Abstract
BACKGROUND
Preterm birth (PTB) is a leading cause of neonatal morbidity and mortality and is not uncommonly associated with chorioamnionitis. We recently have demonstrated that the placenta harbors a unique microbiome with similar flora to the oral community. We also have shown an association of these placental microbiota with PTB, history of antenatal infection, and excess maternal weight gain. On the basis of these previous observations, we hypothesized that the placental membranes would retain a microbiome community that would vary in association with preterm birth and chorioamnionitis.
OBJECTIVE
In the current study, we aimed to examine the differences in the placental membrane microbiome in association with PTB in both the presence and absence of chorioamnionitis and/ or funisitis using state-of-the-science whole-genome shotgun metagenomics.
STUDY DESIGN
This was a cross-sectional analysis with 6 nested spontaneous birth cohorts (n = 9–15 subjects/cohort): Term gestations without chorioamnionitis, term with chorioamnionitis, preterm without chorioamnionitis, preterm with mild chorioamnionitis, preterm with severe chorioamnionitis, and preterm with chorioamnionitis and funisitis. Histologic analysis was performed with Redline's criteria, and inflammatory cytokines were analyzed in the cord blood. DNA from placental membranes was extracted from sterile swabs collected at delivery, and whole-genome shotgun sequencing was performed on the Illumina HiSeq platform. Filtered microbial DNA sequences were annotated and analyzed with MG-RAST (ie, Metagenomic Rapid Annotations using Subsystems Technology) and R.
RESULTS
Subjects were assigned to cohorts on the basis of gestational age at delivery and independent scoring of histologic chorioamnionitis. We found that preterm subjects with severe chorioamnionitis and funisitis had increases in cord blood inflammatory cytokines. Of interest, although the placental membrane microbiome was altered in association with severity of histologic chorioamnionitis (permutational multivariate analysis of variance P = .005), there was no observable impact with either beta-methasone or antibiotic treatment. In preterm subjects with chorioamnionitis, we found a high abundance of both urogenital and oral commensal bacteria. These alterations in the microbiome were accompanied by significant variation (P < .05) in microbial metabolic pathways important in the glucose-fed pentose phosphate pathway (term subjects), or glycerophopholipid metabolism, and the biosynthesis of the siderophore group nonribosomal peptides (preterm subjects).
CONCLUSION
Consistent with ours and others previous findings, women who experienced spontaneous PTB harbor placental microbiota that further differed by severity of chorioamnionitis. Integrative meta-genomic analysis revealed significant variation in distinct bacterial metabolic pathways, which we speculate may contribute to risk of preterm birth with and without severe chorioamnionitis.
Keywords: chorioamnionitis, funisitis, microbiome, preterm birth, whole-genome shotgun metagenomics
Preterm birth (PTB; <37 weeks of gestation) is among the leading causes of global neonatal morbidity and mortality. On average, 9.6% of births in the United States are preterm, and an increased incidence of PTB in the United States occurred from 1981 to 2012, with a recent stabilization reported.1,2 Although the etiology of PTB remains elusive, a near majority (40%–70%) of PTBs may be associated with chorioamnionitis that is clinically diagnosed by the occurrence of a sustained (>2 hours) maternal fever of greater than 100.4°C and at least 1 of the following symptoms: maternal tachycardia (>100 bpm), fetal tachycardia (>160 bpm), foul-smelling amniotic fluid, or uterine tenderness.3 Of women diagnosed with clinical chorioamnionitis, however, less than 70% receive confirmation of histologic chorioamnionitis.4,5 Conversely, a previous study demonstrated the presence of histologic chorioamnionitis without clinical symptoms, which suggests that placental inflammation may occur in the absence of an infectious agent.4 In addition to maternal complications associated with chorioamnionitis, severe cases of chorioamnionitis are associated with neonatal sepsis, funisitis (inflammation of the umbilical cord), and fetal inflammatory response syndrome.3,4
Previously, it has been proposed that an ascending infection from the vagina to the placenta induces chorioamnionitis and PTB.6 This hypothesis was proposed as a result of the association of bacteria of the urogenital tract, such as Mycoplasma species, Ureaplasma species, and group B Streptococcus, with chorioamnionitis and with colonization of placental/fetal membranes.7-18 Although previous studies describing ascending infection have used primarily culture-based techniques,8,11-15,17-20 the advent of next-generation metagenomics sequencing has allowed for the culture-independent characterization of the vaginal microbiome.21-26 Similar to previous studies that use culture-based techniques, the vaginal microbiome in the majority of women is dominated by Lactobacillus species in both nonpregnant and pregnant populations.21-26 In addition to characterizing pregnancy-associated changes in the vaginal microbiome, recent studies that investigated the vaginal microbiome in association with PTB were performed. These studies from several distinct patient populations have similarly pyrosequenced the vaginal microbiome between term and preterm subjects longitudinally but with mixed and disparate findings.25-27 Altogether, these studies emphasize the importance for further investigation into the role of other body niche microbiota in PTB.
Intriguingly, bacterial species not associated with the vagina have been implicated in chorioamnionitis and with placental/fetal colonization, such as the oral bacteria of Streptococcus species and Fusobacterium species.7,9,28-31 Recently, bacteria has been found within the placenta of term and preterm subjects,32-34 and we have previously provided an in-depth metagenomic characterization of the placental parenchyma micro-biome.35,36 We determined that the bacteria harbored in the placenta are associated most closely with the microflora of the oral cavity and vary in association with PTB and excess maternal gestational weight gain.35,36 The association between the placental and oral microbiomes provides a potential explanation for the presence of commensal oral bacteria (such as Streptococcus and Fusobacterium spp) in chorioamnionitis, with a potential mechanism being hematogenous spread of bacteria during pregnancy.31,37 When we further investigated the placental parenchyma microbiome, we found significant differences between term placentas and preterm placentas.35,36 Given the known association between inflammation and PTB, we aimed to further examine the placental membrane microbiome in an independent cohort in the context of inflammation in the chorion and amnion by investigating term and preterm subjects with and without chorioamnionitis and funisitis undergoing spontaneous labor.
Materials and Methods
Subjects
The gravidae provided consent from 2009 to 2012 under a protocol approved by the Institutional Review Boards (IRBs) of Good Samaritan Hospital, Cincinnati, Ohio (09105-09-067) and Cincinnati Children's Hospital Medical Center (2009-0236); subsequent IRB approval for metagenomics studies was obtained from the Baylor College of Medicine IRB (H-27393). Informed consent was requested from mothers admitted for imminent preterm delivery. Inclusion criteria for the preterm cohort were mothers delivering between 320 and 366 weeks’ gestation because of preterm premature rupture of membranes, spontaneous preterm labor, or clinically diagnosed chorioamnionitis. The inclusion criteria for the term infants were women presenting with spontaneous labor at ≥38 weeks of gestation. We excluded mothers delivering infants for medical indications, such as maternal hypertensive disorders including preeclampsia and HELLP syndrome, because these disorders would be expected to have a very low incidence of chorioamnionitis and therefore skew the comparisons. Only 3 subjects (Preterm 381, Term 225, and Term 239) had findings of incidental hypertension, which was not an indication for induction of labor or delivery. Also excluded were the following: gravidae with HIV; delivered infants with acute, life-threatening illness or requiring extensive resuscitation; or fetuses with congenital malformations or infections, such as syphilis or cytomegalovirus. Maternal and neonatal demographics were collected by interview and chart review.
Sample collection
All sample collection was performed with the use of standard operating procedures and followed a strict uniform protocol established before study initiation. Nurses specifically trained in study procedures performed sample collection. After delivery of the infant and before delivery of the placenta, cord blood was collected from the umbilical vein with a sterile ViaCord collection kit (ViaCord, Waltham, MA) containing citrate phosphate dextrose anticoagulant. Placenta was delivered by standard obstetrical practice and immediately collected in sterile bags. These were kept in a refrigerator until sampling. Placental sampling was performed in a dedicated room via strict sterile precautions to prevent exogenous contamination. Placenta was kept with the fetal surface facing the operator. The amnion surface was cleaned by swabbing with 70% ethanol and drying immediately. Then, the surface of the amnion was cut with sterile instruments. A second set of sterile instruments was used to perform a blunt dissection to create a pocket on the underside of the amnion without puncturing the amnion. Swabs for microbial collection were swirled to collect samples from fetal chorion and/or villous placental membranes adjacent to the fetal side while taking care to avoid contamination from the maternal side.
The following swabs were collected and subsequently cryofrozen until analyses: (1) Dry Dacron swab for DNA collection (part 220115; BD, Franklin Lakes, NJ), (2) UP transport medium (clear media, part 220221; BD), and (3) Port-A-Cul (for anaerobic and aerobic culture; pink media, part 221607; BD). This collection method differs from our previous study involving the placental tissue microbiome.35 In our previous study, placental parenchyma tissue was collected 4 cm from the cord insertion site.35 In our current study, we examined the placental membrane microbiome in association with chorioamnionitis. The niche site is ideal for examining associations between the placental micro-biome and inflammation because of the presence of decidual leukocytes.
Histology
The placenta and umbilical cord were sampled from specific tissue locations via an aseptic-standardized protocol for histologic examination.38 Sections of chorioamnion, umbilical cord, and placental tissues were stained with hematoxylin and eosin; all 32-366 week preterm and term tissue samples were scored for histologic chorioamnionitis based on Redline's criteria39 by authors T.G. and S.G.K., who were blinded to the clinical history and placental pathology reports. Redline's criteria determines the stage of chorioamnionitis on the basis of the depth of plane of neutrophil infiltration and the grade as the degree of neutrophilic infiltration.39 We classified chorioamnionitis as mild or severe on the basis maternal inflammation. Mild was denoted as stage 1 and grade 1 chorioamnionitis, and severe was denoted >stage 1 and/or >grade 1 chorioamnionitis. Funisitis indicating fetal inflammation was defined as neutrophilic infiltration surrounding the umbilical cord vessels or into Wharton's jelly. Neonates with chorioamnionitis were compared with neonates born preterm with and without chorioamnionitis.
DNA isolation and whole-genome shotgun (WGS) sequencing
Using manufacturer's instructions, we extracted DNA from the Dacron swab with the Power Soil DNA isolation Kit (cat. no. G-3246-100; MO BIO Laboratories Inc, Carlsbad, CA). This DNA was subsequently used for microbiome analyses that used WGS sequencing as previously described.35,36,40,41 Resulting sequences were quality filtered, and contaminating host genomic DNA (>99%) was removed with the use of Best Match Tagger (BMTagger; ftp://ftp.ncbi.nlm.nih.gov/pub/agarwala/bmt agger/) via a heuristic approach. After the removal of contaminating host genomic DNA, sequences were uploaded to MG-RAST (ie, Metagenomic Rapid Annotations using Subsystems Technology; http://metagenomics.anl.gov/) for taxonomic and pathway identification using the M5NR database with a maximum e-value of 1, minimum percent identity cutoff of 50%, and minimum length alignment cutoff of 15.42 Taxonomy and pathway abundance were normalized by the number of reads per sample. QIIME (ie, Quantitative Insights Into Microbial Ecology; http://qiime.org/) was used for the generation of principal coordinates analysis (PCoA) plots using Canberra and Bray-Curtis distance.43 LEfSe and Wilcoxon rank sum test were used to identify the pathway with differential abundance between cohorts. Reconstructed metabolic pathways were correlated to taxonomic abundance via the use of R. Those with significant differences in association with chorioamnionitis were examined further.
Bacterial cultures
The presence of Ureaplasma or Myco-plasma species was determined by both traditional culture methods and by polymerase chain reaction optimized to detect all the serovars.44
Cytokine/chemokine concentration
Cytokine/chemokine concentrations from the plasma separated from umbilical cord blood were determined by Luminex using MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel (Millipore, Bill-erica, MA). Concentrations were calculated from standard curves using recombinant proteins and expressed in pg/mL.
Statistical analysis
The statistical significance of beta diversity displayed by PCoA plots was determined by permutational multivariate analysis of variance (PERMANOVA) and permutational analysis of multivariate dispersion (PERMDISP) using QIIME.43 Both PERMAVONA and PERMDISP were used to determine clustering between cohorts (PERMANOVA) and to determine the clustering within a cohort (PERMDISP). Statistical analysis determined by t test or 1-way analysis of variance (ANOVA) was generated using GraphPad Prism (La Jolla, CA) software.
Results
Chorioamnionitis diagnosis and subject demographics
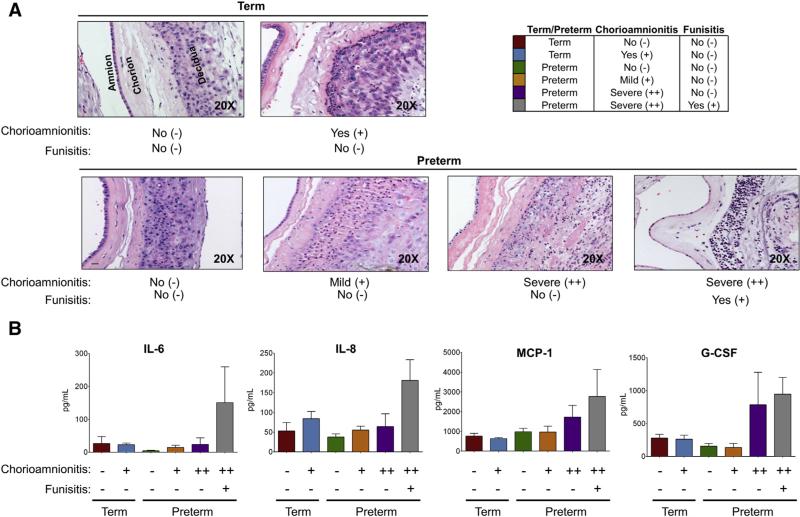
Term subjects were enrolled on the basis of a spontaneous birth ≥38 weeks of gestation, and preterm subjects were enrolled if they spontaneously delivered between 320 and 366 weeks of gestation. Late preterm subjects were the focus of this study in an attempt to further separate inflammation from infectious, pathogenic agents. By design, our study allowed for a week between term and preterm subjects, resulting in significant differences in the gestational age (P < .0001) and neonatal birthweight (P < .0001) between term and preterm cohorts (t test; Table 1). After enrollment and sample collection, placental tissue underwent histologic analysis with hematoxylin and eosin staining. Placentas were scored blindly on the basis of Redline's criteria.39 On the basis of the histologic scores, we had the following 2 term cohorts: spontaneous term birth (cohort 1) and spontaneous term birth with chorioamnionitis (cohort 2). We also had the following spontaneous PTB cohorts: PTB (cohort 3), PTB with mild chorioamnionitis (cohort 4), PTB with severe chorioamnionitis (cohort 5), and PTB with severe chorioamnionitis and funisitis (cohort 6). In subjects with histologic chorioamnionitis, we were able to detect significant leukocyte infiltration into the chorion (Figure 1, A). In addition, we found that inflammatory cytokines were increased in the cord blood of subjects with the most severe chorioamnionitis (Figure 1, B), which is consistent with previous studies examining inflammation and chorioamnionitis.5,45-53 Overall, these data are consistent with long-standing findings by others demonstrating pathologic inflammation occurring in the placenta of subjects with severe histologic chorioamnionitis.
TABLE 1.
Subject demographics
| Cohort | 1 (n = 15) | 2 (n = 12) | 3 (n = 13) | 4 (n = 11) | 5 (n = 9) | 6 (n = 11) |
|---|---|---|---|---|---|---|
| Gestational age, weeks (days) | Term | Term | Preterm | Preterm | Preterm | Preterm |
| Average | 39 (5) | 39 (6) | 35 (1) | 35 (5) | 35 (1) | 34 (6) |
| Range | 38 (1)–41 (3) | 38 (4)–41 (1) | 32 (3)–36 (3) | 32 (4)–36 (3) | 32 (6)–36 (6) | 32 (0)–36 (6) |
| Birthweight, g | 3283.9 ± 351.6 | 3359.3 ± 376.8 | 2725.0 ± 362.7 | 2950.5 ± 322.1 | 2552.5 ± 568.1 | 2425.5 ± 425.9 |
| Betamethasone | 0% (0) | 0% (0) | 23.1% (3) | 18.2% (2) | 55.6% (5) | 27.3% (3) |
| Antibiotics (72 hours predelivery) | 20% (3) | 25% (3) | 76.9% (10) | 54.5% (6) | 55.6% (5) | 72.7% (8) |
| Chorioamnionitis (histologic) | No | Yes | No | Mild | Severe | Severe |
| Funisitis (histologic) | No | No | No | No | No | Yes |
| Ureaplasma positive | 0% (0) | 16.7% (2) | 15.4% (2) | 9.1% (1) | 22.2% (2) | 27.3% (3) |
| Mode of delivery | ||||||
| Vaginal | 73.3% (11) | 75% (9) | 84.6% (11) | 100% (11) | 77.8% (7) | 72.7% (8) |
| Cesarean | 26.7% (4) | 25% (3) | 15.4% (2) | 0% (0) | 22.2% (2) | 27.3% (3) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 80% (12) | 75% (9) | 69.2% (9) | 63.6% (7) | 55.6% (5) | 36.4% (4) |
| African-American/black | 20% (3) | 8.3% (1) | 15.4% (2) | 36.4% (4) | 33.3% (3) | 63.6% (7) |
| Asian | – | 8.3% (1) | – | – | – | – |
| Other | – | 8.3% (1) | 15.4% (2) | – | 11.1% (1) | – |
Prince et al. Placental microbiota and their metabolic pathways in PTB and chorioamnionitis. Am J Obstet Gynecol 2016.
FIGURE 1. Subjects with chorioamnionitis have increased inflammation.
A, Histology was performed with hematoxylin and eosin staining and was scored blindly and according to Redline's criteria. Leukocyte infiltration into the chorion and amnion is present in subjects with chorioamnionitis and absent in subjects without chorioamnionitis. Magnification is 20×. B, Plasma from cord blood was analyzed for inflammatory cytokines (IL-6, IL-8, MCP-1, and G-CSF) with the use of Luminex. Preterm subjects with severe chorioamnionitis (++) had increases in inflammatory cytokines compared with subjects without (−) or with mild (+) chorioamnionitis.
G-CSF, granulocyte-colony stimulating factor; IL-6, interleukin-6; IL-8, interleukin-8; MCP-1, monocyte chemoattractant protein-1.
Prince et al. Placental microbiota and their metabolic pathways in PTB and chorioamnionitis. Am J Obstet Gynecol 2016.
Subject characteristics and treatment summaries are provided in Table 1. Few neonatal comorbidities were seen in each cohort, with the most frequent comorbidities seen in subjects with severe chorioamnionitis and funisitis (cohort 6). The most prevalent neonatal comorbidity was respiratory distress with 15.4% (n = 2) for preterm subjects without chorioamnionitis (cohort 3), 27.3% (n = 3) for preterm subjects with mild chorioamnionitis (cohort 4), 22.2% (n = 2) for preterm subjects with severe chorioamnionitis (cohort 5), and 54.5% (n = 6) for preterm subjects with severe chorioamnionitis and funisitis (cohort 6). High-frequency ventilation was used on 22.2% (n = 2) of neonates born to preterm subjects with severe chorioamnionitis (cohort 5). Preterm subjects with severe chorioamnionitis and funisitis (cohort 6) was the only cohort to have neonates with pneumonia (9.1%; n = 1), early-onset sepsis (9.1%; n = 1), or malformations (9.1%; n = 1). Intriguingly, preterm subjects without histologic chorioamnionitis (cohort 3) had 2 subjects that test positive for Ureaplasma species using culture-based and polymerase chain reaction methodologies. Thus, all cohorts with histologic chorioamnionitis had subjects with cultures positive for Ureaplasma species; however, not all subjects were positive for Ureaplasma. This finding is consistent with the findings in an experimental ovine model of intra-amniotic infection.54 These results bolster the idea of a causative agent other than the vaginal microflora and thus warranted subsequent investigation into the placental membrane microbiome.
Species-level resolution of the preterm placental microbiome with and without chorioamnionitis
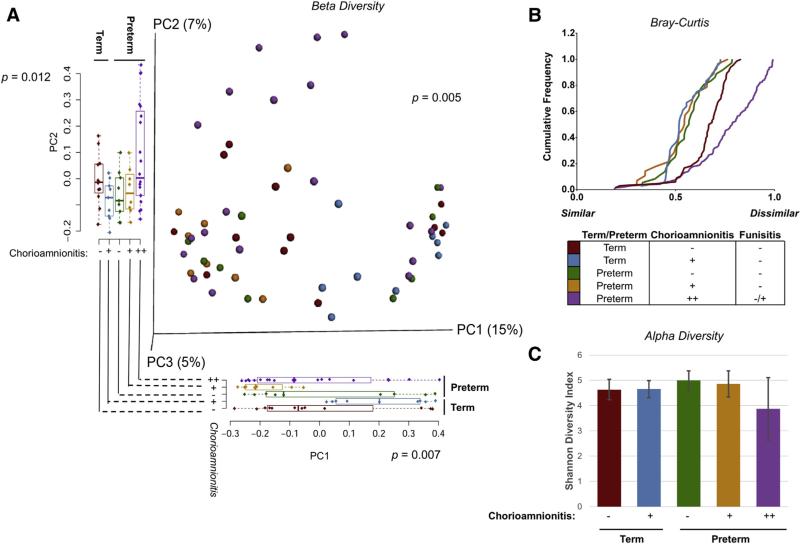
DNA was isolated from placental membrane swabs and subjected to WGS sequencing. For each cohort, DNA from placental membranes of subjects in each was sequenced (n≥8 subjects per group). The resultant sequences were quality filtered and analyzed, and only those with high read yield and without concern for contamination were included in further analysis. On the basis of placental histology and the increase in inflammatory cytokines between preterm subjects with severe chorioamnionitis without and with funisitis (Figure 1), we combined these groups for analysis because funisitis, which was determined by umbilical cord histology, did not appear to differentiate these 2 cohorts. Upon analyzing beta diversity (diversity between cohorts) at the species level using Canberra distance, we found significant differences in clustering by PERMANOVA (P = .005) of our PCoA plots (Figure 2, A). Furthermore, on close examination of the PC1 and PC2 axis of the term cohorts, we found significant differences in both the PC1 (P = .012 by ANOVA) and PC2 axis (P = .007 by ANOVA; Figure 2, A). In addition, our analysis of beta diversity on the PC1 and PC2 axis demonstrates significant differences in our term cohorts without and with chorioamnionitis (Figure 2, A, P = .05 by t test on PC1, P = .01 by t test on PC2).
FIGURE 2. Subjects with chorioamnionitis have alterations in the placental membrane microbiome.
Preterm subjects with severe chorioamnionitis without and with funisitis were combined for analysis on the basis of histology and expression of inflammatory cytokines in the cord blood from Figure 1. A, Beta diversity (diversity between cohorts) of all cohorts using Canberra distance. Significant differences were seen between the cohorts by PERMANOVA (P = .005). Additionally, significant differences between cohorts were seen on the PC1 and PC2 axis by ANOVA (P = .012 and P = .007, respectively). B, Bray-Curtis dissimilarity was analyzed within each cohort. Term subjects with chorioamnionitis (blue) were most similar to preterm subjects without and with mild chorioamnionitis (green and orange, respectively). Term subjects without chorioamnionitis (red) had dissimilarity within the cohort, but subjects with severe chorioamnionitis (purple) had the greatest dissimilarity between subjects. C, Alpha diversity (diversity within cohorts) was measured between subject cohorts by use of the Shannon diversity index. There was a decrease in preterm subjects with severe chorioamnionitis (purple) compared with subjects without chorioamnionitis (term: red, preterm: green) or with mild chorioamnionitis (term: blue, preterm: orange). This indicates that preterm subjects with severe chorioamnionitis have fewer bacterial constituents of their placental microbiome.
ANOVA, analysis of variance; PERMANOVA, permutational multivariate analysis of variance.
Prince et al. Placental microbiota and their metabolic pathways in PTB and chorioamnionitis. Am J Obstet Gynecol 2016.
Given this significance of difference of the placental membrane microbiome community by both preterm and chorioamnionitis, we next examined Bray-Curtis dissimilarity (beta diversity) within our term and preterm cohorts by severity of chorioamnionitis (Figure 2, B). We found that the term cohort with chorioamnionitis was most similar to our preterm cohort without chorioamnionitis or with mild chorioamnionitis (Figure 2, B). Interestingly, our term cohort without chorioamnionitis had a greater dissimilarity within subjects compared with term subjects with chorioamnionitis, preterm subjects without chorioamnionitis, and preterm subjects with mild chorioamnionitis (Figure 2, B). Again, we found that pre-term cohorts with severe chorioamnionitis had the greatest dissimilarity within the cohort (Figure 2, B). When we alternately examined alpha diversity (within sample diversity), we found that preterm subjects with severe chorioamnionitis manifest as diminished species diversity (Shannon diversity index; Figure 2, C). These data indicate that preterm subjects with chorioamnionitis have fewer bacterial constituents of their placental membrane microbiome (Figure 2, C). Our results are in agreement with previous gut microbiome studies that indicate that alpha diversity is decreased in association with clinical infection and histologically significant inflammation.55,56
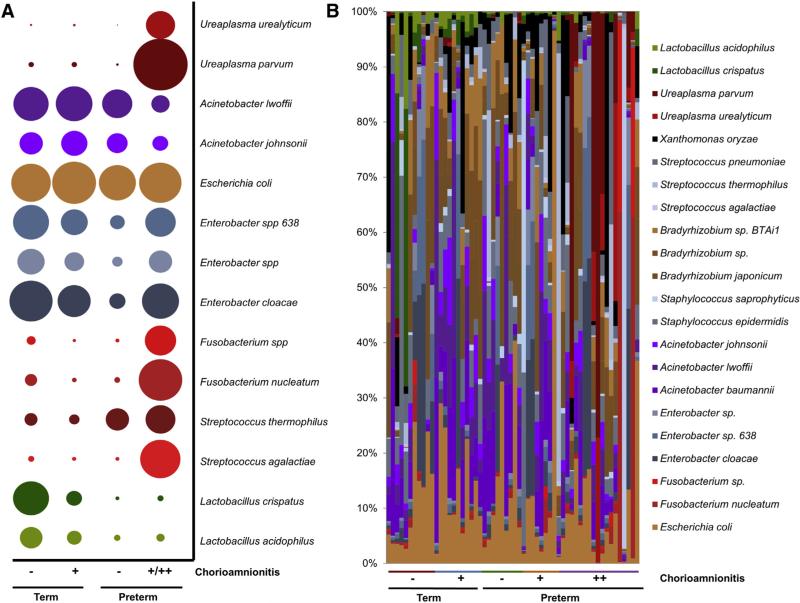
One of the distinct advantages of WGS metagenomics is the high resolution of bacterial taxa at the species level between cohorts. As shown in Figure 3, A, we observed significant differences in overall taxonomy that was not individual subject dependent (Figure 3, B). Specifically, preterm subjects with severe chorioamnionitis had high abundances of Ureaplasma parvum, Fusobacterium nucleatum, and Streptococcus agalactiae (Figure 3). This finding is in agreement with our previous study in which we found enrichment of Streptococcus species in the placental parenchyma microbiome of subjects with a remote history of antenatal infection.35 In addition, these data bolster the results obtained from examining Bray-Curtis dissimilarity (Figure 2, B) and alpha diversity (Figure 2, C) and are in concordance with previous studies of PTB and chorioamnionitis.7-10,16,17,19,20
FIGURE 3. Bacterial taxa at the species level differs between cohorts.
A, Bubble plots depicting the top bacterial species that differ between term and preterm cohorts with and without chorioamnionitis. B, Examination of bacterial taxa between individual subjects. Term subjects appear to have an increase in Lactobacillus crispatus over subjects in other cohorts. Preterm subjects with chorioamnionitis have wide variety of bacteria present in the placenta, including taxa associated with vaginal microbiome (ie, Ureaplasma species and Streptococcus agalactiae) and with the oral microbiome (ie, Fusobacterium species and Streptococcus thermophilus).
Prince et al. Placental microbiota and their metabolic pathways in PTB and chorioamnionitis. Am J Obstet Gynecol 2016.
By contrast, among term subjects we found a greater abundance of Enterobacter species (gammaproteobacteria) and Lactobacillus crispatus (prevalent vaginal species) among term subjects inclusive of those with a cesarean delivery (n = 3, term cohorts). Although we have previously detected Lactobacillus species as a minor constituent of the placental microbiome,35 here we detected that L. crispatus has an increased relative abundance in term placentas without and with chorioamnionitis (Figure 3). This result may be attributable to niche specification within the placenta. We further examined this possibility by comparing our previous placental parenchyma study with our current placental membrane study (Supplemental Figure 1). We found that Escherichia coli was still prevalent within the placental membrane microbiome (Figure 3), but other bacterial taxa, such as Xanthomonas campestris, Propionibacterium acnes, and Lactobacillus species, were also present in high abundance (Supplemental Figure 1). However, despite some detected differences in bacterial taxa, inferred metabolic function was similar between the placental parenchyma and the placental membrane microbiomes (Supplemental Figure 1).
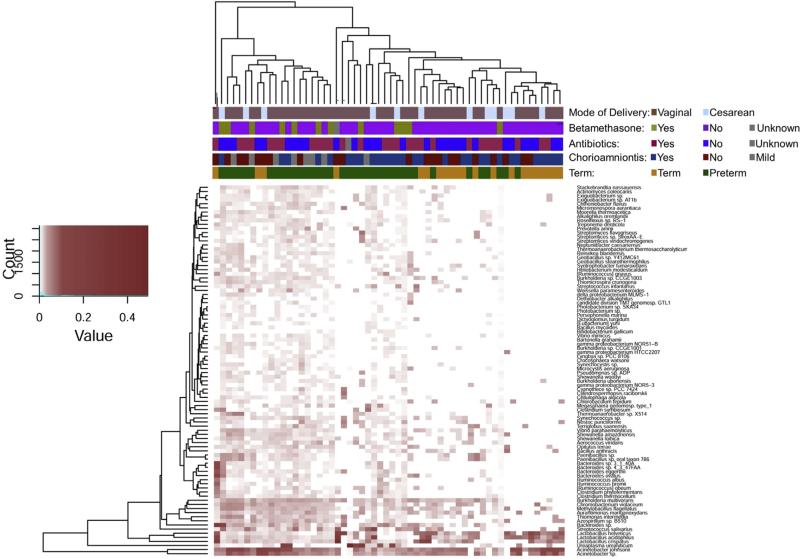
We next wanted to examine clinical confounders to determine their impact on the placental membrane micro-biome. Differences in beta diversity between cohorts were not biased by mode of delivery (Figure 4, P = .82 and Supplemental Figure 2, P = .82) nor antenatal betamethasone (Figure 4 P =.26). In addition, term cohorts were unaltered by antibiotic use (P = .37), as were genus level of bacterial taxa prevalent in our preterm cohorts that have been previously associated with intrauterine infection (Table 2).
FIGURE 4. Abundant taxa in the placental membrane microbiome are unaltered by treatment or mode of delivery.
Differences in bacterial taxa at the species level were determined by Manhattan distance and visualized using a heat map. Subjects clustered by virtue of gestational age (term [orange] vs preterm [green]) and by histologic chorioamnionitis (yes: dark blue, no: dark red, mild: gray). Significance based on antibiotic treatment (yes: blue, no: red, unknown: gray), use of betamethasone (yes: green, no: pink, unknown: gray), or mode of delivery (vaginal: brown, cesarean delivery: light blue) was not detected.
Prince et al. Placental microbiota and their metabolic pathways in PTB and chorioamnionitis. Am J Obstet Gynecol 2016.
TABLE 2.
Prevalent taxa at the genus level in the preterm placental microbiome are not influenced by antibiotic treatment
| Genus | No antibiotics, mean abundance (±SD) | Antibiotics, mean abundance (±SD) | P value (t test) |
|---|---|---|---|
| Mycobacterium | 0.0084 (± 0.0049) | 0.0090 (± 0.0065) | .76 |
| Burkholderia | 0.0086 (± 0.0046) | 0.0077 (± 0.0052) | .59 |
| Mycoplasma | 0.0004 (± 0.0006) | 0.0005 (± 0.0015) | .89 |
| Ureaplasma | 0.0242 (± 0.0585) | 0.0126 (± 0.0319) | .52 |
| Fusobacterium | 0.0610 (± 0.210) | 0.0419 (± 0.191) | .79 |
| Streptococcus | 0.0451 (± 0.0903) | 0.0663 (± 0.168) | .62 |
Prince et al. Placental microbiota and their metabolic pathways in PTB and chorioamnionitis. Am J Obstet Gynecol 2016.
Distinct bacterial metabolic pathways
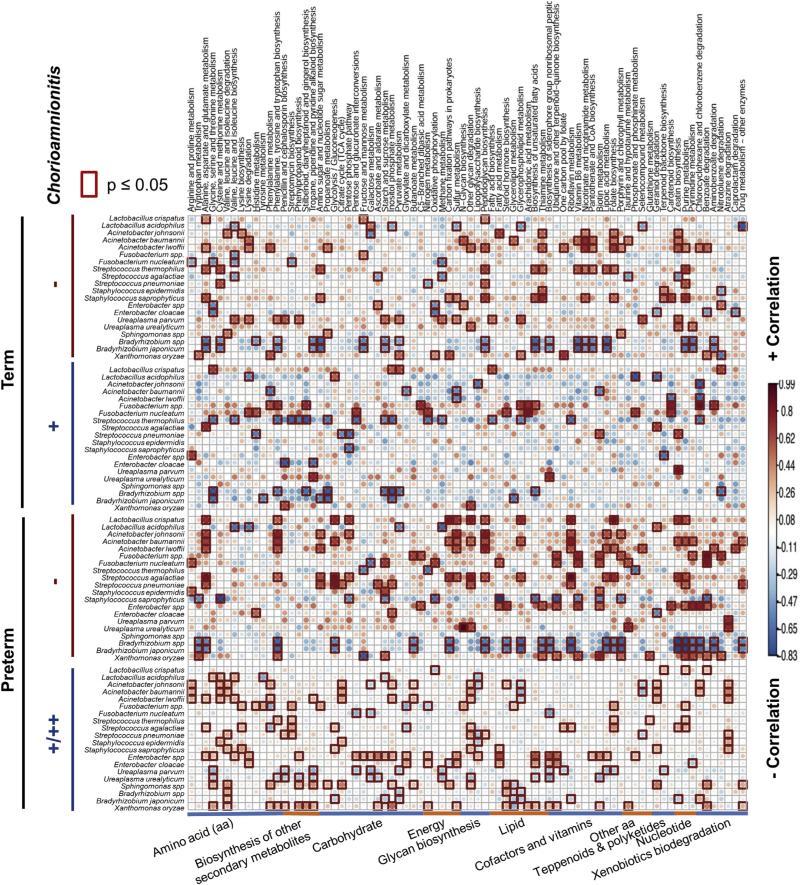
A second advantage to WGS meta-genomics is the capacity to reassemble and annotate (map) bacterial metabolic pathways by gene count. Thus, we next determined functional differences in metabolic pathways between our term and preterm subjects with and without chorioamnionitis by using the Kyoto Encyclopedia of Genes and Genomes. Because our previous analysis demonstrated that chorioamnionitis resulted in different alterations in the placental membrane microbiome between term and preterm subjects (Figures 2 and 3), we first separated the examination of bacterial metabolic pathways on the basis of gestational age (term and preterm). Overall, term subjects without chorioamnionitis had Acinetobacter spp. and Streptococcus thermophilus significantly positively correlated to the functional pathways of metabolism of cofactors and vitamins (Figure 5). Similarly, these pathways were correlated with L. crispatus, A. johnsonii, Fusobacterium species, and Enterobacter species in pre-term subjects without chorioamnionitis (Figure 5). Conversely, in term and preterm subjects with chorioamnionitis that have an increased abundance in S. thermophilus and Fusobacterium species (Figure 3), we detected alterations in the biosynthesis of secondary metabolites and in lipid metabolism associated with these oral commensal bacteria (Figure 5).
FIGURE 5. Distinct associations of bacterial taxa with metabolic pathways.
Metabolic sequences were reconstructed by the use of Kyoto Encyclopedia of Genes and Genomes pathways, and the inferred pathways were correlated to bacterial taxa with R. Red indicates a positive correlation in the metabolic pathway in association with the bacteria whereas blue indicates a negative correlation in a metabolic pathway associated with bacteria. The red box is indicative of significance (P < .05) between the association of the bacterial species and the metabolic function within each cohort. Although many metabolic pathways were altered by virtue of preterm birth and chorioamnionitis, no distinct bacterial taxa were associated with alterations in metabolism by virtue of preterm birth and chorioamnionitis.
Prince et al. Placental microbiota and their metabolic pathways in PTB and chorioamnionitis. Am J Obstet Gynecol 2016.
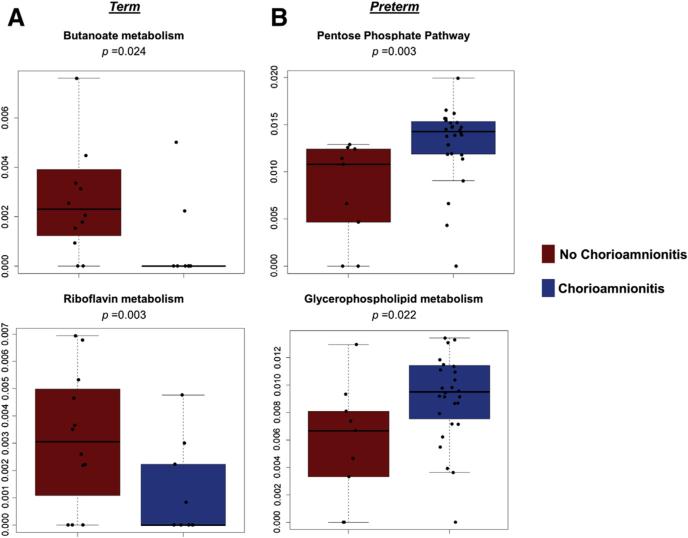
When examining term cohorts without and with chorioamnionitis, we found significant decreases (P < .05) in the following metabolic pathways: amino sugar and nucleotide sugar metabolism, butanoate metabolism, riboflavin metabolism, and amino-benzoate degradation (Figure 6, A). The butanoate metabolism is intriguing because butyrate has been shown to reduce inflammation in the intestine.57-59 In addition, a decrease in riboflavin metabolism also has been associated with inflammation. Thus, decreases in butyrate and riboflavin may explain the inflammation present in the placenta of term subjects with chorioamnionitis.
FIGURE 6. Bacterial metabolic pathways are altered in association with histologic chorioamnionitis.
Because of differences in bacterial taxa, cohorts were analyzed by gestational age (term and pre-term). Alterations in inferred bacterial metabolic pathways were analyzed using Wilcoxon rank sum test. A, Term subjects without chorioamnionitis (red) were compared with term subjects with chorioamnionitis (blue). Butanoate metabolism (top) and riboflavin metabolism (bottom) is significantly decreased in term subjects without chorioamnionitis (P = .024 and P = .003, respectively). B, Preterm subjects without chorioamnionitis (red) were compared with preterm subjects with chorioamnionitis (blue). The pentose phosphate pathway and glycerophospholipid metabolism are significantly increased (P = .003 and P = .022, respectively).
Prince et al. Placental microbiota and their metabolic pathways in PTB and chorioamnionitis. Am J Obstet Gynecol 2016.
When we alternately examined our preterm subjects, we found that subjects with chorioamnionitis have a significant increase (P < .05) in the pentose phosphate pathway, glycerophopholipid metabolism, and the biosynthesis of the siderophore group nonribosomal peptides (Figure 6, B). Interestingly, glucose feeds into the pentose phosphate pathway, which may explain the decrease in amniotic fluid glucose long known to be both associated with chorioamnionitis and serving as clinical diagnostic criteria.60 Furthermore, glycerophospholipids are metabolized to arachidonic acid, which in turn promotes inflammation and the synthesis of prostanoids that are associated with labor. Thus, similar to our previous study examining the placental parenchyma,35 we have demonstrated a unique placental membrane microbiome, which varies in both community members and their metabolic pathways by virtue of PTB. Moreover, herein we show that the bacterial taxa present in the placental microbiomes of our preterm subjects with chorioamnionitis have gene carriage patterns that are enriched for distinct molecular mediators of inflammation.
Discussion
In our present study, we optimized noncultured-based metagenomics techniques by performing state-ofthe-science WGS sequencing. We hypothesized that the placental membrane microbiome would be altered in association with inflammation as determined by histologic chorioamnionitis and expression of inflammatory cytokines in the cord blood. Our results demonstrate that not only is the placental membrane microbiome altered in association with PTB35,36 but its microbial community existing in the layer between the chorion and amnion is also altered in association with histologically evident inflammation and clinically evident PTB. Our current study significantly extends our previous findings by in-depth characterization of the impact of concomitant diagnoses of chorioamnionitis and PTB on the placental membrane microbiome.
There are multiple strengths to our study. First, we have used WGS meta-genomics sequencing to discover bacterial species-level associations between PTB and choriomanionitis. When we closely examined term placentas without chorioamnionitis, we found that the abundance of L. crispatus was increased in these subjects (Figure 3). Although there are no data to date regarding the presence of L. crispatus in the placental tissue per se,35,36 the role of L. crispatus in the vagina has been investigated previously with conflicting results.23,27 We have previously characterized L. crispatus as a constituent of the vaginal microbiome during a healthy, term pregnancy,23 and the abundance of L. crispatus has been shown to increase as gestational age increases.24,25 These previous studies bolster our findings here and suggest that in the chorionamnion space L. crispatus is not detected among subjects presenting with a PTB and chorioamnionitis. This finding is further consistent with a recent report demonstrated that individuals with high bactericidal activity against E. coli in the vagina had a distinct vaginal microbiome from individuals with low bactericidal activity against E. coli.61 These differences in bactericidal activity were attributed to the presence of L. crispatus.61 Intriguingly, E. coli has been shown to be the dominant bacterial species in a healthy placenta,35 and we did not detect significant differences in E. coli in the placental membrane interface among the 6 cohorts described here (Figure 3). Conversely, L. crispatus has been associated with PTB in the vagina,27 but an independent, longitudinal study found little evidence for associations in the vaginal microflora and PTB.25 More recently, DiGiulio et al.26 demonstrated that a decrease in Lacto-bacillus species with an increase in Gardenella and/or Ureaplasma species in the vagina was predictive of PTB. These previous studies combined with our results demonstrate the need for investigation into the role of L. crispatus in modulating PTB and the need for site or niche clarification (ie, posterior vaginal fornix vs vaginal introitus; placental parenchyma vs intermembrane space between the chorion and amnion).
In preterm subjects with chorioamnionitis, bacteria previously associated with PTB were identi-fied,8,11-15,17-20 inclusive of Ureaplasma species and Streptococcus agalactiae (group B Streptococcus). However, we also found traditionally oral bacteria associated with histologic chorioamnionitis, such as Fusobacterium species and Streptococcus thermophilus. These data are in concordance with a previous study in which subjects testing positive for Fusobacterium and Streptococcus species in the amniotic fluid also tested positive for these bacteria within the oral cavity but not the vaginal cavity.62 In addition, F. nucleatum has been implicated in term stillbirth,29 and a separate study found that a patient with Bergeyella infection of the amniotic fluid also had this bacteria present in the subgingival plaque but not the vagina.63 Previously, we have determined that the placental parenchyma microbiome is most similar to the oral microbiome and found taxa normally associated with the oral cavity, such as Prevotella tannerae and nonpathogenic Neisseria species, present in the placental tissue.35 Thus, in our current study, we have further demonstrated the presence of oral commensal bacteria within the placenta. However, our data suggest that the ectopic presence of oral commensal bacteria in the placenta may have a pathogenic effect. Notably, although we were able to detect bacterial taxa normally associated with the gut micro-biome, such as Escherichia and Enterobacter species, but these taxa were similar in abundance between term and preterm subjects with and without chorioamnionitis. Although the literature demonstrates that bacterial taxa associated with the gut have been implicated in intrauterine infection,64 our findings demonstrate taxa predominantly associated with the oral and urogenital cavity. Further, studies involving alterations in the gut microbiome associated with pregnancy are inconsistent26,65 and again emphasize the need for further studies examining niche specificity and the microbiome during pregnancy.
A second significant strength to our study is our established capacity to use WGS metagenomics data and project bacterial metabolic pathways. Doing so, we found that bacterial metabolic changes occurred as the result of chorioamnionitis. Term subjects with chorioamnionitis had a decrease in butanoate and riboflavin metabolism (Figure 6, A). Butyrate has been shown to suppress inflammation in the intestine,57-59 and a decrease in riboflavin also has been associated with an increase in inflammation. Therefore, decreases in butyrate and riboflavin in the placenta of our term subjects may result in histologic inflammation. In our preterm subjects, we found that the pentose phosphate pathway and glycerophospholipid metabolism were significantly decreased in association with chorioamnionitis (P < .05). These results are intriguing because glucose is needed for the pentose phosphate pathway, and preterm subjects with chorioamnionitis have a decrease in glucose in the amniotic fluid.60 In addition, a decrease in glycerophosopholipid metabolism may result in an increase in arachidonic acid that promotes inflammation and the synthesis of prostanoids, which may induce labor. Therefore, not only are alterations in the microbiome promoting inflammation, but these alterations also may promote prostanoids to induce preterm labor. These pathways may differ between term and preterm subjects because of the alterations in bacterial taxa associated with these cohorts. Our results demonstrate that term subjects with chorioamnionitis may have more uniform alterations in the placental membrane microbiome in association with inflammation (Figure 3). Conversely, our preterm subjects, particularly those with severe chorioamnionitis, had high variability in the taxa associated with severe inflammation (Figure 3). These differences between our term and preterm cohorts may account for the resulting differences in the metabolic pathways between the cohorts.
Notably, we were unable to associate alterations in these metabolic pathways with bacterial taxa prevalent in the placental microbiome (Figure 5). However, the results of the metabolic data differ from our previous study, in which we examined the association of antenatal infection with alterations in the placental parenchyma microbiome, which may be attributable to the timing of infection. Our previous study examined alterations in the placental parenchyma micro-biome in association with antenatal infection during the first or second trimester.35 Here, we examined alterations in the placental membrane microbiome in association with histologic chorioamnionitis in the third trimester. These differences can be accounted for by many reasons, including niche specification of the placenta, the timeline of infection/inflammation during pregnancy (ie, first, second, or third trimester), and infection vs inflammation. Although chorioamnionitis is associated with infection, our data suggest that inflammation, rather than infectious agents, is associated with alterations in the placental membrane microbiome. In particular, our data suggest that inflammation may be associated with alterations in the placental membrane microbiome of our term subjects with chorioamniontis and our preterm subjects with mild chorioamnionitis. Altogether, our inferred metabolic data suggest that the specific bacterial taxa present may be less important in placental inflammation than the metabolic activity of the placental microbiome.
Additional strengths of our study include the ability to compare our 6 nested cohorts through WGS sequencing and extensive linked clinical metadata. This allowed us to examine the potential impact of additional clinically indicated interventions, such as antibiotic treatment and administration of antenatal glucocorticoids. In sum, we found no evidence of confounding of our primary observations with these common interventions. Our study is not without limitations, however; one such weakness of our study was the inability to distinguish between the classes of antibiotics used to treat subjects. Our current data suggest that antibiotics are minimally effective in altering the placental microbiome; however, we were unable to distinguish between antibiotic classes, which may have a greater influence in the placenta. Common treatment for chorioamnionitis includes ampicillin, gentamicin, and clindamycin.66,67 A recent study, however, examined treatment with an antibiotic regimen of ceftriaxone, clarithromycin, and metro-nidazole, which the authors found reduced histologic chorioamnionitis and funisitis.68 Therefore, further investigation is necessary to understand how antibiotics may or may not alter the placental membrane microbiome.
Altogether, we find that the placental membrane microbiome is influenced by inflammation, and our study has furthered the understanding of which bacteria, both oral and urogenital commensals, have a role in the etiology of chorioamnionitis. This study demonstrates that a commensal bacteria that is ectopically predominant in the placental membrane varies its abundance and presumptively results in substantial inflammation in association with PTB. Moreover, we have examined how bacterial metabolic pathways are influenced by the bacterial species present. Intriguingly, we found little effect on predominant bacterial taxa from treatment with betamethasone and antibiotics; however, these results warrant further investigation. In sum, the significant findings resulting from this study will likely provide a basis for future mechanistic investigation into the progression, and potential novel therapeutics, of chorioamnionitis and PTB.
Supplementary Material
Acknowledgments
The authors thank the Alkek Center for Metagenomics and Microbiome Research (CMMR) and the Human Genome Sequencing Center (HGSC) at Baylor College of Medicine for performing the whole genome shotgun sequencing (WGS) on the described samples. We thank the Hatton Research Center, Rita Doerger, RN, Peggy Walsh, RN, Laurie Bambrick, RN, and labor and delivery obstetricians and nurses at Good Samaritan Hospital, Cincinnati, for help with recruitment of subjects and study procedures. Karen Henderson and Thomas Panke, MD, helped us process placental specimens for histology.
The effort and resources for this study was funded partially by the National Institutes of Health (grant numbers R01NR014792 and DP2OD001500 to K.M.A, HL97064 to A.H.J. and S.G.K, 1F32HD082969-01A1 to A.L.P.), The Burroughs Welcome Fund Preterm Birth Initiative(s) (K.M.A. and S.G.K., C.A.C. and S.G.K.), and the March of Dimes Prematurity Research Initiative (K.M.A).
Footnotes
The authors report no conflict of interest.
Presented orally at the 36th annual meeting of the Society for Maternal-Fetal Medicine, Atlanta, GA, Feb. 1–6, 2016.
References
- 1.Faye-Petersen OM. The placenta in preterm birth. J Clin Pathol. 2008;61:1261–75. doi: 10.1136/jcp.2008.055244. [DOI] [PubMed] [Google Scholar]
- 2.Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol. 2010;34:408–15. doi: 10.1053/j.semperi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Tita ATN, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–54. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smulian JC, Shen-Schwarz S, Vintzileos AM, Lake MF, Ananth CV. Clinical chorioamnionitis and histologic placental inflammation. Obstet Gynecol. 1999;94:1000–5. doi: 10.1016/s0029-7844(99)00416-0. [DOI] [PubMed] [Google Scholar]
- 5.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 7.Doyle RM, Alber DG, Jones HE, et al. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta. 2014;35:1099–101. doi: 10.1016/j.placenta.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Oh KJ, Lee KA, Sohn YK, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203:211, e1–8. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones HE, Harris KA, Azizia M, et al. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 2009;4:e8205. doi: 10.1371/journal.pone.0008205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–58. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witt A, Berger A, Gruber CJ, et al. Increased intrauterine frequency of Ureaplasma urealyticum in women with preterm labor and preterm premature rupture of the membranes and subsequent cesarean delivery. Am J Obstet Gynecol. 2005;193:1663–9. doi: 10.1016/j.ajog.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: Umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198:1–5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kundsin RB, Leviton A, Allred EN, Poulin SA. Ureaplasma urealyticum infection of the placenta in pregnancies that ended prematurely. Obstet Gynecol. 1996;87:122–7. doi: 10.1016/0029-7844(95)00376-2. [DOI] [PubMed] [Google Scholar]
- 14.Kundsin RB, Driscoll SG, Monson RR, Yeh C, Biano SA, Cochran WD. Association of Ureaplasma urealyticum in the placenta with perinatal morbidity and mortality. N Engl J Med. 1984;310:941–5. doi: 10.1056/NEJM198404123101502. [DOI] [PubMed] [Google Scholar]
- 15.Shurin PA, Alpert S, Bernard Rosner BA, Driscoll SG, Lee YH. Chorioamnionitis and colonization of the newborn infant with genital mycoplasmas. N Engl J Med. 1975;293:5–8. doi: 10.1056/NEJM197507032930102. [DOI] [PubMed] [Google Scholar]
- 16.Randis TM, Gelber SE, Hooven TA, et al. Group B Streptococcus β-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J Infect Dis. 2014;210:265–73. doi: 10.1093/infdis/jiu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desa DJ, Trevenen CL. Intrauterine infections with group B beta-haemolytic streptococci. Br J Obstet Gynaecol. 1984;91:237–9. doi: 10.1111/j.1471-0528.1984.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 18.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 19.Feikin DR, Thorsen P, Zywicki S, Arpi M, Westergaard JG, Schuchat A. Association between colonization with group B streptococci during pregnancy and preterm delivery among Danish women. Am J Obstet Gynecol. 2001;184:427–33. doi: 10.1067/mob.2001.109936. [DOI] [PubMed] [Google Scholar]
- 20.Regan JA, Klebanoff MA, Nugent RP, et al. Colonization with group B streptococci in pregnancy and adverse outcome. Am J Obstet Gynecol. 1996;174:1354–60. doi: 10.1016/s0002-9378(96)70684-1. [DOI] [PubMed] [Google Scholar]
- 21.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aagaard K, Riehle K, Ma J, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7:e36466. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Hassan SS, Gajer P, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero R, Hassan SS, Gajer P, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiGiulio DB, Callahan BJ, McMurdie PJ, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci USA. 2015;112:11060–5. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyman RW, Fukushima M, Jiang H, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci. 2014;21:32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohrer JC, Kamemoto LE, Almeida PG, Ogasawara KK. Acute chorioamnionitis at term caused by the oral pathogen fusobacterium nucleatum. Hawaii J Med Public Health. 2012;71:280–1. [PMC free article] [PubMed] [Google Scholar]
- 29.Han YW, Fardini Y, Chen C, et al. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol. 2010;115:442–5. doi: 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han Y, Redline R, Li M, Yin L. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fardini Y, Chung P, Dumm R, Joshi N, Han YW. Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun. 2010;78:1789–96. doi: 10.1128/IAI.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steel JH, Malatos S, Kennea N, et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57:404–11. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 33.Stout MJ, Conlon B, Landeau M, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208:226, e1–7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney EL, Kallapur SG, Gisslen T, et al. Placental infection with Ureaplasma species is associated with histologic chorioamnionitis and adverse outcomes in moderate and late preterm infants. J Infect Dis. 2016;213:1340–7. doi: 10.1093/infdis/jiv587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obs Gynecol. 2014:13–8. doi: 10.1016/j.ajog.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198:110, e1–7. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 39.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–48. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 40.Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Li Y, Li S, Hu N, He Y, Pong R, et al. Comparison of next-generation sequencing systems. J Biomed Biotechnol. 2012;2012:251364. doi: 10.1155/2012/251364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glass EM, Wilkening J, Wilke A, Antonopoulos D, Meyer F. Using the meta-genomics RAST server (MG-RAST) for analyzing shotgun metagenomes. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5368. pdb.prot5368. [DOI] [PubMed] [Google Scholar]
- 43.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dando SJ, Nitsos I, Kallapur SG, et al. The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy? PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med. 2015;213(4 Suppl):S29–52. doi: 10.1515/jpm-2015-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lencki SG, Maciulla MB, Eglinton GS. Maternal and umbilical cord serum interleukin levels in preterm labor with clinical chorioamnionitis. Am J Obstet Gynecol. 1994;170:1345–51. doi: 10.1016/s0002-9378(94)70154-7. [DOI] [PubMed] [Google Scholar]
- 47.Miralles R, Hodge R, Kotecha S. Fetal cortisol response to intrauterine microbial colonisation identified by the polymerase chain reaction and fetal inflammation. Arch Dis Child Fetal Neonatal Ed. 2008;93:F51–4. doi: 10.1136/adc.2006.110130. [DOI] [PubMed] [Google Scholar]
- 48.Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A. Relationship between neonatal blood protein concentrations and placenta histologic characteristics in extremely low GA newborns. Pediatr Res. 2011;69:68–73. doi: 10.1203/PDR.0b013e3181fed334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shim S-S, Romero R, Hong J-S, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 50.Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–9. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 51.Mestan K, Yu Y, Thorsen P, et al. Cord blood biomarkers of the fetal inflammatory response. J Matern Fetal Neonatal Med. 2009;22:379–87. doi: 10.1080/14767050802609759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrams ET, Milner DA, Kwiek J, et al. Risk factors and mechanisms of preterm delivery in Malawi. Am J Reprod Immunol. 2004;52:174–83. doi: 10.1111/j.1600-0897.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 53.Chaiworapongsa T, Romero R, Berry SM, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. J Perinat Med. 2011;39:653–66. doi: 10.1515/JPM.2011.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knox CL, Dando SJ, Nitsos I, et al. The severity of chorioamnionitis in pregnant sheep is associated with in vivo variation of the surface-exposed multiple-banded antigen/gene of Ureaplasma parvum. Biol Reprod. 2010;83:415–26. doi: 10.1095/biolreprod.109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 56.Yu G, Fadrosh D, Ma B, Ravel J, Goedert JJ. Anal microbiota profiles in HIV-positive and HIV-negative MSM. AIDS. 2014;28:753–60. doi: 10.1097/QAD.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 57.Zimmerman MA, Singh N, Martin PM, et al. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. AJP Gastrointest Liver Physiol. 2012;302:G1405–15. doi: 10.1152/ajpgi.00543.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–76. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segain JP, Raingeard de la Blétière D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buhimschi CS, Dulay AT, Abdel-Razeq S, et al. Fetal inflammatory response in women with proteomic biomarkers characteristic of intraamniotic inflammation and preterm birth. BJOG. 2009;116:257–67. doi: 10.1111/j.1471-0528.2008.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghartey JP, Smith BC, Chen Z, et al. Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PLoS One. 2014;9:e96659. doi: 10.1371/journal.pone.0096659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid microorganism infection and microflora in the mouth. BJOG. 2002;109:527–33. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 63.Han Y, Ikegami A, Bissada N. Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. J Clin Microbiol. 2006;44:1475–83. doi: 10.1128/JCM.44.4.1475-1483.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mendz GL, Kaakoush NO, Quinlivan JA. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front Cell Infect Microbiol. 2013;3:58. doi: 10.3389/fcimb.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–80. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman E, Reveiz L, Bonfill Cosp X. Antibiotic regimens for management of intraamniotic infection. Cochrane Database Syst Rev. 2014;12:CD010976. doi: 10.1002/14651858.CD010976.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenberg MB, Anderson BL, Schulkin J, Norton ME, Aziz N. A first look at chorioamnionitis management practice variation among US obstetricians. Infect Dis Obstet Gynecol. 2012;2012:628362. doi: 10.1155/2012/628362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee J, Romero R, Kim SM, et al. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J Matern Neonatal Med. 2015;7058:1–14. doi: 10.3109/14767058.2015.1020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.