Abstract
Introduction
Patients with peritoneal dissemination of pancreatic adenocarcinoma do not benefit from surgical resection, but radiologic and cytologic detection of peritoneal cancer lack sensitivity. This trial sought to determine if an oncolytic virus may be used as a diagnostic agent to detect peritoneal cancer.
Methods
Peritoneal washings from patients with pancreatic adenocarcinoma were incubated with the enhanced green fluorescent protein (eGFP)-expressing oncolytic herpes simplex virus (HSV) NV1066. eGFP-positive or negative status was recorded for each specimen and compared to results obtained by conventional cytologic evaluation. These results were correlated with recurrence and survival for patients who underwent R0 resection.
Results
Of 82 patients entered in this trial, 12 (15%) had positive cytology and 50 (61%) had virally-mediated eGFP positive cells in peritoneal washings. All cytology-positive patients were also eGFP positive. HSV-mediated fluorescence detection had sensitivities of 94% and 100% for detection of any and peritoneal metastatic disease; respectively. Median recurrence free and disease specific survival were 6.5 and 18.3 months for eGFP positive patients, versus 12.2 and 36.2 months for eGFP negative patients (P = 0.01 and 0.19); respectively.
Conclusions
A genetically modified HSV can be used as a highly sensitive diagnostic agent for detection of micro-metastatic disease in patients with pancreatic adenocarcinoma and may improve patient selection for surgery.
Keywords: Pancreatic adenocarcinoma, Oncolytic viral therapy, Herpes simplex virus, Peritoneal fluid cytology, Diagnostic laparoscopy
Highlights
-
•
Oncolytic virus-mediated fluorescence is a sensitive assay for detection of cancer cells in peritoneal fluid.
-
•
Pancreatic cancer patients with eGFP-positive cells in peritoneal washings had a poor prognosis following surgery.
Pancreatic cancer is an aggressive disease. Even with complete surgical removal of a pancreatic tumor, recurrence is common. Patients with microscopic spread of cancer cells into the abdomen, or peritoneum, do not benefit from surgery. Current methods of detection of this kind of spread are not very sensitive. This study utilized a virus that specifically infects cancer cells and expresses a green fluorescent protein within them to detect peritoneal disease. Viral fluorescence was more sensitive than standard methods for detecting peritoneal disease and may help to identify which patients with pancreas cancer will benefit from surgery.
1. Introduction
Pancreatic cancer is a highly aggressive disease with dismal prognosis. Patients with localized disease benefit from multimodality therapy including surgical resection. Patients with metastatic disease, however, gain no benefit from a potentially morbid surgical procedure. Staging laparoscopy (SL) has been shown to identify occult metastatic disease in 14% to 31% of patients, effectively sparing these patients unnecessary, aggressive therapies (Ahmed et al., 2006, Doucas et al., 2007, Enestvedt et al., 2008, Jimenez et al., 2000a, Jimenez et al., 2000b, White et al., 2008). One component of DL that is increasingly acknowledged as important is the cytological analysis of peritoneal cells harvested. Free cancer cells in the peritoneum are thought to arise from exfoliation of malignant cells from the primary tumor and their presence is thought to lead to the peritoneum as a frequent site of recurrence (Foo et al., 1993, Gold et al., 2007). Patients with positive peritoneal cytology (PPC) as the only evidence of metastasis have the same outcome as patients with grossly visible metastases (Ferrone et al., 2006, Merchant et al., 1999). Thus, the American Joint Commission on Cancer (AJCC) staging system for pancreatic cancer includes PPC as a criterion for M1 disease.
While conventional cytology is currently the gold standard for detection of malignant cells in peritoneal fluid because it is both highly specific and clinically relevant, it is thought to lack sensitivity, Many patients with negative peritoneal cytology at the time of resection with curative intent still develop early intraperitoneal recurrence(Abe et al., 1995, Kodera et al., 2005, Selvaggi, 2003). Additional techniques including immunohistochemistry (IHC), microarray analysis, and reverse-transcription polymerase chain reaction (RT-PCR) for tumor markers have been investigated to improve the sensitivity of detection of peritoneal cancer cells (Kodera et al., 2002, Kodera et al., 2005, Dalal et al., 2007, Fukumoto et al., 2006, Hoffmann et al., 2007, Katsuragi et al., 2007, Mori et al., 2007, Oyama et al., 2004, Tamura et al., 2007, To et al., 2003, Zhang et al., 2006, Schmidt et al., 2001). Limitations of these techniques include cost and time consuming nature of their use, and lack of reproducibility. RT-PCR is also limited by its exquisite sensitivity, which can lead to false-positive, clinically irrelevant results (Timar et al., 2002, Kammula et al., 2004, Wong and Coit, 2012).
Viral oncolytic therapy is a field that seeks to genetically design viruses that specifically infect, replicate within, and kill cancers. Many promising viruses so designed are now in clinical trials as cancer therapy (Geevarghese et al., 2010, Heo et al., 2013, Kaufmann and Chiocca, 2014, Kemeny et al., 2006, Reid et al., 2001). The herpes-based virus, T-VEC, recently became the first oncolytic virus approved for clinical use in the Western world for the treatment of metastatic melanoma. NV1066 is a replication-competent, tumor specific herpes virus that carries the marker gene encoding enhanced green fluorescent protein (eGFP). In vitro, NV1066 has been shown to infect over 111 different human cancer cell lines, and can detect as few as 1 cancer cell in a background of 1 million normal cells (Adusumilli et al., 2011). In animal models, NV1066 can circulate the blood stream and identify occult metastatic deposits of cancer (Adusumilli et al., 2005, Adusumilli et al., 2006a, Adusumilli et al., 2006b, Eisenberg et al., 2006, Stanziale et al., 2004). The aim of the current study was to evaluate use of this virus in rapid detection of peritoneal dissemination of pancreatic cancer. This human clinical trial sought to determine if the presence of virally-detected, rare peritoneal cancer cells predict peritoneal recurrence and patient outcome.
2. Methods
2.1. Virus
NV1066 is a replication-competent, attenuated oncolytic HSV type 1 (HSV-1), derived from wild-type HSV-1 (F strain) as previously described (Wong et al., 2002). This virus was attenuated by deletions of UL23, an internal repeat sequence containing single copies of the genes encoding ICP-0, ICP-4, and the ICP34.5 neurovirulence gene. This virus also carries the marker gene for eGFP, which fluoresces 35 times brighter compared with the wild-type GFP from which it was derived, inserted under the control of a constitutively expressed cytomegalovirus (CMV) promoter (Cormack et al., 1996). The eGFP transgene was inserted in place of another deleted internal repeat sequence. As a result of these genetic alterations, NV1066 has decreased virulence relative to the wild-type virus and can only successfully infect and replicate in transformed, malignant cells. The virus is remarkably cancer specific and infected cells display unmistakable bright green fluorescence (major peak at 509 nm) (Chalfie et al., 1994).
2.2. Patients
From February 2007 to May 2008, peritoneal washings were obtained prospectively from 96 patients undergoing diagnostic laparoscopy for evaluation and staging of pancreatic adenocarcinoma at Memorial Sloan-Kettering Cancer Center (MSKCC). All patients were at least 18 years of age and all presented to the Surgical Service with known or presumed pancreatic cancer based on imaging studies and/or tissue biopsy. Only patients who had histologically confirmed pancreatic adenocarcinoma were included in the clinical trial. Those pancreatic adenocarcinoma patients with an additional concomitant malignancy were excluded from the study. For outcome results, all patients were followed for recurrence of cancer and survival for a minimum of 5 years.
2.3. Ethics
This study was reviewed and approved by the MSKCC institutional review board (IRB). All patients were required to provide informed consent for participation in the study.
2.4. Laparoscopic Evaluation
Patients underwent staging laparoscopy under general anesthesia as previously described (Wong and Coit, 2012, Kelly et al., 2009). In all cases, a systemic examination of the peritoneal cavity was performed after trocar insertion and CO2 insufflation. Peritoneal washing was then performed immediately, prior to any surgical manipulation of tissues. Normal saline was introduced into the right upper abdomen, left upper abdomen, and pelvis. Gentle agitation was performed and the fluid was aspirated and collected separately from each site. Each of the three patient samples was divided into two parts: half of the fluid (approximately 30 mL) was sent to the pathology department for cytologic examination, and the other half was transported to the laboratory on ice for evaluation with virus. For patients found to have ascites at laparoscopy, ascites fluid was aspirated and collected from the three sites without instillation of normal saline.
2.5. Cytologic Assay
Samples brought to the pathology department for cytologic evaluation were placed in Cytolyte fixative (Cytyc Corp., Marlborough, MA) and centrifuged for 10 min. The resulting cell pellet was fixed with PreservCyte (Cytyc Corp). Two slide preparations were made from each sample using the Thin Prep procedure. One was stained with H&E and the other with Papanicolaou stain. All cytologic preparations were evaluated for the presence of malignant cells by attending cytopathologists at Memorial Sloan-Kettering Cancer Center (MSKCC).
2.6. Virally Mediated Fluorescence Detection Assay
The samples were centrifuged for 5 min (8000 rpm) at 4 °C. After centrifugation, the cell pellets were resuspended in 500 μL warm Roswell Park Memorial Institute (RPMI) media supplemented with 10% fetal calf serum (FCS), 100 μg/mL penicillin, and 100 μg/mL streptomycin. Samples were then infected with 1 × 106 PFU NV1066. Samples were maintained at room temperature for 30 min after infection, at which time an additional 500 μL of RPMI media was added. All samples were then transferred to a humidified incubator supplied with 5% CO2 at 37 °C. Samples were incubated with virus for 12 to 18 h prior to evaluation by fluorescence microscopy or flow cytometry.
2.7. Fluorescence Microscopy
Samples to be evaluated by fluorescence microscopy were incubated with virus in chamber slides (Lab-Tek II; Nalge Nunc, Rochester, NY). After incubation, samples were systematically evaluated with a Zeiss Axiovert 200 M inverted stand microscope (Carl Zeiss, Inc., Oberkochen, Germany) with a 100 W mercury arc lamp light source and Retiga EX CCD digital camera. Selective excitation of eGFP was produced through a Chroma 41017 filter set after placement of both excitation and emission filters to detect eGFP. Images were processed and analyzed with the MetaMorph Imaging System (Universal Imaging Corporation, Downingtown, PA).
2.8. Flow Cytometry
Samples to be evaluated by flow cytometry were incubated with virus in polystyrene round-bottom tubes (BD Falcon, San Jose, CA). Standard flow cytometry was performed in accordance with guidelines outlined in the 1995 US–Canadian consensus conference. Data acquisition analyses were performed on a FACScan flow cytometer (BD Biosciences, San Jose, CA). CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA) was used for data analysis.
2.9. Characterization of eGFP-Positive Cells
To further molecularly characterize the green cells identified in peritoneal washings, virus-infected, eGFP-positive samples were incubated with immunofluorescent antibodies to cell surface markers. R-PE conjugated mouse antihuman anti-CD66 (BD Pharmingen, Franklin Lakes, NJ) and mouse antihuman anti-HLA-DR (BD Pharmingen, Franklin Lakes, NJ) were used to characterize eGFP-expressing cells in peritoneal washings. Samples were incubated with each antibody for 30 min at room temperature prior to fluorescence microscopy examination. R-PE conjugated antibody was identified by detection of red fluorescence under TRITC filter. Immunohistochemistry was also employed for characterization of eGFP-expressing cells. Cells were fixed and stained with a mouse anti-human antibody to c-kit.
2.10. Statistical Analysis
eGFP status was reported as positive or negative and was correlated with clinical and pathologic factors using the Chi-Square test for categorical variables, and the Mann–Whitney U test for continuous variables. The probability of recurrence was estimated by the Kaplan–Meier method and compared across groups using a log-rank test. Statistical significance was defined by P < 0.05.
3. Results
3.1. Patient Demographics
From February 2007 through April 2008, peritoneal washings were obtained from 96 patients undergoing diagnostic laparoscopy for presumed or biopsy-proven pancreatic cancer. Four patients were found to have neuroendocrine tumors, ten had peri-pancreatic tumors (arising from distal common bile duct or duodenal mucosa), and two had benign disease. The remaining 82 patients had pancreatic adenocarcinoma and comprised the study population. The median age of this cohort was 67.5 years (range, 29–87 years). A total of 42 patients (51%) were female. Within the pancreatic adenocarcinoma group, 49% of patients underwent laparoscopy with or without biopsy and/or celiac plexus block (n = 40), 45% underwent resection (pancreaticoduodenectomy, n = 30; distal pancreatectomy, n = 7). Lastly, 6% of patients (n = 5) underwent a palliative bypass procedure with cholecystojejunostomy, choledochojejunostomy, and/or gastrojejunostomy.
The tumor was in the head of the pancreas in 68% (n = 56) of pancreatic adenocarcinoma patients. The tumor was well differentiated in 2%, moderately differentiated in 54%, poorly differentiated in 18%, and not assessed in the remaining 26%. Four percent (n = 3) had T1; 5% (n = 4) had T2; 68% (n = 56) had T3; and 10% (n = 8) had T4 tumors. A total of 11 patients (13%) had unknown T stage. Disease stages for the pancreatic adenocarcinoma patients were as follows: IA—3 (4%), IB—2 (2%), IIA—19 (23%), IIB—27 (33%), III—4 (5%), and IV—27 (33%).
3.2. Conventional Cytology
Of the 82 patients with pancreatic adenocarcinoma, 12 (15%) had positive cytology. Of these 12 patients, ten had visible liver (n = 2) and/or peritoneal metastases (n = 8) confirmed by tissue biopsy. Two of the 12 had positive cytology as the only evidence of metastasis. These two patients had T3 and T4 disease, and would have been stages IIA and III, respectively, if not for the finding of positive cytology. Of 16 total patients with isolated liver metastases, two had positive cytology. Of nine patients with isolated peritoneal or peritoneal and liver metastases, eight had positive cytology. Cytology showed sensitivities of 44% and 89% for detection any metastatic disease and for detection of peritoneal metastases, respectively.
3.3. eGFP Expression
Of the 82 patients with pancreatic adenocarcinoma, 50 (61%) had virally-mediated strongly eGFP positive cells in peritoneal washings (Fig. 1). Of these 50 patients, 26 (52%) had stage IV disease. Disease stages for the remaining eGFP-positive patients were as follows: IA—2 (4%), IB—1 (2%), IIA—7 (14%), IIB—12 (24%), and III—2 (4%). All twelve cytology-positive patients were also eGFP positive. Of the 16 patients with isolated liver metastases, 15 were eGFP positive. All nine patients with isolated peritoneal or peritoneal and liver metastases were eGFP positive. HSV-mediated fluorescence detection had sensitivities of 94% and 100% for detection of any metastatic disease and for detection of peritoneal-based metastasis, respectively.
Fig. 1.
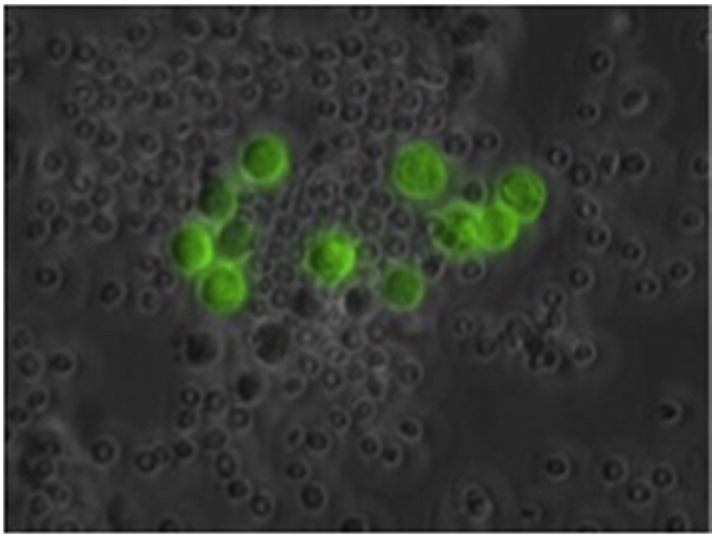
Fluorescent microscopic image of strongly eGFP-positive cells in peritoneal washing from a patient with pancreatic cancer who had negative cytology (magnification 100 ×).
3.4. Correlation of Clinicopathologic Factors and eGFP Expression in Peritoneal Washings
Table 1 summarizes the correlation between eGFP expression in peritoneal washings and various clinicopathologic features including T stage, presence of intraperitoneal metastases (hepatic and peritoneal), cytology, stage, lymph node status, degree of differentiation, and the presence of vascular/perineural invasion. Molecular diagnosis with virally mediated fluorescence correlated significantly with the presence of hepatic (P < 0.005) and peritoneal (P = 0.01) metastases, cytology results (P < 0.005), and disease stage (P = 0.027).
Table 1.
Univariate analysis of clinicopathologic variables associated with eGFP positivity in all patients.
| Clinicopathologic features | Total (n = 82) | eGFP positive (n = 50) | eGFP negative (n = 32) | P |
|---|---|---|---|---|
| T-stagea | 1.00 | |||
| Tx | 11 | 11 | 0 | |
| T1–2 | 7 | 4 | 3 | |
| T3–4 | 64 | 35 | 29 | |
| LN metastasisa | 29 | 13 | 16 | 0.78 |
| Peritoneal metastasis | 9 | 9 | 0 | 0.01 |
| Hepatic metastasis | 16 | 15 | 1 | < 0.01 |
| Positive cytology | 12 | 12 | 0 | < 0.01 |
| Stagea | 0.03 | |||
| I–IIA | 24 | 10 | 14 | |
| IIB–IV | 58 | 40 | 18 | |
| Differentiation | 0.76 | |||
| Unknown | 21 | 11 | 10 | |
| Well/moderate | 46 | 30 | 16 | |
| Poor | 15 | 9 | 6 | |
| Vascular invasion | 27 | 15 | 12 | 0.07 |
| Perineural invasion | 34 | 16 | 18 | 1.00 |
Values in bold font are statistically significant.
Pathologic information was used when available for defining T and N stages. When pathology was not available, clinical staging information was used.
3.5. Molecular Characterization of eGFP-Positive Cells
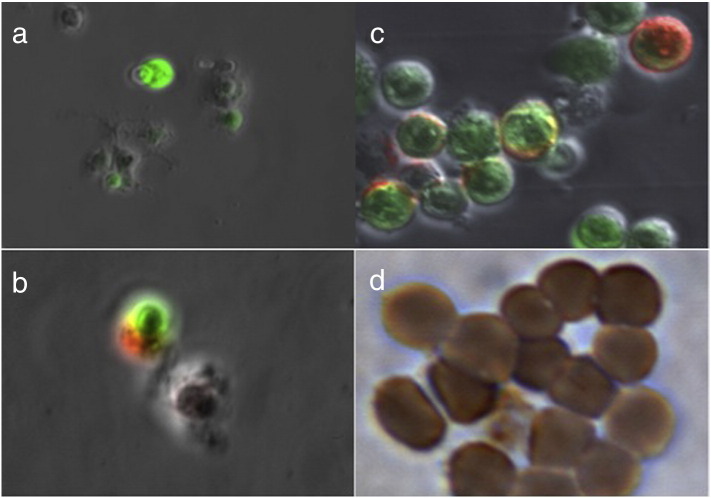
eGFP-positive cells isolated by flow cytometry were able to be further characterized using fluorescent antibodies to cell-surface markers, and immunohistochemistry. Fig. 2A demonstrates strongly and weakly-eGFP expressing cells in peritoneal washings from a patient with pancreatic cancer. The weakly-eGFP positive cells had dendrites and were occasionally observed in both benign and malignant washings. These cells were counter-stained with a fluorescent antibody to HLA-DR, confirming them to be dendritic cells (Fig. 2B). Conversely, the round, strongly positive cells were counterstained with a fluorescent antibody to CEA (Fig. 2C). Lastly, eGFP-positive cells isolated from a patient with metastatic GIST were positive for c-KIT on immunohistochemistry (Fig. 2D).
Fig. 2.
(A) Fluorescent microscopic image of cells in peritoneal washing from a patient with pancreatic cancer, demonstrating large, round, strongly eGFP positive cells, and rare, weakly eGFP positive cells with dendritic processes (10 ×). The cells were subsequently sorted by eGFP expression. The weakly eGFP positive cells were counter-stained with a fluorescent antibody to a human leukocyte antigen (HLA-DR), confirming them to be dendritic cells (B; 100 ×). Conversely, the strongly eGFP positive cells counter-stained with an antibody to CEA (C; 100 ×). eGFP positive cells isolated from a patient with metastatic gastrointestinal stromal tumor (GIST) were fixed and stained with an antibody to c-KIT (D; 100 ×).
3.6. Recurrence and Survival
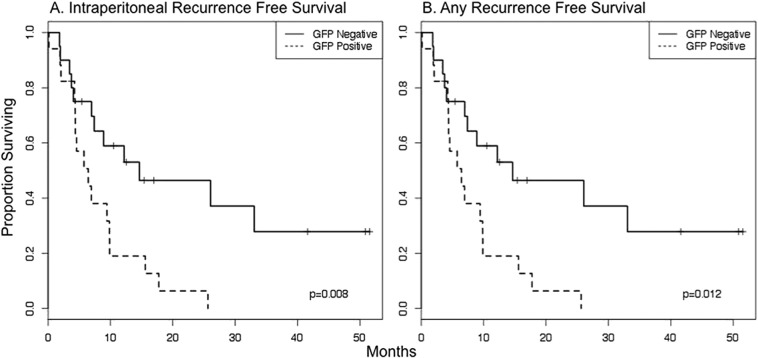
Of the 82 pancreatic adenocarcinoma patients, a total of 33, all of whom were cytology negative, underwent R0 resection. This group was analyzed for intraperitoneal and any recurrence free survival (RFS). At a median follow up of 15.4 months, 20 patients (61%) went on to develop clinical recurrence. Within the eGFP-positive group, 75% of patients developed recurrence. The most common site of first recurrence was the liver (n = 12), followed by the peritoneum (n = 4), and the lung (n = 2) or local/nodal (n = 2). Of those patients with recurrent disease first detected in the liver, nearly half also had evidence of peritoneal disease. Median intraperitoneal and any recurrence free survival were significantly shorter in the eGFP positive group (6.5 versus 14.6 months, P < 0.01; 6.5 versus 12.2 months, P = 0.01; respectively) (Fig. 3A and B). Disease specific survival was also shorter in the eGFP positive patients but this difference did not reach statistical significance (18.3 versus 36.2 months; P = 0.19) (Fig. 4). On univariate analysis of factors associated with recurrence and survival in 33 patients who underwent R0 resection, eGFP positivity was the only variable associated with recurrence (O.R. 2.64, 95% C.I. 1.21–5.78; P = 0.02). None of the variables evaluated had a statistically-significant association with disease specific survival (Table 2).
Fig. 3.
Kaplan–Meier curve demonstrating recurrence free survival for pancreatic cancer patients who underwent R0 resection who were eGFP positive (n = 16) or negative (n = 17). At a median follow up of 15.4 months, eGFP positive patients had significantly shorter time to intraperitoneal recurrence (A) and any recurrence (B) (P = 0.05, 0.02; respectively).
Fig. 4.
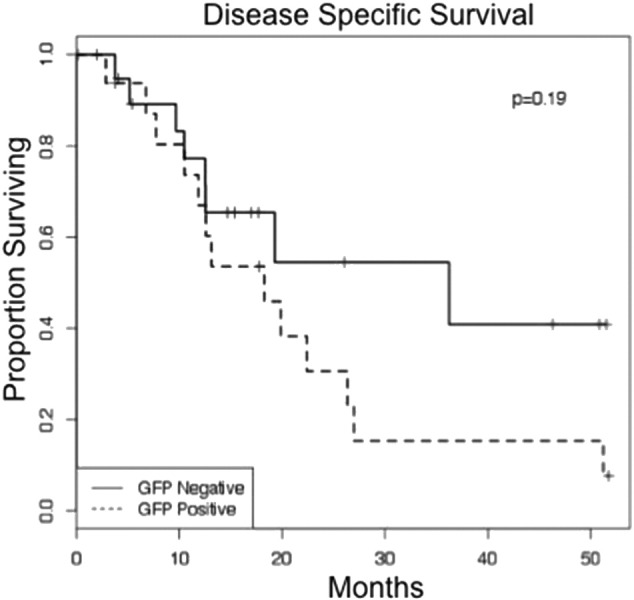
Kaplan–Meier curve demonstrating disease specific survival for pancreatic cancer patients who underwent R0 resection stratified by eGFP status. Median DSS was clinically, but not statistically, significantly shorter in the eGFP positive patients (18.3 versus 36.2 months; P = 0.19).
Table 2.
Univariate analysis of pathologic variables associated with recurrence and survival in patients undergoing R0 resection (N = 33).
| Variable | Recurrence free survival |
Disease specific survival |
||||
|---|---|---|---|---|---|---|
| O.R. | 95% C.I. | P | O.R. | 95% C.I. | P | |
| eGFP positive | 2.64 | 1.21–5.78 | 0.02 | 1.72 | 0.74–4.00 | 0.21 |
| Tumor depth | 0.25 | 0.46 | ||||
| T1–2 | REF | REF | ||||
| T3 | 2.33 | 0.55–9.86 | 1.73 | 0.40–7.45 | 0.46 | |
| Positive lymph nodes | 1.11 | 0.50–2.43 | 0.80 | 1.25 | 0.51–3.06 | 0.62 |
| Differentiation | 0.11 | 0.64 | ||||
| Well | REF | REF | ||||
| Moderate/poor | 2.05 | 0.85–4.94 | 1.21 | 0.52–2.85 | 0.65 | |
| Vascular invasion | 1.97 | 0.79–4.93 | 0.15 | 1.53 | 0.52–4.53 | 0.44 |
| Perineural invasion | 1.37 | 0.18–10.17 | 0.76 | 1.81 | 0.23–14.35 | 0.57 |
Values in bold font are statistically significant.
4. Discussion
Peritoneal micro-metastases are a common occurrence and portend poor prognosis for many malignancies including ovarian, gastric, and pancreatic cancer (Ferrone et al., 2006, Merchant et al., 1999, Bentrem et al., 2005, Burke et al., 1998, Yachida et al., 2002, Yoshimura et al., 1984). The current standard method of detection relies on cytologic evaluation of harvested cells according to for cytomorphologic characteristics of malignant transformation. This test is highly specific and clinically relevant: positive results are associated with very poor prognosis, early intraabdominal recurrence, and death (Jimenez et al., 2000a, Gold et al., 2007, Ferrone et al., 2006, Merchant et al., 1999, Bentrem et al., 2005, Burke et al., 1998, Yachida et al., 2002, Yoshimura et al., 1984). However, when very few free cancer cells are present in the peritoneum, they may be difficult to identify by cytomorphologic criteria alone. In addition, mesothelial atypia and inflammatory infiltrates can complicate cytologic analysis of peritoneal fluid. Pancreatic and gastric cancer patients undergo DL with the goal of accurate preoperative staging and are often found to have false negative cytology. Many of these patients then undergo resection with curative intent only to develop early postoperative recurrence in the peritoneal cavity.
NV1066-mediated eGFP expression has been shown to aid in detection of malignant deposits in preclinical animal models of pleural, peritoneal, and lymphatic disease, and in preparations of malignant cells mixed with benign cells from various organs in vitro (Adusumilli et al., 2005, Adusumilli et al., 2006a, Adusumilli et al., 2006b, Adusumilli et al., 2011, Eisenberg et al., 2006, Stanziale et al., 2004). In the current study, we clinically test a virally-mediated fluorescence detection assay with NV1066 was employed to detect peritoneal micro-metastases in pancreatic cancer patients. In this cohort of pancreatic adenocarcinoma patients evaluated prospectively, virally-mediated fluorescence increased the yield of diagnosis of peritoneal micro-metastasis by 46% over conventional cytology. This increased sensitivity appears to be clinically relevant. Patients undergoing resection with curative intent, all of whom had negative conventional cytology, had a median recurrence free survival of only 6.5 months if eGFP positive, versus 12.2 months if eGFP negative (P = 0.01). This finding confirms that the presence of eGFP positive cells in peritoneal washings is a sensitive indicator of micrometastasis and that these cells do go on to develop into early recurrence. As is true for pancreas cancer in general, the most common site of first clinical recurrence after curative-intent resection for patients in this trial was the liver, followed by the peritoneum. eGFP positivity predicted not only intraperitoneal, but any recurrence. This finding suggests that the presence of eGFP positive cells may be an indicator of aggressive tumor biology. Median disease specific survival for the eGFP-negative patients was 36.2 months, double that of eGFP-positive patients. Although not statistically significant, this survival is comparable if not superior to the 20–24 month median survival expected after curative resection and adjuvant therapy. Median survival of this magnitude for pancreatic cancer patients has only been reported in neoadjuvant therapy trials. This finding suggests that virally-mediated fluorescence detection of peritoneal micrometastasis may have an important role in patient selection for surgery.
Virally-mediated fluorescence detection can be incorporated into the DL procedure with essentially no increased morbidity or risk to the patient, and can enhance the sensitivity of detection of peritoneal micrometastatic disease. This assay is inexpensive, rapid, and easy to perform and interpret. eGFP positivity could potentially be incorporated into DL for pancreatic cancer patients. If adopted into clinical practice, eGFP positive patients, like those with positive cytology, could be offered neoadjuvant or definitive systemic therapy. While this pilot study focuses on pancreatic adenocarcinoma, there is reason to believe that this highly sensitive and specific assay is applicable to essentially all intraabdominal malignancies. Virally mediated fluorescence has the potential to enhance the sensitivity of DL for the detection of occult micrometastatic disease for many cancers (Kemeny et al., 2006).
The virus tested in this study is one of a family of genetically engineered viruses designed for specific infection and killing of cancer. These were meant as therapeutic agents. In preclinical studies, these viruses infect and kill many cancer cell lines, including many pancreatic cancer cell lines (Buijs et al., 2014, Dai et al., 2014, Haddad et al., 2012, Liu et al., 2013, Yamamura et al., 2014). The current study indicates that this virus infects and replicates in primary pancreatic cancer cells from many different patients. In preclinical studies, the ability to kill tumor is directly correlated to infectivity and replication (Adusumilli et al., 2006b, Stanziale et al., 2004, Wong et al., 2002). These observations suggest that herpes simplex oncolytic viruses should be investigated not only as agents to improve staging and patient selection, but especially as novel treatment agents for pancreatic cancer, a disease that is highly resistant to cytotoxic chemotherapy.
Declaration of Interests
The authors have declared no conflicts of interest.
Author Contributions
Kaitlyn Kelly: Literature search, data collection, data analysis and interpretation, figures, manuscript writing.
Joyce Wong: Data collection, manuscript review.
Mithat Gönen: Data analysis and interpretation, statistics.
Peter Allen: Patient recruitment, data interpretation, manuscript review.
Murray Brennan: Patient recruitment, data interpretation, manuscript review.
Daniel Coit: Patient recruitment, data interpretation, manuscript review.
Yuman Fong: Study design, patient recruitment, data analysis and interpretation, figures, manuscript writing and reviewing.
Acknowledgments and Funding
This study was funded by a National Institute of Health R01 grant (CA 75416; Fong (PI)#6437803).
References
- Abe S., Yoshimura H., Nagaoka S. Long-term results of operation for carcinoma of the stomach in T1/T2 stages: critical evaluation of the concept of early carcinoma of the stomach. J. Am. Coll. Surg. 1995;181:389–396. [PubMed] [Google Scholar]
- Adusumilli P.S., Eisenberg D.P., Chun Y.S. Virally directed fluorescent imaging improves diagnostic sensitivity in the detection of minimal residual disease after potentially curative cytoreductive surgery. J. Gastrointest. Surg. 2005;9:1138–1146. doi: 10.1016/j.gassur.2005.06.029. (discussion 46-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adusumilli P.S., Eisenberg D.P., Stiles B.M. Intraoperative localization of lymph node metastases with a replication-competent herpes simplex virus. J. Thorac. Cardiovasc. Surg. 2006;132:1179–1188. doi: 10.1016/j.jtcvs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Adusumilli P.S., Gholami S., Chun Y.S. Fluorescence-assisted cytological testing (FACT): ex vivo viral method for enhancing detection of rare cancer cells in body fluids. Mol. Med. 2011;17:628–634. doi: 10.2119/molmed.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adusumilli P.S., Stiles B.M., Chan M.K. Imaging and therapy of malignant pleural mesothelioma using replication-competent herpes simplex viruses. J. Gene Med. 2006;8:603–615. doi: 10.1002/jgm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.I., Bochkarev V., Oleynikov D., Sasson A.R. Patients with pancreatic adenocarcinoma benefit from staging laparoscopy. J. Laparoendosc. Adv. Surg. Tech. A. 2006;16:458–463. doi: 10.1089/lap.2006.16.458. [DOI] [PubMed] [Google Scholar]
- Bentrem D., Wilton A., Mazumdar M., Brennan M., Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann. Surg. Oncol. 2005;12:347–353. doi: 10.1245/ASO.2005.03.065. [DOI] [PubMed] [Google Scholar]
- Buijs P.R., van Eijck C.H., Hofland L.J., Fouchier R.A., van den Hoogen B.G. Different responses of human pancreatic adenocarcinoma cell lines to oncolytic Newcastle disease virus infection. Cancer Gene Ther. 2014;21:24–30. doi: 10.1038/cgt.2013.78. [DOI] [PubMed] [Google Scholar]
- Burke E.C., Karpeh M.S., Jr., Conlon K.C., Brennan M.F. Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann. Surg. Oncol. 1998;5:411–415. doi: 10.1007/BF02303859. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W.W., Prasher D.C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Cormack B.P., Valdivia R.H., Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Dai M.H., Liu S.L., Chen N.G. Oncolytic vaccinia virus in combination with radiation shows synergistic antitumor efficacy in pancreatic cancer. Cancer Lett. 2014;344:282–290. doi: 10.1016/j.canlet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Dalal K.M., Woo Y., Galanis C. Detection of micrometastases in peritoneal washings of pancreatic cancer patients by the reverse transcriptase polymerase chain reaction. J. Gastrointest. Surg. 2007;11:1598–1605. doi: 10.1007/s11605-007-0283-z. (discussion 605-6) [DOI] [PubMed] [Google Scholar]
- Doucas H., Sutton C.D., Zimmerman A., Dennison A.R., Berry D.P. Assessment of pancreatic malignancy with laparoscopy and intraoperative ultrasound. Surg. Endosc. 2007;21:1147–1152. doi: 10.1007/s00464-006-9093-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg D.P., Adusumilli P.S., Hendershott K.J. Real-time intraoperative detection of breast cancer axillary lymph node metastases using a green fluorescent protein-expressing herpes virus. Ann. Surg. 2006;243:824–830. doi: 10.1097/01.sla.0000219738.56896.c0. (discussion 30-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enestvedt C.K., Mayo S.C., Diggs B.S. Diagnostic laparoscopy for patients with potentially resectable pancreatic adenocarcinoma: is it cost-effective in the current era? J. Gastrointest. Surg. 2008;12:1177–1184. doi: 10.1007/s11605-008-0514-y. [DOI] [PubMed] [Google Scholar]
- Ferrone C.R., Haas B., Tang L. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J. Gastrointest. Surg. 2006;10:1347–1353. doi: 10.1016/j.gassur.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Foo M.L., Gunderson L.L., Nagorney D.M. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation +/− 5 fluorouracil. Int. J. Radiat. Oncol. Biol. Phys. 1993;26:483–489. doi: 10.1016/0360-3016(93)90967-z. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Ikeguchi M., Matsumoto S. Detection of cancer cells and gene expression of cytokines in the peritoneal cavity in patients with gastric cancer. Gastric Cancer. 2006;9:271–276. doi: 10.1007/s10120-006-0390-7. [DOI] [PubMed] [Google Scholar]
- Geevarghese S.K., Geller D.A., de Haan H.A. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum. Gene Ther. 2010;21:1119–1128. doi: 10.1089/hum.2010.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.S., Jaques D.P., Bentrem D.J. Outcome of patients with known metastatic gastric cancer undergoing resection with therapeutic intent. Ann. Surg. Oncol. 2007;14:365–372. doi: 10.1245/s10434-006-9059-z. [DOI] [PubMed] [Google Scholar]
- Haddad D., Zanzonico P.B., Carlin S. A vaccinia virus encoding the human sodium iodide symporter facilitates long-term image monitoring of virotherapy and targeted radiotherapy of pancreatic cancer. J. Nucl. Med. 2012;53:1933–1942. doi: 10.2967/jnumed.112.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J., Reid T., Ruo L. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K., Kerner C., Wilfert W. Detection of disseminated pancreatic cells by amplification of cytokeratin-19 with quantitative RT-PCR in blood, bone marrow and peritoneal lavage of pancreatic carcinoma patients. World J. Gastroenterol. 2007;13:257–263. doi: 10.3748/wjg.v13.i2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez R.E., Warshaw A.L., Fernandez-Del Castillo C. Laparoscopy and peritoneal cytology in the staging of pancreatic cancer. J. Hepato-Biliary-Pancreat. Surg. 2000;7:15–20. doi: 10.1007/s005340050148. [DOI] [PubMed] [Google Scholar]
- Jimenez R.E., Warshaw A.L., Rattner D.W., Willett C.G., McGrath D., Fernandez-del Castillo C. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch. Surg. 2000;135:409–414. doi: 10.1001/archsurg.135.4.409. (discussion 14-5) [DOI] [PubMed] [Google Scholar]
- Kammula U.S., Ghossein R., Bhattacharya S., Coit D.G. Serial follow-up and the prognostic significance of reverse transcriptase-polymerase chain reaction—staged sentinel lymph nodes from melanoma patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004;22:3989–3996. doi: 10.1200/JCO.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Katsuragi K., Yashiro M., Sawada T., Osaka H., Ohira M., Hirakawa K. Prognostic impact of PCR-based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. Br. J. Cancer. 2007;97:550–556. doi: 10.1038/sj.bjc.6603909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann J.K., Chiocca E.A. Glioma virus therapies between bench and bedside. Neuro-Oncology. 2014;16:334–351. doi: 10.1093/neuonc/not310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K.J., Wong J., Gladdy R. Prognostic impact of RT-PCR-based detection of peritoneal micrometastases in patients with pancreatic cancer undergoing curative resection. Ann. Surg. Oncol. 2009;16:3333–3339. doi: 10.1245/s10434-009-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny N., Brown K., Covey A. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum. Gene Ther. 2006;17:1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- Kodera Y., Nakanishi H., Ito S. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer. 2002;5:69–76. doi: 10.1007/s101200200012. [DOI] [PubMed] [Google Scholar]
- Kodera Y., Nakanishi H., Ito S. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer. 2005;8:142–148. doi: 10.1007/s10120-005-0318-7. [DOI] [PubMed] [Google Scholar]
- Liu H., Yuan S.J., Chen Y.T. Preclinical evaluation of herpes simplex virus armed with granulocyte-macrophage colony-stimulating factor in pancreatic carcinoma. World J. Gastroenterol. 2013;19:5138–5143. doi: 10.3748/wjg.v19.i31.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant N.B., Conlon K.C., Saigo P., Dougherty E., Brennan M.F. Positive peritoneal cytology predicts unresectability of pancreatic adenocarcinoma. J. Am. Coll. Surg. 1999;188:421–426. doi: 10.1016/s1072-7515(98)00327-5. [DOI] [PubMed] [Google Scholar]
- Mori K., Suzuki T., Uozaki H. Detection of minimal gastric cancer cells in peritoneal washings by focused microarray analysis with multiple markers: clinical implications. Ann. Surg. Oncol. 2007;14:1694–1702. doi: 10.1245/s10434-006-9321-4. [DOI] [PubMed] [Google Scholar]
- Oyama K., Terashima M., Takagane A., Maesawa C. Prognostic significance of peritoneal minimal residual disease in gastric cancer detected by reverse transcription-polymerase chain reaction. Br. J. Surg. 2004;91:435–443. doi: 10.1002/bjs.4455. [DOI] [PubMed] [Google Scholar]
- Reid T., Galanis E., Abbruzzese J. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 2001;8:1618–1626. doi: 10.1038/sj.gt.3301512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P., Thiele M., Rudroff C. Detection of tumor cells in peritoneal lavages from patients with gastrointestinal cancer by multiplex reverse transcriptase PCR. Hepato-Gastroenterology. 2001;48:1675–1679. [PubMed] [Google Scholar]
- Selvaggi S.M. Diagnostic pitfalls of peritoneal washing cytology and the role of cell blocks in their diagnosis. Diagn. Cytopathol. 2003;28:335–341. doi: 10.1002/dc.10290. [DOI] [PubMed] [Google Scholar]
- Stanziale S.F., Stiles B.M., Bhargava A., Kerns S.A., Kalakonda N., Fong Y. Oncolytic herpes simplex virus-1 mutant expressing green fluorescent protein can detect and treat peritoneal cancer. Hum. Gene Ther. 2004;15:609–618. doi: 10.1089/104303404323142051. [DOI] [PubMed] [Google Scholar]
- Tamura N., Iinuma H., Takada T. Prospective study of the quantitative carcinoembryonic antigen and cytokeratin 20 mRNA detection in peritoneal washes to predict peritoneal recurrence in gastric carcinoma patients. Oncol. Rep. 2007;17:667–672. [PubMed] [Google Scholar]
- Timar J., Csuka O., Orosz Z., Jeney A., Kopper L. Molecular pathology of tumor metastasis. II. Molecular staging and differential diagnosis. Pathol. Oncol. Res. 2002;8:204–219. doi: 10.1007/BF03032397. [DOI] [PubMed] [Google Scholar]
- To E.M., Chan W.Y., Chow C., Ng E.K., Chung S.C. Gastric cancer cell detection in peritoneal washing: cytology versus RT-PCR for CEA transcripts. Diagn. Mol. Pathol. 2003;12:88–95. doi: 10.1097/00019606-200306000-00004. [DOI] [PubMed] [Google Scholar]
- White R., Winston C., Gonen M. Current utility of staging laparoscopy for pancreatic and peripancreatic neoplasms. J. Am. Coll. Surg. 2008;206:445–450. doi: 10.1016/j.jamcollsurg.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Wong J., Coit D. Detection of gastric cancer peritoneal metastases by peritoneal lavage: current limitations and future perspectives. Surgery. 2012;152:1–4. doi: 10.1016/j.surg.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Wong R.J., Joe J.K., Kim S.H., Shah J.P., Horsburgh B., Fong Y. Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Hum. Gene Ther. 2002;13:1213–1223. doi: 10.1089/104303402320138998. [DOI] [PubMed] [Google Scholar]
- Yachida S., Fukushima N., Sakamoto M., Matsuno Y., Kosuge T., Hirohashi S. Implications of peritoneal washing cytology in patients with potentially resectable pancreatic cancer. Br. J. Surg. 2002;89:573–578. doi: 10.1046/j.1365-2168.2002.02061.x. [DOI] [PubMed] [Google Scholar]
- Yamamura K., Kasuya H., Sahin T.T. Combination treatment of human pancreatic cancer xenograft models with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib and oncolytic herpes simplex virus HF10. Ann. Surg. Oncol. 2014;21:691–698. doi: 10.1245/s10434-013-3329-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Scully R.E., Taft P.D., Herrington J.B. Peritoneal fluid cytology in patients with ovarian cancer. Gynecol. Oncol. 1984;17:161–167. doi: 10.1016/0090-8258(84)90072-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y.S., Xu J., Luo G.H. Detection of carcinoembryonic antigen mRNA in peritoneal washes from gastric cancer patients and its clinical significance. World J. Gastroenterol. 2006;12:1408–1411. doi: 10.3748/wjg.v12.i9.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]