.7 |
| TBI13 | 63 | M | 15 | MVA | N/A | No | No | No | N/A | No | Mild | 37.3 |
| TBI14 | 43 | F | 16 | Hit | N/A | A few minutes | 2 months | 5 min | SAH, occipital skull fracture | Right frontal lobe | Moderate-severe | 18.9 |
| TBI15 | 63 | M | 14 | Fall | N/A | N/A | 12 h | 4–5 h | SAH | No | Moderate-severe | 20.2 |
| TBI16 | 42 | F | 16 | MVA | N/A | N/A | N/A | Several hours | Intracranial hemorrhage (require craniotomy) | Right middle frontal gyrus | Moderate-severe | 297.4 |
| TBI17 | 69 | F | 12 | UVA, unhelmeted | N/A | A few seconds | A couple of weeks | A few minutes | N/A | Left inferior temporal gyrus | Moderate-severe | 374.8 |
| TBI18 | 54 | F | 12 | UVA, unhelmeted | N/A | None | A couple of weeks | 20 min | Negative | No | Moderate-severe | 29.5 |
| TBI19 | 52 | M | 19 | MVA, helmeted | 13 | N/A | N/A | 3–5 min | SAH | No | Moderate-severe | 17.5 |
| TBI20* | 60 | M | 14 | Fall/MVA | N/A / 15 | N/A / No | N/A / No | A few minutes/No | N/A | No | Mild | 52.7/37 |
| TBI21 | 55 | M | 13 | MVA, helmeted | 15 | 14 h | 2 weeks | Duration unclear | SAH | No | Moderate-severe | 37 |
| TBI group (mean ± SD) | 51.76 (±14.7) | 11 (M) | 14.19 (±2.27) | |||||||||
| NC group (mean ± SD) | 52.19 (±14.6) | 11 (M) | 14.79 (±2.22) |
Sustained two TBIs in two different occasions. All information was collected via patient/collateral report or, when available, via inspection of medical records.
GCS, Glasgow Coma Scale; PTA, post-traumatic amnesia; LOC, loss of consciousness; CT, computed tomography; MRI, magnetic resonance imaging; F, female; TBI, traumatic brain injury; SAH, subarachnoid hemorrhage; M, male; N/A, not available; SDH, subdural hemorrhage; EDH, epidural hemorrhage; UVA, unmotored vehicle accident.
Participants were classified as A) mild when GCS was 13–15, acute CT findings were unremarkable and no focal lesions were visible on a chronic MRI, LOC was 30 min or less and PTA was shorter than 24 h; or B) moderate-severe, when GCS was less than 12, positive acute CT findings or chronic intracranial abnormality defined as focal lesions visible on MRI were present, LOC was longer than 30 min, and PTA longer than 24 h. If only one of these criteria was met (i.e., GCS lower than 13, LOC longer than 30 min, PTA longer than 24 h or presence of any trauma-related abnormality), it was sufficient to exclude the patient from the mild TBI group and to classify them as moderate-severe (Table 1). As these are all common occurrences following TBI, perhaps not surprisingly 18 out of 21 participants were assigned to the moderate to severe group and only three to the mild group. However, it is important to mention that other classification systems would lead to different characterizations of the sample; in particular, those participants who are assigned to the moderate-severe group merely due to the presence of skull or intracranial abnormalities would be defined as complicated-mild TBI or high-risk mild TBI patients.37,38 All TBI participants were cleared for contraindication to MRI scanning.
Normal healthy comparison participants (NC; n = 21) were sampled from a large existing database of participants scanned at the University of Illinois on the same scanner model. Participants in this database were recruited from the community of Urbana-Champaign, Illinois. For NC participants, eligibility criteria included: 1) right handedness (at least 75% on the Edinburgh Handedness Questionnaire); 2) age between 18 and 80; 3) no previous history of psychiatric and neurological illness or traumatic brain injury; 4) a score >27 on the Mini-Mental State Exam; 5) normal or corrected-to-normal vision of at least 20/40 and no color blindness; and 6) suitability for MRI environment. Each TBI patient was matched pairwise with a NC participant for sex, age, education and handedness. A two-tailed t-test revealed that the TBI and NC groups did not significantly differ in age (t = 20, p > 0.5) or education (t = 20, p > 0.5; Table 1).
All participants signed a written informed consent and were compensated for their participation. The study was approved by the Institutional Review Boards at University of Iowa and the University of Illinois-Urbana Champaign.
Neuroimaging data
For both groups, all neuroimaging data were collected during a single session. Data for TBI participants were acquired at the University of Iowa on a 3T whole-body MRI scanner (Magnetom TIM Trio; Siemens Healthcare, Erlangen, Germany) operated with a 12-channel RF head receive coil. High resolution T1-weighted brain images were acquired using a three-dimensional (3D) Magnetization Prepared Rapid Gradient Echo Imaging (MPRAGE) protocol with 208 contiguous coronal slices, echo time (TE) = 3.04 msec, repetition time (TR) = 2530 msec, field of view (FOV) = 256 mm2, voxel size = 1 mm3, and flip angle = 10°. T2*-weighted resting state data were collected with a fast echo planar imaging (EPI sequence) with BOLD contrast (6 min, TR = 2000 msec, TE = 30 msec, 31 slices acquired in ascending ordervoxel size: 3.4 × 3.4 × 3.5mm, 64 × 64 matrix, flip angle = 75°).
MRI data for the NC group was collected using a 3T Siemens Trio Tim system at the University of Illinois-Urbana Champaign. High resolution T1-weighted brain images were acquired using a 3D MPRAGE protocol with 192 slices (TE = 3.32 msec, TR = 1900 msec, FOV = 230 mm2, voxel size = 0.9 mm3, and flip angle = 9°). Resting state images were acquired with a fast EPI sequence (6 min, TR = 2000 sec, TE = 25 msec, 35 slices acquired in ascending order, voxel size 3.4 × 3.4 × 4 mm, 64 × 64 matrix, flip angle = 80°). During resting state data collection, all participants were instructed to keep their eyes closed.
Pre-processing
Functional MRI data pre-processing was carried out using FSL 5.0.4 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl).40 The investigator who conducted the analysis was not blinded to whether the data analyzed was collected from TBI or NC participants. High-resolution T1 images were skull stripped using BET and the resulting masks were further manually inspected and corrected.41 EPI data were then motion corrected using MCFLIRT, and brain extracted and spatially smoothed using a full width at half maximum 6.0 mm Gaussian kernel. Single-subject independent component analysis (ICA) was computed with MELODIC and each component was visually inspected and manually classified as signal (components of interest) or noise (e.g., collection artifacts, signal of non-neural origin) by two independent raters using a custom-made graphic user interface (https://github.com/ktera/fmri-ica-gui).42–44 A moderator, who made the final classification, further rated components on which the two raters did not achieve agreement. Inter-rater reliability analysis using Cohen's kappa statistics was performed to determine consistency among raters, and it was found that κ = 0.759, with a disagreement of 9%, interpreted as substantial agreement.45 The following steps included temporal filtering for frequencies below 0.008 and above 0.1 Hz, nuisance regression with FEAT (using nuisance ROIs placed in white matter and cerebrospinal fluid of the left ventricle and six motion parameters), global signal regression and volume censoring based on a conjunction of BOLD signal spikes and motion.46 No volumes were scrubbed from any of the participants EPI data, likely due to ICA denoising and the use of the simultaneous nuisance regression approach suggested by Hallquist and colleagues.47 Spatial normalization of the functional images to the Montreal Neurological Institute (MNI) 2-mm template brain was computed using the boundary based registration algorithm.48 Registration from MPRAGE to MNI space was computed using FNIRT with the default 10 mm warp resolution. The two resulting transformations were concatenated and applied to the original EPI data to transform it into standard MNI space. This process allowed to better account for local anatomical variability due to atrophy and ventricle deformation.49,50
A research specialist with extensive experience in processing and analyzing neuroimaging data collected on neurological patients with focal lesions and blinded to diagnosis examined each participant's T1 for visible trauma-related focal lesions and identified eight participants who clearly showed distinct regions of atrophy possibly due to head contusion. Focal lesions were hand traced using FSLVIEW and the resulting masks were used during spatial normalization with FNIRT in order to increase the quality of the fit to the MNI brain.
Data for the TBI and NC groups were collected in separate scanners and with two slightly different protocols. In order to clarify the possible confounding effect of the multi-site acquisition, signal-to-noise (SNR) analysis was performed to determine if the marked differences between the two groups could be explained by different scanner properties (supplementary text and Tables S1 and S2; see online supplementary material at www.liebertpub.com).
Seed analysis
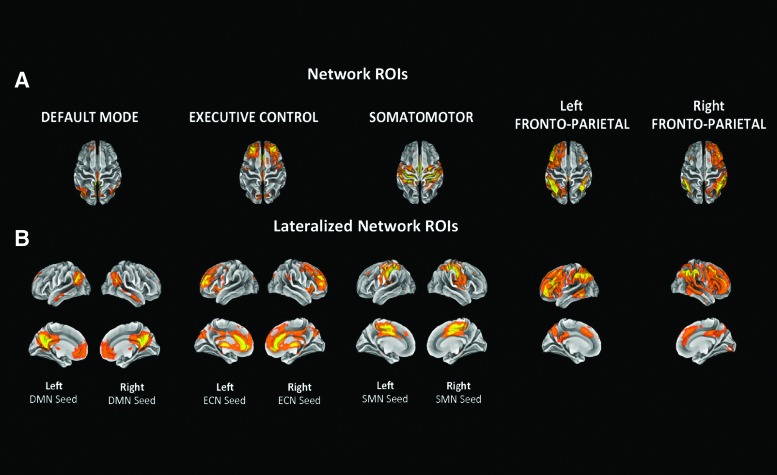
RSNs generated by Smith and colleagues9 and available at http://fsl.fmrib.ox.ac.uk/analysis/brainmap+rsns were used as network ROIs (also known as “seeds”) during the seed analysis. The RSNs were derived applying group ICA to extract 20 components from a 36 subjects rs-fMRI dataset, and were found to match considerably to 10 components resulting from group ICA carried out on the 29,671-subject BrainMap activation database.9 In addition, these components highly resemble the ones generated by the Group ICA analysis performed by Stevens and colleagues18 on a mild TBI sample, thus eliminating the potential concern in employing FC maps derived from a healthy sample to study a clinical population dataset.18 In particular, from the Smith database we selected as seeds RSN 420 (DMN), 620 (SMN), 820 (ECN), and 920 and 1020 (right and left FPN, respectively; Fig. 1A).9 Both ECN and FPN have been found to be crucial for externally-focused attention, and thus were selected as examples of EONs, while the DMN served as the ION, and the SMN as a sensory network.12,15 In addition, in order to better investigate inter-hemispheric FC differences between TBI patients and control participants, for each RSN, FSL was used to create symmetrical left and right lateralized seeds, only including network regions situated in one hemisphere (excluding the left and right FPNs, as they already showed strong lateralization; Fig. 1B). Lateralized maps were utilized for the seed analysis. All ROIs were generated from the Smith network maps with a threshold of Z > 2.33.
FIG. 1.
Five resting state networks (RSNs)9 were selected as regions of interest (ROIs) for the seed analysis. For each RSN, FSL was used to generate symmetrical left and right lateralized seeds, only including network regions situated in one hemisphere. Given their already strongly lateralized nature, left and right fronto-parietal network map were not modified before being used as ROIs. A threshold of Z > 2.33 was applied to all maps to create network-based ROIs. The networks are shown on the transverse plane (A) and on the sagittal plane (B). Color image is available online at www.liebertpub.com/neu
The concatenated transform obtained during pre-processing was applied to transform the seeds from MNI to native space and again to transform the seed correlation maps to MNI space. ROI correlation analysis included computing voxel-wise Pearson coefficients between the corrected average time series extracted from a given seed network with the corrected time series for each voxel in the brain; in order to ensure a normal distribution of the r values, MATLAB (2014a, The MathWorks, Inc., Natick, Massachusetts) was used to transform the resultant statistical maps to Fisher's z-scores.51 A four-dimensional image file was created by concatenating individual network seed maps and FSL's flameo tool was used to perform a between participants ordinary least-square regression.52
To determine statistical significance and account for multiple comparisons, each network seed map was thresholded at Z > 3.11, with a cluster significance of p < 0.05. For each network seed, the resulting Z-statistic map represented a group average and a contrast of group differences. After visual inspection, anatomical labels were assigned by referencing the Harvard-Oxford Cortical and Subcortical Structural Atlases in the FSL analysis package.53,54
In order to obtain correlation values between different ROI pairs, the mean time series of all voxels contained in each ROI was extracted and correlated with the mean time series of all other ROIs. The resultant Pearson coefficient was then converted into a Fisher's z-score as was done for the voxel-wise analysis. Functional connectivity matrices and connectivity dendrograms were separately generated for each group using the obtained Fisher's z-values and Matlab's heatmap, linkage, and dendrogram functions. Further statistical analysis on these data was carried out using SPSS (released 2012, IBM SPSS Statistics for MacIntosh, Version 21.0; IBM Corp., Armonk, NY).
Lastly, in order to verify whether inter-hemispheric FC differences between groups were due to the presence of trauma-related focal lesions in the TBI sample, the same analyses were repeated eliminating the participants with lesions from the TBI sample and their match from the NC group.
Experiment 2
Participants and behavioral data
Participants included the same 21 TBI participants from experiment 1. Each of the TBI participants were administered a paper version of the Rey Osterrieth Complex Figure Test-Copy (ROCFT-C) and the Rey Osterrieth Complex Figure Test-Delayed Recall (ROCFT-DR). As no comparable neuropsychological behavioral data were collected for all of the NC group, TBI participants' ROCFT scores were z transformed in order to compare their performance with the population mean.
The ROCFT score collected for the TBI participants were z-transformed using internally generated normative data.55 Inspection of the standard scores revealed that 75% of the sample placed below average (Z < 0), with 29% one SD below average (Z < 1) and 15% two deviation standards below mean (Z < −2; Table 2). For the subset of the TBI group for whom GCS information was available (n = 10), there was no correlation between GCS and performance on the ROCFT-C (r = −0.211, p = 0.559), nor between GCS and ROCFT-DR (r = 0.254, p = 0.478). As the ROCFT–DR has been found to be more strongly associated with executive functions than ROCFT-C, the former measure was regarded as more inclusive and globally representative of TBI patients' functioning, and used as a covariate of interest to investigate how inter-hemispheric FC may correlate with neuropsychological measures.56
Table 2.
Performance on the Rey Osterrieth Complex Figure Test
| Rey Osterrieth Complex Figure Test (z-scores) | ||
|---|---|---|
| Copy | Delayed recall | |
| Mean | −0.641 | −0.705 |
| SD | 1.294 | 1.114 |
| Z > 1 (N) | 2 | 2 |
| −2 < Z < −1 (N) | 3 | 6 |
| Z < −2 (N) | 2 | 3 |
SD, standard deviation.
Analysis of the relationship between FC and RCFT-delayed performance
FSL's flameo tool was used to perform an ordinary least square regression entering as a factor the demeaned z-scores for the ROCFT-DR.52 The association between FC and performance of TBI participants in the ROCFT-DR was assessed by examining the correlation between normalized performance scores and FC in network seed maps. Z-statistic maps were thresholded at Z > 3.11, with a cluster significance of p < 0.05. All reported results are corrected for age, sex, and education.
Results
Experiment 1
Group differences in FC
Peaks for the FC maps obtained for each ROI network seed are reported in Table 3.
Table 3.
Functional Connectivity Clusters and Peaks
| TBI > NC | NC > TBI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak (mm) | Peak (mm) | |||||||||||
| Seed network | Cluster size | x | y | z | Peak Z statistic | Anatomical location* | Cluster size | x | y | z | Peak Z statistic | Anatomical location* |
| Left FPN | 887 | 26 | 26 | 56 | 4.35 | Right superior frontal gyrus | ||||||
| 566 | 26 | 50 | 12 | 4.36 | Right frontal pole | |||||||
| 429 | 60 | −52 | 38 | 4.91 | Right angular gyrus | |||||||
| 298 | −42 | −64 | −40 | 4.02 | Left lateral superior occipital cortex | |||||||
| 235 | 8 | −28 | −14 | 4.35 | Right brain stem | |||||||
| Right FPN | 214 | −36 | −88 | 2 | 4.09 | Left lateral inferior occipital cortex | 1351 | −22 | 26 | 54 | 4.58 | Left superior frontal gyrus |
| 599 | −6 | −12 | 10 | 4.81 | Left thalamus | |||||||
| 585 | −44 | −62 | 48 | 4.47 | Left lateral superior occipital cortex | |||||||
| 285 | 52 | −60 | 38 | 4.14 | Right lateral superior occipital cortex | |||||||
| 245 | −52 | 30 | 10 | 4.32 | Left inferior frontal gyrus | |||||||
| 215 | −34 | −76 | −42 | 4.6 | Left cerebellum | |||||||
| Left ECN |
354 | 46 | −44 | 52 | 4.06 | Right supramarginal gyrus | ||||||
| 202 | 40 | −80 | 36 | 4.32 | Right superior lateral occipital cortex | |||||||
| 169 | 46 | 52 | 4 | 3.77 | Right frontal pole | |||||||
| Right ECN | 779 | −8 | −84 | 44 | 5.21 | Left superior lateral occipital cortex | 570 | −46 | 4 | 44 | 5.22 | Left precentral gyrus |
| 308 | 58 | −28 | −2 | 4.76 | Left posterior superior temporal gyrus | 204 | −48 | 36 | 2 | 4.3 | Left inferior frontal gyrus | |
| 186 | −38 | −78 | 48 | 3.85 | Left superior lateral occipital cortex | |||||||
| 186 | −36 | −64 | −42 | 4.51 | Left Cerebellum | |||||||
| Left SMN | 1259 | 10 | −50 | 24 | 4.5 | Right posterior cingulate gyrus | ||||||
| 286 | 58 | −10 | −20 | 4.11 | Right middle temporal gyrus | |||||||
| 272 | −22 | 24 | 48 | 4.23 | Left superior frontal gyrus | |||||||
| 181 | 44 | −56 | 22 | 3.8 | Right angular gyrus | |||||||
| 179 | 20 | 32 | 46 | 4.56 | Right superior frontal gyrus | |||||||
| Right SMN | 395 | 6 | −70 | −16 | 4.56 | Right lingual gyrus | 1304 | −14 | −50 | 24 | 4.54 | Left precuneus |
| 263 | −34 | 46 | 12 | 3.81 | Left frontal pole | 534 | −50 | −24 | −2 | 4.79 | Left posterior superior temporal gyrus | |
| 347 | −48 | −58 | 22 | 4.3 | Left angular gyrus | |||||||
| 267 | 4 | 28 | −10 | 4.3 | Right subcallosal cortex | |||||||
| 266 | 60 | −20 | 0 | 4.55 | Right posterior superior temporal gyrus | |||||||
| Left DMN | 1412 | 2 | −66 | 38 | 4.71 | Right precuneus | 719 | 44 | −30 | 64 | 4.53 | Right postcentral gyrus |
| 400 | −30 | −24 | −20 | 4.76 | Left posterior parahippocampal gyrus | |||||||
| 221 | 28 | −16 | −8 | 4.36 | Right amygdala | |||||||
| Right DMN | 1132 | 8 | −60 | 20 | 4.6 | Right precuneus | 267 | −42 | 48 | −4 | 4.01 | Left frontal pole |
| 188 | 28 | −36 | 58 | 4.19 | Right postcental gyrus | |||||||
Anatomical labels were assigned by imposing each peak on two probabilistic atlases, the Harvard-Oxford Cortical and Subcortical Structural Atlases in the FSL analysis package. For the cerebellum, labels were assigned following visual inspection. Cluster units are number of voxels in MNI space.
TBI, traumatic brain injury; NC, normal comparisons; FPN, fronto-parietal network; ECN, executive control network; SMN, somato-motor network; DMN, default mode network; MNI, Montreal Neurological Institute.
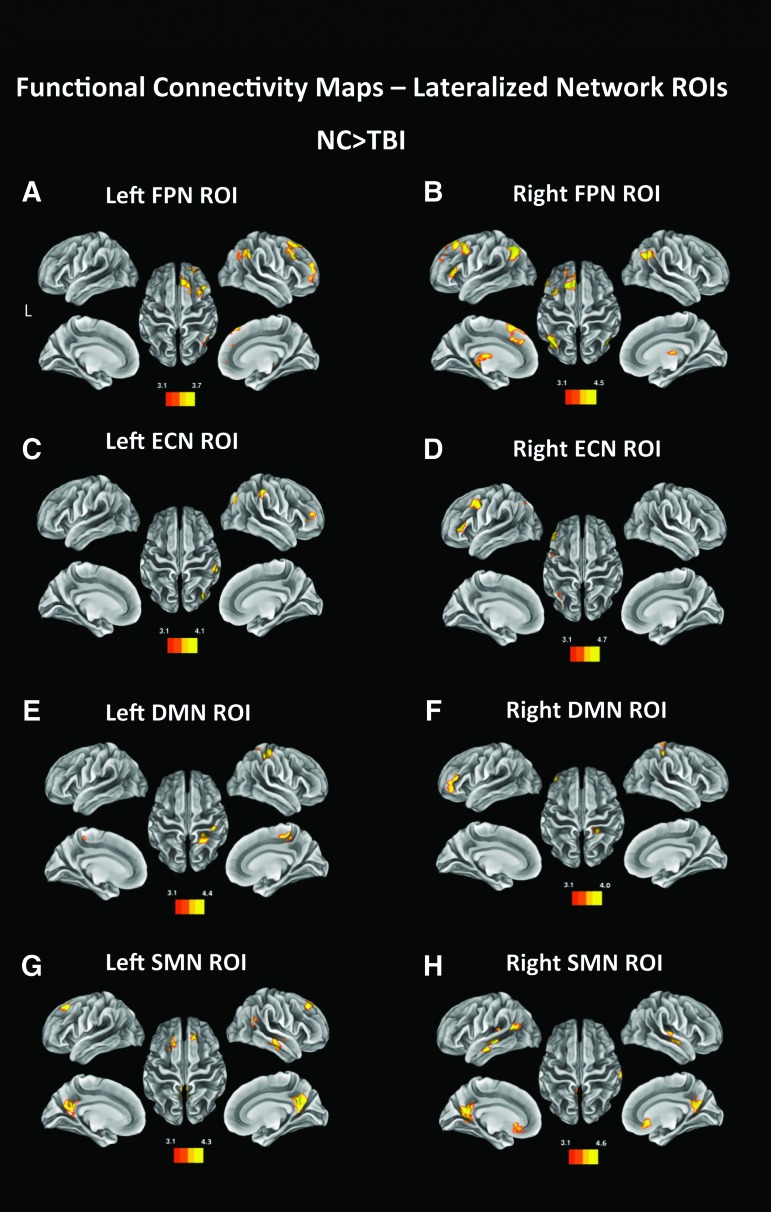
Left FPN
Based on the results of a t-contrast (NC > TBI), NC participants had more FC than the TBI patients between the left FPN lateralized seed and regions of the right FPN in the right frontal and parietal lobes, in particular the right superior and middle frontal gyrus, the right frontal pole, and the right angular gyrus (Table 2). In addition, NC participants showed more FC between the left FPN and bilateral regions in the thalamus (Fig. 2A). A second t-contrast (TBI > NC) revealed no regions of higher FC with the Left FPN in the TBI group compared with NC.
FIG. 2.
Functional connectivity maps generated by performing a seed analysis using left fronto-parietal network (FPN; A), right FPN (B), left executive control network (ECN; C) and right ECN (D), left default mode network (DMN; E), right DMN (F), left SMN (G), and right DMN (H) as regions of interest (ROIs). The images are thresholded at Z > 3.11, with a cluster significance of p < 0.05 and show the regions displaying higher functional connectivity between the seeded ROIs and areas included in the maps in the normal comparison sample than in the traumatic brain injury (TBI) group. Color image is available online at www.liebertpub.com/neu
Right FPN
Similarly to the left FPN seed, for NC participants, the right FPN seed had more FC with the frontal and parieto-occipital lobe of the left hemisphere, with clusters in the medial portion of the superior frontal gyrus, middle and inferior frontal gyrus, superior parietal lobule (angular gyrus), dorso-medial part of the occipital cortex, and left thalamus. NC participants also showed higher FC with the right angular gyrus and right thalamus (Fig. 2B). TBI patients showed significantly more FC between the right FPN and the left inferior occipital cortex, a region not included in the left FPN (Table 2).
Left ECN
Compared with the TBI group, the NC group had significantly more FC between the left ECN lateralized seeds and three right hemisphere regions, the supramarginal gyrus, the occipital cortex and the middle frontal gyrus (Fig. 2C). Interestingly, none of these areas is homologous to regions comprised in the left ECN, nor overlaps with the right ECN. A t-contrast (TBI > NC) showed no clusters of significantly higher FC with the left ECN.
Right ECN
NC participants displayed significantly more FC between the right ECN seed and the left middle and inferior frontal gyrus, and some smaller clusters in the left superior parieto-occipital cortex (Fig. 2D). Conversely, TBI patients showed more FC with the right superior temporal gyrus and with a large cluster in the superior occipital cortex (Table 2).
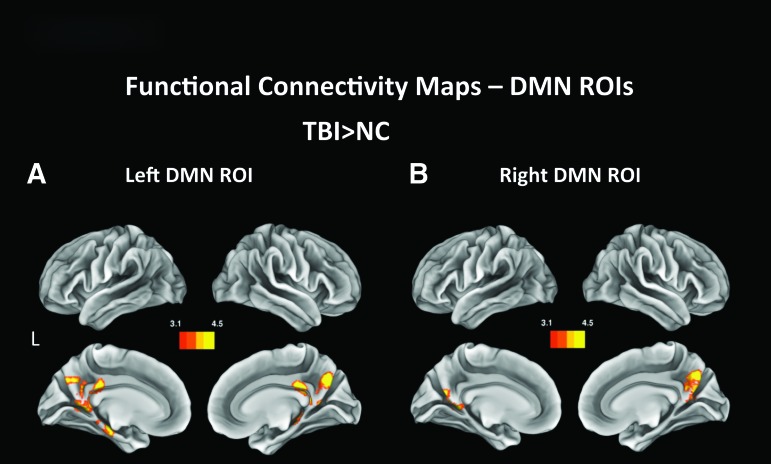
Left DMN
NC participants displayed significantly more FC between the left DMN and the right postcentral gyrus and superior parietal lobule than TBI individuals (Fig. 2E). TBI patients had more FC between the left DMN lateralized seed and bilateral hippocampi and parahippocampal gyri, bilateral precuneus and PCCs (Fig. 3A).
FIG. 3.
Functional connectivity maps generated by performing a seed analysis using the left (A) and right (B) default mode network (DMN) as regions of interest (ROIs). The images are cluster thresholded for p < 0.05 and for Z < 3.11 and show regions displaying higher functional connectivity between the seeded ROI and areas included in the bilateral DMN. Color image is available online at www.liebertpub.com/neu
Right DMN
NC showed higher FC with a cluster in the inferior frontal gyrus (Fig. 2F), while TBI exhibited significantly more FC between the right DMN seed and both left and right precuneus (Fig. 3B).
Left SMN
FC maps revealed more FC between the left SMN and clusters in bilateral precuneus, middle and superior frontal gyri, right angular gyrus and right middle temporal gyrus in NC participants (Fig. 2G); none of these regions is comprised in the right SMN (Table 2). The TBI group showed no regions of higher FC.
Right SMN
Comparably (but symmetrically) to the left SMN seed, NC participants displayed more FC between the right SMN seed and bilateral precuneus, the right middle and superior temporal gyrus, and the left angular and supramarginal gyrus. In addition, they had more FC with the anterior cingulate cortex and the left superior and middle temporal gyri (Fig. 2G; Table 2). TBI participants revealed more FC with the right superior temporal gyrus and the cerebellum (Table 2).
ROI analysis
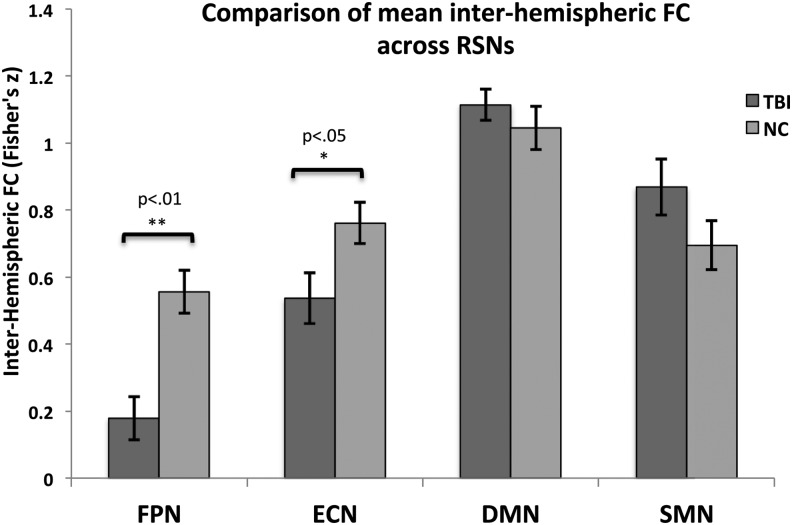
Following analyses at the voxel-wise level, in order to better quantify the inter-hemispheric disconnection effect and its relative strength across RSNs, differences in inter-hemispheric FC between TBI and NC groups were investigated with an a priori ROI approach using the Smith and colleagues' network ROI seeds.9
Independent sample two-tailed t-tests were performed to assess between-group differences in FC between homologous ROIs. Statistical analyses revealed that TBI participants had significantly less ROI to ROI FC between lateralized seeds of the FPN (t[40] = −4.134, p < 0.01) and the ECN (t[(40] = −2.313, p < 0.05), but not between left and right DMN seeds (t[40] = 0.860, p > 0.05) or SMN seeds (t[40] = 1.561, p > 0.05; Fig. 4). In order to examine the impact of the presence of mild TBI patients on these findings, we repeated the analysis removing the three mild TBI participants and their respective NC. A t-test revealed comparable results to the original sample: the TBI group had significantly less ROI to ROI FC between lateralized seeds of the FPN (t[(34] = −3.397, p < 0.01) and the ECN (t[34] = −2.129, p < 0.05), but not lateralized DMN seeds (t[34] = 1.156, p > 0.05) or SMN seeds (t[34] = 1.433, p > 0.05).
FIG. 4.
Comparison of mean functional connectivity (FC; ± standard error) between lateralized resting state network (RSN) seeds in traumatic brain injury (TBI) and normal comparison (NC) participants.
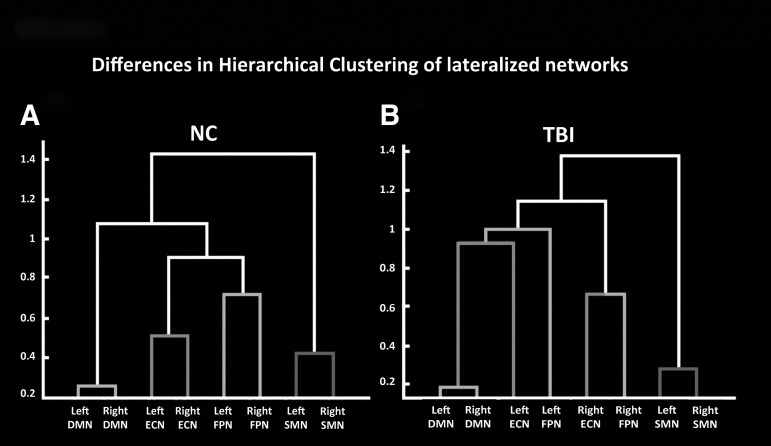
To visualize the inter-relationships between all lateralized ROIs and how they differ between groups, FC dendrograms were generated separately for NC (Fig. 5A) and TBI (Fig. 5B). This approach allows us to explore where a given network ROI may have more FC for the TBI group, compared with the NC group, and examine this across all possible inter-ROI relationships. A visual inspection of the two dendrograms revealed marked differences in lateralized RSNs clustering, especially for the ECN and the FPN. In the NC group, lateralized ROIs for the two EONs are shown to be most strongly associated with their own homologous counterparts, and secondarily with the other EON. Conversely, TBI participants exhibited clustering seemingly more based on hemispherical lateralization than on RSN membership, with higher FC between right ECN and right FPN and left ECN with DMN seeds and left FPN.
FIG. 5.
Dendrograms showing differences in network clustering between the normal comparison NC (A) and traumatic brain injury TBI (B) groups.
Experiment 2
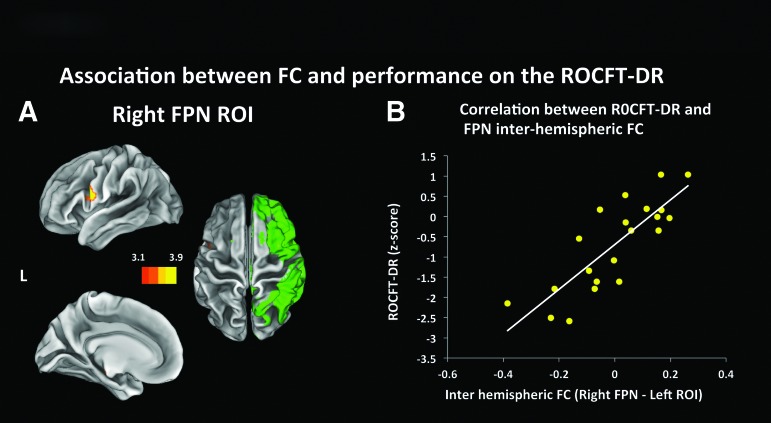
The map resulting from the addition of the ROCFT-DR performance as a covariate of interest revealed a positive linear relationship between behavioral data and the FC between the right FPN and a cluster extending from the pars opercularis of the left inferior frontal gyrus to the left ventral part of the precentral gyrus (peak at MNI coordinates (x,y,z) of (-54, 6, 16), Z = 3.97, 252 voxels in cluster), a region included in the homologous left FPN (Fig. 6A). No correlations were found for the left FPN, or the left and right ECN.
FIG. 6.
Functional connectivity maps generated by performing a seed analysis using the right fronto-parietal network (FPN) as regions of interest (ROIs) and adding the demeaned performance on the Rey Osterrieth Complex Figure Test-Delayed Recall (ROCFT-DR) as a covariate. The maps (Fig. 6A) show regions displaying a positive temporal correlation with the seeded ROIs (in green), which is in turn associated with performance on the ROCFT-DR. Figure 6B was obtained by masking the areas included in the z-statistic maps shown in Figure 6A and using it to extract the time series; Figure 6B is only reported for visualization purposes. Figure 6A is thresholded at Z > 3.11, with a cluster significance of p < 0.05. Color image is available online at www.liebertpub.com/neu
In order to visualize the association between the behavioral variables and FC and ensure that it was not driven by the presence of outliers, the average time series was extracted from ROIs defined based on the peaks in FC, and its correlation with said variables was calculated and plotted. FC between the right FPN network and the frontal ROI had a positive correlation of r = 0.818 (p > 0.001) with performance on the ROCFT-DR (Fig. 6B).
Discussion
Our analyses using both voxel wise and ROI correlation approaches revealed significantly less inter-hemispheric FC in TBI patients for EONs (FPN and ECN), but not for the DMN and the SMN. Given the selectively abnormal inter-hemispheric FC in the TBI group, we were interested in the clinical and behavioral significance of this pattern. As the ultimate goal of our research is to establish the utility of FC at rest as a potential rehabilitation-informing biomarker of cognitive impairment following TBI, we decided to investigate the parallels between FC at rest and specific behavioral impairments within TBI individuals.
To explore the relation between inter-hemispheric FC strength and behavioral performance, we examined performance of the TBI participants on a standardized neuropsychological test, the Rey-Osterrieth Complex Figure Test (ROCFT), administered outside of the scanner. We chose this measure as it is one of the four tests recommended by the American Brain Injury Consortium to assess neuropsychological outcome following TBI, and because the abilities measured by the ROCFT are commonly impaired in individuals with TBI, who often perform poorly on this task.57,58 The ROCFT is a test sensitive to detection of impairment in visuo-spatial, visuo-perceptive and visuo-constructive abilities, memory, organizational skills, and highly associated with processing speed and executive functioning, and has the potential for providing insights into the mechanisms that underlie cognitive dysfunctions after TBI. We hypothesized that reduced inter-hemispheric FC would be associated with worse performance on the ROCFT within the TBI group.56,59–61 As the aim of this analysis was exploring the relationship between inter-hemispheric connectivity and behavior, the addition of the ROCFT as a covariate of interest focused on the lateralized seeds of the two networks that had shown significantly less inter-hemispheric connectivity in the TBI group: the left and right FPN and the left and right ECN.
To our knowledge, this is the first study to investigate how inter-hemispheric FC at rest may be altered with specificity at the level of functional networks. Our finding generalizes across a broad literature because we measured inter-hemispheric FC with lateralized RSN ROIs generated by meta-analytic ICA (i.e., derived from the literature as highly replicable functional networks). The use of such a template allowed us to maintain a network perspective throughout the analysis; in addition, in a high variability population such as the one analyzed in this study, where several changes in brain structure may occur following a traumatic injury, the use of more extensive seeds addresses some potential problems that might arise with the use of smaller ROIs (e.g., the sensitivity of resting state fMRI results to ROIs coordinates, size and shape, the difficulty of establishing the most representative ROIs for a given RNS and, specifically in the TBI population, the possible confounds due to changes and shifts in brain structure following head injury).62,63
Our results with chronic TBI patients are partially consistent with results on acute and sub-acute samples by Sours and colleagues29 and Marquez de la Plata and colleagues28 using more restricted frontal and parietal ROIs. In particular, we found that TBI participants display significantly less inter-hemispheric FC, but only between networks more involved in externally oriented cognition (FPN and ECN).28,29 These RSNs repeatedly have been found to activate during tasks demanding cognitive flexibility, and to support cognitive functions necessary for successful interaction with the environment including goal directed action, set maintenance, attentional selection, encoding of salience, working memory and mental representations.12,22,64,65 Given the across-the-board impairment in attention and cognitive functions found in TBI patients of all severities and the extensive neuropsychiatric, emotional, and behavioral problems displayed in everyday life, it is crucial to uncover how these complaints map onto specific aberrant patterns of FC in these large scale networks.66,67 The marked inter-hemispheric disconnection found in our sample, coupled with the association between inter-hemispheric FC and performance on the ROCFT, has the potential of being informative about the mechanisms underlying TBI-related cognitive impairment. In particular, given the pattern of inter-hemispheric FC differences and the correlations with behavior found only in EONs, our results further our understanding of how impaired cognitive functions may selectively map onto specific attributes of specific functional systems; our findings reveal that not only the cognitive functions supported by a certain system (e.g., executive functions for the ECN), but also the specific nature of the aberrant connectivity patterns found in said system (e.g., reduced functional connectivity between homologous regions vs. globally reduced functional connectivity within a network) should be taken into consideration in the study of how behavioral dysfunction following TBI corresponds to neural activity at rest.
In our sample, the DMN inter-hemispheric FC was not reduced following TBI. We took a large-scale network-based approach and thus we did not directly use a hippocampal seed; however, the hippocampus is usually considered part of the MTL sub-network of the DMN.16 Contrary to previous reports, which found less hippocampal connectivity for TBI, compared with controls, our analyses did not reveal less inter-hemispheric FC between lateralized DMN seeds for TBIs, compared with controls.28,30 There are two possible explanations for this: first, the network maps we used as seeds might have led to different results, especially since when thresholded at Z > 2.33 they did not include the hippocampus. Consequently, although the hippocampus is generally considered part of the DMN, our investigation focused on large-scale networks and not on small circular seeds, and this might have caused our discordant findings. Secondly, the failure to replicate might be related to the chronic stage of our TBI sample, whereas the mentioned studies focused mainly on acute TBI populations.
Although inter-hemispheric FC in the ION appeared unaffected, our TBI group did show greater FC between left DMN ROI and the right precuneus and the left parahippocampal gyrus and between the right DMN ROI and the right precuneus, replicating previous results on chronic samples.17 Unfortunately, based on our findings it is impossible to conclude whether the stronger FC reflects an adaptive response to brain injury, as we did not administer tasks sensitive to DMN integrity to our TBI sample such as a sustained-attention task.17,68 It is not surprising, then, that FC between the DMN and precuneus, PCC and hippocampi did not correlate with behavioral performance on the ROCFT. Recent reports indicate that focus on the DMN and the regions it comprises, as well as their disruption following disease or injury may be key to advancing the study of how different disorders can influence brain functions and cognition.21,69,70 For this reason, evidence on the mechanisms through which DMN intra-network FC is associated with cognitive outcomes (inter-hemispheric FC vs. FC with nodes such as hippocampi and precuneus) can guide future research and inform targeted rehabilitation procedures.
Our findings regarding the SMN did not replicate the inter-hemispheric disconnection reported by Kasahara and colleagues using an event-related design, probably due to the different methodologies employed.27 Although TBI patients tend to regain their gross neuro-motor skills over time, persistence of certain abnormalities, such as tandem gait problems and postural instability can cause severe distress and decrease quality of life.27,71,72 The fact that no motor task performance was assessed for either group prevents us from making claims, but lack of a deficit in inter-hemispheric FC shown by NC, compared with screened healthy NC, suggest that subtle motor deficits following TBI may not be mediated by functional disconnection of homologous motor regions; future research should investigate this possibility by comparing FC during rest compared to tasks with respect to their predictive utility for TBI outcomes.
Finally, the differences reported in network clustering clearly demonstrate TBI can disturb network architecture and is associated with changes both within RSNs and in the relationship between RSNs.29 Previous studies have highlighted the importance of characterizing the way networks interact with each other to reach a deeper understanding of the pathophysiological mechanisms of TBI outcome.21,68 Notably, in our patient sample, FC between areas located in the same hemisphere was higher than in regions belonging to the same RSNs. Furthermore, as all our TBI patients were in the chronic stage of their injury, our results clearly demonstrate that these alterations in inter and intra network balance are long-term. Thus, our findings could serve as a starting point for future research that focuses on how specific changes in hierarchical clustering of RSNs may predict behavior following a TBI, and how FC between distinct networks and lateralized ROIs vary in their activation patterns during demanding cognitive tasks.
Limitations
The work presented here explored inter-hemispheric connectivity following TBI using an innovative large-scale network approach, which lead to robust findings important in the study of TBI, its sequelae and their biomarkers. Yet, some limitations should be noted. The first limitation concerns the structural and functional neuroimaging data being collected in different scanners for the TBI and NC groups. This could serve as a potential confound, although given the results of our SNR analysis and the nature of the results, we believe it is very unlikely that the specific group differences in inter-hemispheric connectivity could have been driven by differences in the hardware or scanning procedure. However, it is important to mention that at this stage we do not have the means to precisely quantify how much inter-scanner effects are contributing to the significant inter-group differences detected in the study. Furthermore, given the vast amount of resting state data that has and is still being collected by multicenter efforts such as the Human Connectome Project, and the growing necessity to produce studies with large effect sizes on patient groups, the comparison of data acquired in different sites and scanners to study different populations is soon to become a frequent reality; this situation will leave to each research team the responsibility of performing data analysis (e.g., SNR analysis) which can give insights on the possible confounds of different acquisition sites. The results of the SNR analysis we performed on our data clearly shows no significant differences between sites, thus increasing confidence in our findings.
While we found a significant relationship between FC and performance on a neuropsychological measure that captures the hallmark deficits of TBI, having a more extensive battery of neuropsychological tests and NC comparison data would strengthen the findings reported here. For this reason, the lack of extensive neuropsychological testing and behavioral indices for the NC poses another limitation: the two groups cannot be compared from a behavioral viewpoint, and conclusions drawn on whether the TBI group was cognitively impaired in one or more specific domains. Subsequently, without the possibility of investigating the association between behavior and FC profiles, it is difficult to interpret the clinical significance of our FC findings. However, the use of normative data allowed us to place our TBI participants below the mean, compared with a healthy population for the ROCFT, replicating previous work. The correlations found by adding behavioral measures as covariates are restricted to the TBI sample, precluding inter group comparison and begging the question of whether the relationship between cognitive abilities and FC changes qualitatively or quantitatively after TBI. Thus, future research should focus on expanding the present study's findings using a comparative framework. That said, the robust findings we reported represent a step forward in the investigation of the relationship between cognitive impairment and resting state FC in specific brain networks and in demonstrating the feasibility of a large scale network approach to the study of TBI.
Although our sample included three mild TBI participants, the vast majority was moderate to severe TBI patients. This invites caution in generalizing our findings to mild TBI populations. Similarly, all our participants were scanned over 6 months after their injury. Therefore, our findings cannot be generalized to TBI patients in the acute or sub-acute phase of their TBI, and no information on the evolution of the aberrant patterns of FC reported can be made.
Conclusion
The present study revealed that mild-to-severe chronic TBI is associated with network-specific aberrant patterns of FC: two externally oriented networks, the FPN and the ECN, showed significantly less inter-hemispheric FC in TBI patients than in normal controls, while the DMN, an internally oriented network, had more FC with PCC and hippocampi in TBI patients. In addition, the fact that inter-hemispheric FC between regions of the FPN in TBI individuals who perform better in a task that measures visuo-spatial, memory and executive function points toward the clinical relevance of our findings and their potential usefulness. The robustness of our findings shows the usefulness of employing a network perspective in the study of the relationship between FC in brain systems and complex behavior and takes us further in the search for biomarkers that will serve as a diagnostic tool and allow more precise subtyping, classification and prediction of disorder trajectories following TBI.
Supplementary Material
Acknowledgments
This work was supported in part by start-up funds provided by the University of Iowa to MD and MV, and grant funding from NIH R37 AG025667 and NIH RO1 HD069381 supported image acquisition for the normal control participants. The authors would like to thank Joel Bruss for his advice during visual inspection of the anatomical images, Jocelyn Cole for the hand-tracing of the brain masks and Ruth Henson for logistic support in scheduling TBI participants.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Roozenbeek B., Maas A.I., and Menon D.K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nature Rev. Neurol. 9, 231–236 [DOI] [PubMed] [Google Scholar]
- 2.Selassie A.W., Zaloshnja E., Langlois J.A., Miller T., Jones P. and Steiner C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 23, 123–131 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. (2014). Injury Prevention and Control: Traumatic Brain Injury. www.cdc.gov/traumaticbraininjury/
- 4.McDonald B.C., Saykin A.J., and McAllister T.W. (2012). Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav. 6, 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ham T.E. and Sharp D.J. (2012). How can investigation of network function inform rehabilitation after traumatic brain injury? Current opinion in neurology 25, 662–669 [DOI] [PubMed] [Google Scholar]
- 6.Sharp D.J., Scott G. and Leech R. (2014). Network dysfunction after traumatic brain injury. Nature Rev. Neurol. 10, 156–166 [DOI] [PubMed] [Google Scholar]
- 7.Fox M.D. and Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Rev. Neurosci. 8, 700–711 [DOI] [PubMed] [Google Scholar]
- 8.Biswal B., Yetkin F.Z., Haughton V.M., and Hyde J.S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 [DOI] [PubMed] [Google Scholar]
- 9.Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., and Beckmann C.F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 106, 13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox M.D. and Greicius M. (2010). Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., and Shulman G.L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 98, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., and Greicius M.D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spreng R.N. and Grady C.L. (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of cognitive neuroscience 22, 1112–1123 [DOI] [PubMed] [Google Scholar]
- 14.Spreng R.N., Stevens W.D., Chamberlain J.P., Gilmore A.W., and Schacter D.L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 53, 303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spreng R.N. (2012). The fallacy of a “task-negative” network. Front. Psychol. 3, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., and Buckner R.L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron 65, 550–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp D.J., Beckmann C.F., Greenwood R., Kinnunen K.M., Bonnelle V., De Boissezon X., Powell J.H., Counsell S.J., Patel M.C., and Leech R. (2011). Default mode network functional and structural connectivity after traumatic brain injury. Brain 134, 2233–2247 [DOI] [PubMed] [Google Scholar]
- 18.Stevens M.C., Lovejoy D., Kim J., Oakes H., Kureshi I., and Witt S.T. (2012). Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav. 6, 293–318 [DOI] [PubMed] [Google Scholar]
- 19.Mayer A.R., Mannell M.V., Ling J., Gasparovic C., and Yeo R.A. (2011). Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 32, 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson B., Zhang K., Gay M., Horovitz S., Hallett M., Sebastianelli W. and Slobounov S. (2012). Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. NeuroImage 59, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arenivas A., Diaz-Arrastia R., Spence J., Cullum C.M., Krishnan K., Bosworth C., Culver C., Kennard B., and Marquez de la Plata C. (2014). Three approaches to investigating functional compromise to the default mode network after traumatic axonal injury. Brain imaging and behavior 8, 407–419 [DOI] [PubMed] [Google Scholar]
- 22.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., and Raichle M.E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 102, 9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krmpotich T.D., Tregellas J.R., Thompson L.L., Banich M.T., Klenk A.M., and Tanabe J.L. (2013). Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug and alcohol dependence 129, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald B.C., Flashman L.A., and Saykin A.J. (2002). Executive dysfunction following traumatic brain injury: neural substrates and treatment strategies. NeuroRehabilitation 17, 333–344 [PubMed] [Google Scholar]
- 25.Shumskaya E., Andriessen T.M., Norris D.G., and Vos P.E. (2012). Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology 79, 175–182 [DOI] [PubMed] [Google Scholar]
- 26.Kasahara M., Menon D.K., Salmond C.H., Outtrim J.G., Tavares J.V., Carpenter T.A., Pickard J.D., Sahakian B.J., and Stamatakis E.A. (2011). Traumatic brain injury alters the functional brain network mediating working memory. Brain inj. 25, 1170–1187 [DOI] [PubMed] [Google Scholar]
- 27.Kasahara M., Menon D.K., Salmond C.H., Outtrim J.G., Taylor Tavares J.V., Carpenter T.A., Pickard J.D., Sahakian B.J., and Stamatakis E.A. (2010). Altered functional connectivity in the motor network after traumatic brain injury. Neurology 75, 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marquez de la Plata C.D., Garces J., Shokri Kojori E., Grinnan J., Krishnan K., Pidikiti R., Spence J., Devous M.D., Sr., Moore C., McColl R., Madden C., and Diaz-Arrastia R. (2011). Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch. Neurol. 68, 74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sours C., Rosenberg J., Kane R., Roys S., Zhuo J., Shanmuganathan K., and Gullapalli R.P. (2014). Associations between interhemispheric functional connectivity and the Automated Neuropsychological Assessment Metrics (ANAM) in civilian mild TBI. Brain Imaging Behav. 9, 190–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slobounov S.M., Gay M., Zhang K., Johnson B., Pennell D., Sebastianelli W., Horovitz S., and Hallett M. (2011). Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. NeuroImage 55, 1716–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson B., Zhang K., Gay M., Neuberger T., Horovitz S., Hallett M., Sebastianelli W., and Slobounov S. (2012). Metabolic alterations in corpus callosum may compromise brain functional connectivity in MTBI patients: an 1H-MRS study. Neuroscience Lett. 509, 5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Reilly J.X., Croxson P.L., Jbabdi S., Sallet J., Noonan M.P., Mars R.B., Browning P.G., Wilson C.R., Mitchell A.S., Miller K.L., Rushworth M.F., and Baxter M.G. (2013). Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proc. Natl Acad. Sci. U. S. A. 110, 13982–13987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millis S.R., Rosenthal M., Novack T.A., Sherer M., Nick T.G., Kreutzer J.S., High W.M., Jr., and Ricker J.H. (2001). Long-term neuropsychological outcome after traumatic brain injury. J. Head trauma Rehabil. 16, 343–355 [DOI] [PubMed] [Google Scholar]
- 34.Pagulayan K.F., Temkin N.R., Machamer J., and Dikmen S.S. (2006). A longitudinal study of health-related quality of life after traumatic brain injury. Arch. Phys. Med. Rehabil. 87, 611–618 [DOI] [PubMed] [Google Scholar]
- 35.Teasdale G. and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 36.Malec J.F., Brown A.W., Leibson C.L., Flaada J.T., Mandrekar J.N., Diehl N.N., and Perkins P.K. (2007). The mayo classification system for traumatic brain injury severity. J. Neurotrauma 24, 1417–1424 [DOI] [PubMed] [Google Scholar]
- 37.Williams D.H., Levin H.S., and Eisenberg H.M. (1990). Mild head injury classification. Neurosurgery 27, 422–428 [DOI] [PubMed] [Google Scholar]
- 38.Hsiang J.N., Yeung T., Yu A.L., and Poon W.S. (1997). High-risk mild head injury. J. Neurosurgery 87, 234–238 [DOI] [PubMed] [Google Scholar]
- 39.Stonnington C.M., Tan G., Kloppel S., Chu C., Draganski B., Jack C.R., Jr, Chen K., Ashburner J., and Frackowiak R.S. (2008). Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. NeuroImage 39, 1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., and Matthews P.M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1, S208–S219 [DOI] [PubMed] [Google Scholar]
- 41.Smith S.M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkinson M., Bannister P., Brady M. and Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17, 825–841 [DOI] [PubMed] [Google Scholar]
- 43.Beckmann C.F. and Smith S.M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152 [DOI] [PubMed] [Google Scholar]
- 44.Kelly R.E., Jr, Alexopoulos G.S., Wang Z., Gunning F.M., Murphy C.F., Morimoto S.S., Kanellopoulos D., Jia Z., Lim K.O., and Hoptman M.J. (2010). Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J. Neurosci. Methods 189, 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landis J.R. and Koch G.G. (1977). The measurement of observer agreement for categorical data. Biometrics 33, 159–174 [PubMed] [Google Scholar]
- 46.Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L. and Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallquist M.N., Hwang K. and Luna B. (2013). The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage 82, 208–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greve D.N. and Fischl B. (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage 48, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson J.L.R., Jenkinson M. and Smith S. (2007). Non-linear optimisation. FMRIB technical report TR07JA1. Available at: www.fmrib.ox.ac.uk/analysis/techrep/tr07ja1/tr07ja1.pdf Accessed March18, 2016
- 50.Andersson J.L.R., Jenkinson M., and Smith S. (2007). Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. Available at: www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf Accessed March16, 2016
- 51.J, Z. (1996). Biostatistical Analysis. Prentice-Hall: Upper Saddle River, NJ [Google Scholar]
- 52.Beckmann C.F., Jenkinson M., and Smith S.M. (2003). General multilevel linear modeling for group analysis in FMRI. NeuroImage 20, 1052–1063 [DOI] [PubMed] [Google Scholar]
- 53.Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., and Killiany R.J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980 [DOI] [PubMed] [Google Scholar]
- 54.Eickhoff S.B., Paus T., Caspers S., Grosbras M.H., Evans A.C., Zilles K., and Amunts K. (2007). Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage 36, 511–521 [DOI] [PubMed] [Google Scholar]
- 55.Tranel D. (1996). The Iowa-Benton School of Neuropsychological Assessment. Second ed. Oxford University Press: New York [Google Scholar]
- 56.Schwarz L., Penna S., and Novack T. (2009). Factors contributing to performance on the Rey Complex Figure Test in individuals with traumatic brain injury. Clinical Neuropsychol. 23, 255–267 [DOI] [PubMed] [Google Scholar]
- 57.Bullock M.R., Merchant R.E., Choi S.C., Gilman C.B., Kreutzer J.S., Marmarou A., and Teasdale G.M. (2002). Outcome measures for clinical trials in neurotrauma. Neurosurg. Focus 13, ECP1. [DOI] [PubMed] [Google Scholar]
- 58.Ashton V.L., Donders J., and Hoffman N.M. (2005). Rey Complex Figure Test performance after traumatic brain injury. J. Clin. Exp. Neuropsychol. 27, 55–64 [DOI] [PubMed] [Google Scholar]
- 59.Lezack M.D., Howieson D.B., and David W.L. (2004). Neuropychological Assessment. Fourth Edition ed. Oxford University Press: New York [Google Scholar]
- 60.Shukla D., Devi B.I. and Agrawal A. (2011). Outcome measures for traumatic brain injury. Clin. Neurol. Neurosurg. 113, 435–441 [DOI] [PubMed] [Google Scholar]
- 61.Diamond B.J., DeLuca J., and Kelley S.M. (1997). Memory and executive functions in amnesic and non-amnesic patients with aneurysms of the anterior communicating artery. Brain : a journal of neurology 120 (Pt 6), 1015–1025 [DOI] [PubMed] [Google Scholar]
- 62.Bramlett H.M. and Dietrich W.D. (2002). Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathologica 103, 607–614 [DOI] [PubMed] [Google Scholar]
- 63.Irimia A., Wang B., Aylward S.R., Prastawa M.W., Pace D.F., Gerig G., Hovda D.A., Kikinis R., Vespa P.M., and Van Horn J.D. (2012). Neuroimaging of structural pathology and connectomics in traumatic brain injury: Toward personalized outcome prediction. NeuroImage. Clin. 1, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., and Petersen S.E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 104, 11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ptak R. (2012). The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist 18, 502–515 [DOI] [PubMed] [Google Scholar]
- 66.Arciniegas D.B., Topkoff J., and Silver J.M. (2000). Neuropsychiatric aspects of traumatic brain injury. Curr. Treat. Options Neurol. 2, 169–186 [DOI] [PubMed] [Google Scholar]
- 67.Wortzel H.S. and Arciniegas D.B. (2012). Treatment of post-traumatic cognitive impairments. Curr. Treat. Options Neurol. 14, 493–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonnelle V., Leech R., Kinnunen K.M., Ham T.E., Beckmann C.F., De Boissezon X., Greenwood R.J., and Sharp D.J. (2011). Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 31, 13442–13451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang D. and Raichle M.E. (2010). Disease and the brain's dark energy. Nat. Rev. Neurol. 6, 15–28 [DOI] [PubMed] [Google Scholar]
- 70.Leech R. and Sharp D.J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker W.C. and Pickett T.C. (2007). Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J. Rehabil. Res. Dev. 44, 975–982 [DOI] [PubMed] [Google Scholar]
- 72.Pickett T.C., Radfar-Baublitz L.S., McDonald S.D., Walker W.C., and Cifu D.X. (2007). Objectively assessing balance deficits after TBI: role of computerized posturography. J. Rehabil. Res. Dev. 44, 983–990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.