Abstract
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a poorly understood syndrome affecting up to 6.5% of adult women in the U.S. The lack of broadly accepted objective laboratory markers for this condition hampers efforts to diagnose and treat this condition. To identify biochemical markers for IC/BPS, we applied mass spectrometry-based global metabolite profiling to urine specimens from a cohort of female IC/BPS subjects from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. These analyses identified multiple metabolites capable of discriminating IC/BPS and control subjects. Of these candidate markers, etiocholan-3α-ol-17-one sulfate (Etio-S), a sulfoconjugated 5-β reduced isomer of testosterone, distinguished female IC/BPS and control subjects with a sensitivity and specificity > 90%. Among IC/BPS subjects, urinary Etio-S levels are correlated with elevated symptom scores (symptoms, pelvic pain, and number of painful body sites) and could resolve high- from low-symptom IC/BPS subgroups. Etio-S-associated biochemical changes persisted through 3–6 months of longitudinal follow up. These results raise the possibility that an underlying biochemical abnormality contributes to symptoms in patients with severe IC/BPS.
Highlights
-
•
Unbiased small molecule profiling identified an interstitial cystitis/bladder pain syndrome associated metabolite.
-
•
This urinary metabolite independently identified patients with severe symptoms scores.
-
•
Associated biochemical changes persisted over 3–6 months and hint at broader metabolic dysfunction in patients.
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a poorly understood syndrome associated with chronic bladder or pelvic pain, often accompanied by frequent urination. Identifying biochemical pathways associated with IC/BPS is necessary to understand the disease processes and suggest new therapeutic targets. Here we applied a biochemical approach to compare all detectable urinary metabolites from human subjects with and without IC/BPS. This analysis identified a steroid hormone metabolite that corresponds to patients that report the most severe symptoms. This result offers insight into IC/BPS pathophysiology, and provides a new biochemical clue to guide future investigation into this mysterious condition.
1. Introduction
Urologic chronic pelvic pain syndrome (UCPPS) encompasses two of the least understood syndromes in urology: interstitial cystitis/bladder pain syndrome (IC/BPS) in females and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) in males (van de Merwe et al., 2008). Female IC/BPS patients present with chronic pelvic pain, bladder/urogenital discomfort, and urinary frequency or urgency symptoms. Despite its high prevalence (Berry et al., 2011), the pathophysiologic processes underlying IC/BPS symptoms are poorly understood, and there are no uniformly effective treatments (Hanno et al., 2015). Diagnosis is often made based on exclusion of other conditions, and subjective reports of symptoms. There are no pathognomonic pathologic or cystoscopic findings, or objective biomarkers for this syndrome (Hanno et al., 2015). The absence of distinctive biochemical, neurological, or anatomical explanations for IC/BPS, coupled with a lack of objective biomarkers, has led many to regard it as a functional somatic syndrome (Clemens, 2008).
There is a longstanding interest in identifying biochemical markers of IC/BPS, both to facilitate diagnosis and to identify underlying pathobiological mechanisms. Past studies have proposed a range of putative IC/BPS biomarkers, including antiproliferative factor (Erickson et al., 2002), urinary phenylacetylglutamine (Fukui et al., 2009), interleukin-6 and histamines (Lamale et al., 2006), urinary nerve growth factor (Kim et al., 2014), Tamm–Horsfall protein-associated nucleotides (Argade et al., 2013), and metabolites such as 2-oxoglutarate (Wen et al., 2015). Recent advances in mass spectrometry based metabolomics now raise the prospect that new biomarkers might be discovered from among the hundreds to thousands of metabolite features observable in patient specimens. Comprehensive metabolite profiling offers nuanced insights into the systemic biochemical shifts experienced by patients, and can be linked to clinical metadata to reveal IC/BPS-specific phenotypic effects.
Here, we used mass spectrometry based metabolomics approaches to discover urinary biomarkers in IC/BPS females who underwent extensive urologic and non-urologic phenotyping in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network's central clinical protocol, the Trans-MAPP Epidemiology and Phenotyping Study (Landis et al., 2014, Clemens et al., 2014). Multiple rounds of metabolomics discovery and validation revealed a IC/BPS-associated urinary sulfometabolome profile exemplified by the sulfoconjugated steroid etiocholan-3a-ol-17-one. This distinctive sulfometabolome profile persisted over one year of follow up in female IC/BPS participants. These results reveal a shift in steroid metabolism among IC/BPS females that may be useful for both diagnosis and for better understanding the etiology of this debilitating illness.
2. Materials and Methods
2.1. Human Specimen Collection
All biological samples and clinical data investigated in this study were collected as a part of the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network(Clemens et al., 2014, Landis et al., 2014), The MAPP Network is a multi-site research effort sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) that aims to investigate the disease characteristics, phenotypes, and underlying biological mechanisms associated with urologic chronic pelvic pain syndrome (UCPPS) (i.e., interstitial cystitis/painful bladder pain syndrome [IC/BPS] and chronic prostatitis/chronic pelvic pain syndrome [CP/CPPS]). The central clinical protocol conducted by the MAPP Network is a longitudinal observational examination of UCPPS termed the Trans-MAPP Epidemiology/Phenotyping Study (EPS). This involved extensive urologic and non-urologic phenotyping, and collection of biological samples at baseline and at 6 and 12 month office visits with intervening online data collection (Landis et al., 2014). Participants who qualified for the EPS were 18 years of age or older, indicated a level of pain, pressure, or discomfort in the pelvic region of at least 1 on a 0 to 10 scale, and had a diagnosis of IC/BPS. Two types of controls were also recruited to provide symptom data and biospecimens at baseline: 1) non-IC/BPS participants who reported a 0 on the pain, pressure and discomfort scale, no chronic pain in the pelvic or bladder region and chronic pain in at most one other body region, and no urological symptoms, and 2) positive IC/BPS controls who did not exhibit pelvic pain or urinary symptoms but had at least one of 3 targeted comorbid non-urologic associated conditions (Chronic Fatigue Syndrome, Irritable Bowel Syndrome, or Fibromyalgia). All participants provided informed consent. The institutional review boards of the participating MAPP Network sites approved all experimental procedures described in this manuscript, which conform to standards indicated by the Declaration of Helsinki.
2.2. Patient Demographics and Disease Symptoms
The midstream urine samples (voided bladder 2, or VB2, representative of bladder contents) characterized in this study were derived from female IC/BPS participants who reported high symptoms at the time of their initial visit. Samples from non-IC/BPS participants were used as negative controls for basis comparisons. IC/BPS females were classified as high symptom based on the sum of the Genitourinary Pain Index (GUPI) Pain and Urinary subscales, where included IC/BPS females had a combined score ≥ 22. Symptom metrics were established by questionnaires that interrogated participants' pelvic and bladder symptoms, pain symptoms, and severity of urologic condition (Table 1). Urologic symptoms were assessed using the Genitourinary Pain Index (GUPI) (Clemens et al., 2009), Interstitial Cystitis Symptom Index (ICSI), American Urological Association Symptom Index (AUASI), Brief Pain Inventory (BPI), Symptom and Health Care Utilization Questionnaire (SYM-Q) and the Interstitial Cystitis Problem Index (ICPI) (O'Leary et al., 1997). These indices include subscales for frequency and intensity of pain, urgency and frequency of urination, and nocturia. Psychosocial metrics were also assessed using the Hospital Anxiety and Depression Scale (HADS) (Snaith, 2003).
Table 1.
Patient baseline demographics, continuous variables and metabolic characteristics. This study examined forty IC/BPS female (with high symptom scores) and forty age-matched non-IC/BPS (healthy) control female participants in the MAPP Research Network study.
| Index | Metric | IC/BPS (n = 40) | Non-IC/BPS controls (n = 40) | MAPP cohort (n = 424) |
|---|---|---|---|---|
| Age (years) | 37.7 ± 13.8 | 37.5 ± 13 | 43.4 ± 15.1 | |
| Duration of symptoms | 12.5 ± 7.3 | n/a | 8.5 ± 10.6 | |
| ICSI | IC symptom index | 15.3 ± 3.0 | 0.2 ± 0.6 | 9.7 ± 4.7 |
| ICPI | IC problem index | 13.3 ± 2.0 | 0.1 ± 0.5 | 8.5 ± 4.4 |
| SYM-Q | Pain in pelvic region (0–10) | 7.2 ± 1.4 | 0.0 ± 0.0 | 5.1 ± 2.2 |
| Urgency (0–10) | 7.4 ± 1.5 | 0.2 ± 0.6 | 5.1 ± 2.6 | |
| Frequency (0–10) | 7.0 ± 2.0 | 0.1 ± 0.5 | 4.9 ± 2.6 | |
| Urological symptoms (0–10) | 7.5 ± 1.3 | 0.0 ± 0.0 | 5.2 ± 2.3 | |
| Non-urological pain symptoms (0–10) | 4.7 ± 3.0 | 0.4 ± 0.9 | 3.3 ± 2.7 | |
| GUPI | Pain score (0–23) | 18.0 ± 2.0 | 0.0 ± 0.0 | 12.6 ± 4.5 |
| Total score (0–45) | 36.5 ± 3.9 | 0.3 ± 1.0 | 25.6 ± 8.6 | |
| BPI | Pain severity score (0–10) | 5.9 ± 1.4 | 0.1 ± 0.3 | 4.0 ± 2.0 |
| # of pain sites checked (0–45) | 6.5 ± 2.2 | 0.1 ± 0.3 | 5.8 ± 6.5 | |
| AUASI | Baseline symptom score index (0–35) | 26.3 ± 5.3 | 0.8 ± 1.1 | 15.5 ± 8.5 |
| HADS | Anxiety (0–21) | 10.2 ± 4.9 | 2.7 ± 2.6 | 7.7 ± 4.5 |
| Depression (0–21) | 7.7 ± 5.0 | 1.0 ± 1.2 | 5.4 ± 4.7 |
2.3. Sample Preparation
Biospecimens were initially stored at 4 °C and moved to − 80 °C. All biospecimen shipments were on dry ice. Urine samples were thawed on ice and centrifuged (23,000 g for 10 min) at 4 °C. 200 μl of sample supernatant was diluted with 1 volume HPLC grade H2O (Sigma), and filtered (MillexGP, 0.22 mm) before analysis. Quality control (QC) samples were prepared by pooling equal volume of each analyzed sample.
2.4. Liquid Chromatography–Mass Spectrometry (LC–MS)
The mass spectrometer used for this study was an AB Sciex API 4000 QTrap (AB Sciex, Foster City, CA) operated in the negative ion electrospray ionization (ESI) mode using the Turbo V ESI ion source. The mass spectrometer was coupled to a Shimadzu UFLC (Kyoto, Japan). Samples were injected onto an Ascentis Express fused core phenyl-hexyl column (100 mm × 2 mm × 2.7 μm) with a flow rate of 0.35 ml/min (Ascentis Express, Supelco). The gradient used was as follows: Solvent A (0.1% formic acid) was held constant at 98% and solvent B (90% acetonitrile in 0.1% formic acid) was held constant at 2% for 1 min. Solvent B was increased to 35% by 23 min and then to 98% over 10 min. The column was allowed to equilibrate for 3 min before starting the next run. The ion spray voltage was set to 5 kV. The heater temperature was 600 °C. The declustering potential, nebulizer gas (G1), auxiliary gas (G2) and collision energy were set at − 60, 45, 45 and -5 V respectively. Full scan analysis was performed in the enhanced mass spectrometry (EMS) mode using the electrospray ionization technique with coverage of mass range: 50 to 1000 Da by using a scan rate of 1000 per minute, and the MS/MS screening was accomplished in the combinational mode of EMS_IDA_EPS.
2.5. Chemometric Analysis
To identify IC/BPS -associated molecular features, full scan or sulfate precursor scan profiles from selected urinary specimens were aligned and integrated using XCMS (Scripps) or MarkerView v1.2.0. Pareto scaling of triplicate runs was used to identify differential biomarker expression among clinical subgroups. Unsupervised and discriminate principal component analyses were both performed in MarkerView v1.2.0. Candidate ions were identified by PCA-DA loading plots and visually verified in the mass spectra.
2.6. Etio-S Purification and Identification
To partially purify Etio-S from human urine, we used high performance liquid chromatography (HPLC) on a Shimadzu Prominence UFLC with a gradient of 0.1% formic acid to 90% acetonitrile + 0.1% formic acid. Urine was fractionated on an Ascentis Express phenyl-hexyl column (Supelco). Candidate-containing fractions, as determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS), were further purified over a Kinetex C18 column (Phenomenex). These enriched fractions were then analyzed on an Agilent 6550 Q-TOF mass spectrometer to determine the accurate mass. Selected analytes were characterized by MS/MS product ion scanning over a range of collision energies. For targeted LC–MS/MS assays, the most intense product ion peak(s) were selected with their corresponding optimal collision energy. Further structural information was generated using GC-EI-MS, whose mass spectra permitted the identification of etiocholan-3α-ol-17-one sulfate through spectral matching with the NIST 14 spectral library. Subsequent confirmation was accomplished my confirming identical GC retention time and mass spectrum with a commercially available standard (Sigma).
2.7. Urinary Sulfate Neutral Loss Analysis
We used a UFLC-4000 QTRAP with the chromatography and ion source settings described above to identify compounds with a common neutral fragment loss of 97 m/z units. The collision energy was set to 35 V, and the first mass analyzer (Q1) was set to scan from m/z 100 to 1000 a.m.u., whereas the second mass analyzer (Q3) simultaneously scanned at 97 m/z units less than Q1. Sensitivity was maximized by selecting the low-resolution (2-a.m.u. window, 0.7 full-width at half-height) setting. In this manner, only ions showing a neutral loss of 97 a.m.u. were detected.
2.8. Statistical Analysis
For between-group comparisons, we determined whether the responses were normally distributed by using the Shapiro–Wilk test. When normally distributed, we used t-test for parametric, or Mann–Whitney for non-parametric comparisons (Prism v.6.0d, GraphPad). Principal component analysis-discriminant analysis (PCA-DA) and partial lease squares-discriminant analysis (PLS-DA) were used for discriminant analysis. Two-way analysis of variance (ANOVA) was performed for multiple-group comparisons. Wilcoxon matched pairs signed rank test was used for non-parametric paired longitudinal comparisons. Receiver Operator Characteristic (ROC) curves were used to evaluate Etio-S levels in IC/BPS samples as predictive for subgroup 1 vs. subgroup 2 (SigmaPlot v. 12.3, Systat Software). These ROC curves were also used to determine the threshold to maximize sensitivity and specificity.
3. Results
3.1. Participant Characteristics
To seek signature IC/BPS metabolites, we selected a well-characterized group of female IC/BPS participants and controls enrolled in the MAPP Research Network (Landis et al., 2014) (referred to here as the ‘discovery’ specimens) for metabolomic analysis. The 40 IC/BPS discovery subjects selected in this study are females, had high symptom scores (see Materials and Methods), and had urine specimens available at enrollment. The non-IC/BPS control group consisted of 40 age-matched female specimens (Table 1). IC/BPS females reported symptoms for 12.5 ± 7.3 years. At the time of initial evaluation, IC/BPS females reported a mean interstitial cystitis symptom index (ICSI) of 15.3 ± 3.0, and an interstitial cystitis problem index (ICPI) of 13.3 ± 2.0. They reported similarly high pelvic pain/pressure/discomfort ratings (7.2 ± 1.4 on a 0 to 10 numeric rating scale), and the genitourinary pain index (GUPI; 18.0 ± 2.0). These disease metrics are significantly lower for non-IC/BPS controls (Table 1). While 6/40 (15%) of both the IC/BPS and control groups reported general medication usage, IC/BPS participants reported additional central (19/40, 47.5%) and opioid medication usage (9/40, 22.5%).
3.2. The IC/BPS Patient Group Exhibits a Distinctive Urinary Metabolite Composition
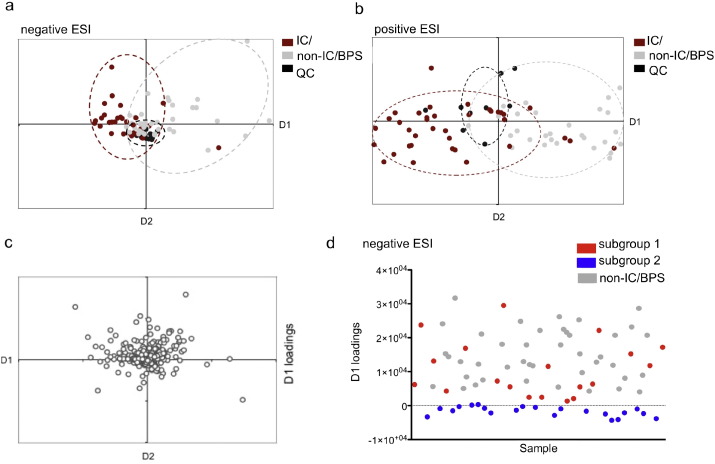
To determine whether there exist signature IC/BPS urinary metabolites, we analyzed urine specimens from the discovery data set using liquid chromatography-mass spectrometry (LC–MS) in both negative and positive electrospray ionization modes (ESI, see Materials and Methods) (Lv et al., 2011). We first used unsupervised principal components analysis (PCA) to overview the metabolomics profiles for each discovery specimen in the study cohort (Supplemental Fig. 1), followed by supervised PCA discriminant analysis (PCA-DA, Fig. 1a and b). While PCA graphically displays overall metabolite variation differences between all subjects, this analysis was not clearly related to IC/BPS-specific differences. To identify metabolite variations that distinguish the two clinical groups (IC/BPS and non-IC/BPS) we used PCA-DA. The resulting PCA-DA plot indicates only partial overlap between IC/BPS and non-IC/BPS specimens, consistent with a distinctive urinary metabolite pattern in some IC/BPS subjects. To determine which metabolites contribute to this distinctive metabolite pattern, we conducted a loadings plot analysis (Fig. 1c). The loadings plot identified multiple metabolites that distinguish IC/BPS subjects and therefore represent preliminary biomarker candidates (Table 3).
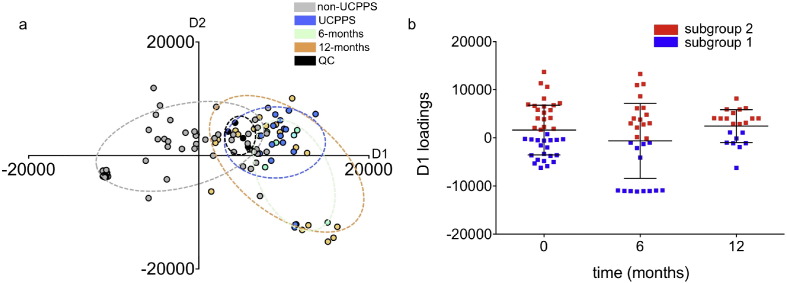
Fig. 1.
PCA-DA distinguishes an IC/BPS participant subgroup by a) negative and b) positive ESI-MS. Score plots of PCA-DA analysis of urine from IC/BPS participants (red) were clustered separately from non-IC/BPS controls (gray). Quality control samples (black) were prepared by pooling equal aliquots from each IC/BPS and non-IC/BPS sample, and run at random intervals. Each data point represents the average of triplicate runs. (c) Loading plot of PCA components 1 and 2 determined by negative ESI-MS. (d) Distribution of values from the loadings plot indicates two distinct subgroups within the IC/BPS population. The D1 loadings values resolve two distinct IC/BPS subgroups, with 19 out of the 40 (47.5%) samples in subgroup 1 (red), and 21 out of the 40 (52.5%) in subgroup 2 (blue).
Table 3.
Putative IC/BPS biomarkers (negative ESI) identified by PCA-DA loadings. Masses (m/z) for candidate molecules are shown along with their retention time in minutes.
| Metabolite (m/z) | Retention time (min) |
|---|---|
| 175.2 | 17.9 |
| 212.2 | 2.6 |
| 379.2 | 5.8 |
| 369.4 | 18.0 |
| 243.3 | 24.9 |
| 181.2 | 7.6 |
Urinary Metabolites Distinguish Symptomatically Distinctive IC/BPS Patient Subgroups
The PCA-DA plot of urinary metabolite profiles suggests the existence of two IC/BPS patient subgroups: subgroup 1, whose metabolome resembles non- IC/BPS controls, and subgroup 2, whose urinary metabolome is distinctive. The parameter that best distinguishes these two subgroups' metabolomic differences is D1 loadings (the x-axis in Fig. 1a). A single parameter created by PCA-DA, the D1 loadings value quantifies the variance in multiple interrelated metabolites that distinguish IC/BPS from non- IC/BPS subjects. Specimens whose D1 values overlap non- IC/BPS controls (Fig. 1d) defined subgroup 1 (19/40, 47.5% IC/BPS subjects). IC/BPS subjects whose D1 values did not overlap non-IC/BPS controls defined subgroup 2 (21/40, 52.5% IC/BPS subjects). To determine whether these subgroups correspond to clinically meaningful differences, we compared their disease activity scores. Symptom and pain scores in IC/BPS subgroup 2 were significantly higher than in subgroup 1 (p-value < 0.05 for SYM-Q, GUPI, BPI, and AUASI scales, Table 2). This association between urinary metabolomic profile and symptom characteristics suggests the existence of phenotypically distinctive IC/BPS patient subgroups with objective biochemical differences.
Table 2.
Clinical characteristics discriminate the IC/BPS subgroups. IC/BPS subjects were divided into two subgroups (1 and 2) based on metabolic differences. These subgroups can be discriminated on the basis of commonly used symptom scores (defined below). Only the statistically significant mean values and standard deviations of each metric are shown for clarity.*
| Index | Metric | Subgroup 1 | Subgroup 2 | p-Value |
|---|---|---|---|---|
| SYM-Q | Non-urological pain symptoms (0–10) | 3.5 ± 2.7 | 5.7 ± 2.9 | 0.024 |
| GUPI | Pain score (0–23) | 17.2 ± 1.7 | 18.7 ± 1.9 | 0.037 |
| BPI | # of painful sites checked (0–45) | 9.5 ± 9.5 | 13.3 ± 9.4 | 0.043 |
| AUASI | Baseline symptom index (0–35) | 24.3 ± 5.9 | 28.1 ± 3.9 | 0.038 |
| CSQ | Ability to decrease pain with coping (0–6) | 2.32 ± 4.2 | 2.95 ± 0.9 | 0.050 |
| PANAS | Negative affect (5–50) | 24.24 ± 2.24 | 26.24 ± 0.24 | 0.042 |
SYM-Q—Symptom and Health Care Utilization Questionnaire; GUPI—Genitourinary Pain Index; BPI—Brief Pain Inventory; AUASI—American Urological Association Symptom Index; CSQ—Client Satisfaction Questionnaire; PANAS—Positive and Negative Affect Schedule.
3.3. Urinary Sulfoconjugated Etiocholan-3α-ol-17-One (Etio-S) is a Correlate of IC/BPS Subgroup Differences
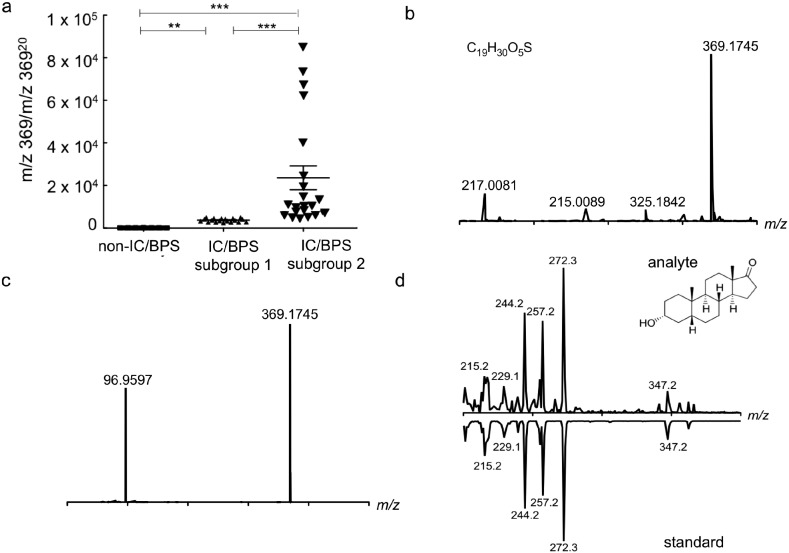
To determine exactly which urinary metabolites and metabolite class(es) distinguish IC/BPS subgroup 2, we used the D1 loadings plot to select six metabolites most closely associated with IC/BPS (Table 3) for detailed chemical characterization. Among these, a molecule at m/z 369 (retention time 18 min) corresponded to a well-resolved, highly reproducible chromatographic peak with greater abundance in subgroup 2 specimens. To more precisely quantify this molecule, we constructed a targeted mass spectrometric assay (LC–MS/MS) and normalized the molecule's peak intensity to that of an internally invariant urinary molecule with a similar m/z ratio and a retention time of 20 min. In this assay, urinary levels of m/z 369 (18 min) discriminated (i) IC/BPS from non- IC/BPS samples and (ii) IC/BPS subgroup 1 from subgroup 2 (p = 0.0003 and 0.0022, respectively, Fig. 2a). These results confirm the presence of a single urinary molecule (m/z 369, 18 min) identified through metabolomic analyses that differentiates IC/BPS subjects (Fig. 2b). Analysis of variance comparing Etio-S levels between healthy, subgroup 1 and subgroup 2 categories show that this correlation is statistically significant (p < 0.005, ANOVA).
Fig. 2.
Targeted analysis confirms urinary etiocholan-3a-ol-17-one sulfate as a IC/BPS subgroup biomarker. (a) Targeted analysis reveals a significantly higher ratio of m/z 36918 to m/z 36920 in IC/BPS subgroup 2 compared to subgroup 1 and non-IC/BPS controls (p = 0.0003 and 0.0022, respectively, t-test). Likewise, levels of m/z 369 were higher in subgroup 1 compared to non-IC/BPS controls (p = 0.0071, t-test). (b) High-resolution positive ion ESI mass spectrum is consistent with the empiric formula C19H30O5S for a [M − H]− species with a difference of − 2.18 ppm. (c) High-resolution negative ion tandem ESI spectrum of the candidate biomarker is consistent with the empiric formula C19H30O5S ([M − H]− at m/z 369.1745) that fragments to give a peak at m/z 96.9597, consistent with the presence of a sulfate group with the empiric formula SO4H. (d) Matching GC-EI-MS spectra of this sulfatase-treated, TMS-derivatized analyte compared to a commercially available reference standard. Retention time for both the analyte and standard was 21.8 min.
Next, we pursued chemical structure analysis of the IC/BPS -associated molecule at m/z 369 (18 min). This molecule was stable for HPLC purification, and accurate mass analysis of a purified urinary fraction yielded an m/z value of 369.1745, − 2.18 ppm, corresponding to the empiric formula C19H30O5S. MS/MS analysis of this ion yielded a fragment at m/z 96.9597 (Fig. 2c). This distinctive negative ion fragmentation is highly characteristic of a sulfate group (SO4H−) fragment from a sulfated aliphatic molecule, which is distinct from the m/z 80 loss associated with sulfated aromatic alcohols or enols (SO3) (Shields-Cutler et al., 2015, Yi et al., 2006). The empiric formula and MS/MS spectrum supported provisional molecular identification as a sulfated androgen isomer (androsterone, dihydrotestosterone, or etiocholanolone). To distinguish from among these isomers, the biomarker candidate was sulfatase-treated and analyzed by gas chromatography–mass spectrometry (GC–MS) as its trimethylsilyl (TMS) derivative. Spectral matching of the electron ionization (EI) spectrum with the NIST14 mass spectral library revealed etiocholan-3α-ol-17-one as the nearest match. This assignment was next confirmed by comparison to a commercially available etiocholan-3α-ol-17-one standard (Fig. 2d). Together, these data support identification of the biomarker candidate at m/z 369 as etiocholan-3α-ol-17-one (Etio-S), a testosterone derivative.
3.4. Etio-S Reliably Classifies IC/BPS Specimens
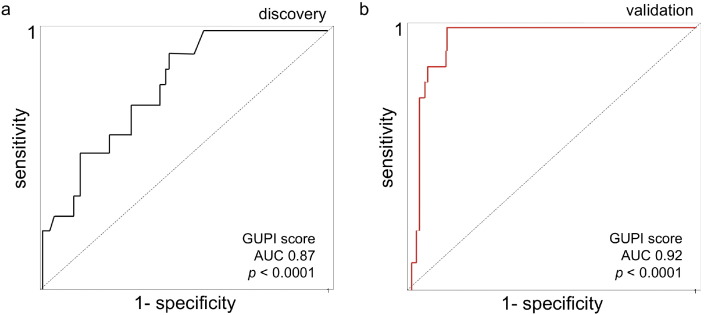
To determine whether urinary Etio-S levels can accurately resolve IC/BPS from non-IC/BPS specimens, we used the discovery dataset to construct a Receiver-Operator Characteristic (ROC) curve. This analysis indicates that urinary Etio-S is a sensitive and specific IC/BPS marker, with an area under the curve (AUC) of 0.87 (p < 0.001, Fig. 3a). To confirm these results, we assessed its predictive value in a separate blinded specimen set composed of forty female IC/BPS samples and forty healthy samples that were not used in the discovery set or for model training (referred to here as the ‘validation’ set). Etio-S was more highly associated with IC/BPS in the validation set (AUC of 0.92, p < 0.0001; Fig. 3b). Elevated Etio-S is a successful predictor of IC/BPS specimens in the validation set, with a specificity of 87.4% and sensitivity of 91.2% (p < 0.0001). Together, these findings demonstrate that Etio-S levels can classify IC/BPS subjects with high accuracy.
Fig. 3.
Urinary Etio-S is associated with IC/BPS subjects. (a) ROC curve analysis on the discovery set of 40 healthy participants and 40 IC/BPS participants' Etio-S levels and GUPI scores. The area under the curve (AUC) value of 0.87 is statistically significant from the null hypothesis (p < 0.001). (b) ROC curve analysis on validation samples using urine Etio-S levels and GUPI scores. These results were confirmed in three independent experiments.
3.5. Urinary Sulfometabolites Distinguish IC/BPS Subjects From Non-IC/BPS Controls
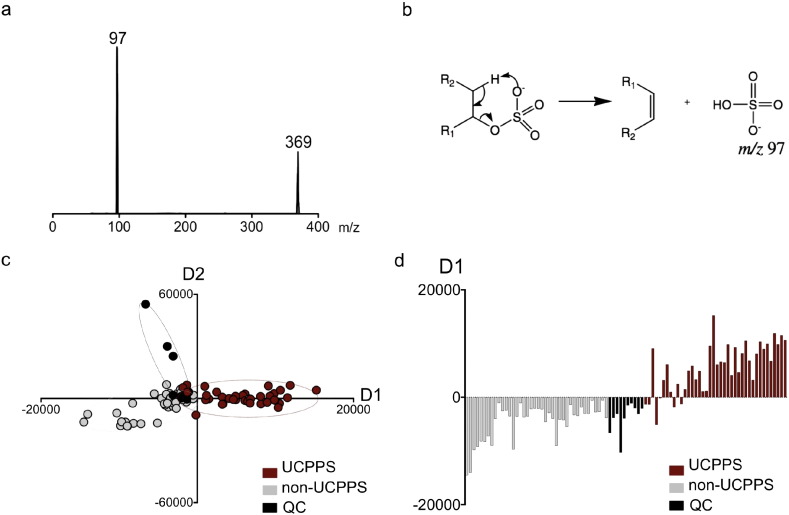
Metabolomic associations with IC/BPS raise the possibility that Etio-S may be the most detectable urinary manifestation of an extended, multistep biochemical pathway. We hypothesized that metabolomic profiling of urinary molecules that share Etio-S's distinctive sulfate group might better resolve IC/BPS subjects from non- IC/BPS controls. We therefore reconfigured our tandem mass spectrometer to selectively detect other similarly sulfoconjugated, non-aromatic urinary molecules. Specifically, we conducted a precursor scan in which only molecules that undergo MS/MS fragmentation to produce an m/z 97 ion (SO4H−) are detected (Fig. 4a). This distinctive negative ion fragmentation is characteristic of sulfated allylic or benzylic alcohols and aliphatic alcohols or enols, and can be used to distinguish alicyclic sulfates from aromatic sulfates (Shields-Cutler et al., 2015, Yi et al., 2006) (Fig. 4b). The resulting PCA-DA analysis of urinary sulfometabolomes resolved IC/BPS and non-IC/BPS individuals more completely (Fig. 4c) than the initial full scan (LC–MS) analysis (Fig. 1a). This urinary sulfometabolomic resolution of IC/BPS subjects is remarkably evident in the D1 loading parameter, which corresponds to multiple metabolites in the loadings plot (Fig. 4d). Together these data show that IC/BPS subjects exhibit stereotypical shifts in multiple sulfoconjugated urinary metabolites.
Fig. 4.
IC/BPS participants exhibit urinary sulfometabolomic shifts. (a) Representative MS–MS scan of Etio-S indicates loss of a sulfate group at m/z 97. Using this information, a precursor scan was designed to yield a metabolomic dataset selective for sulfoconjugated metabolites. (b) The characteristic m/z 97 loss, generated by sulfoconjugated aliphatic alcohols. (c) A PCA-DA plot comparing the differences in sulfoconjugated metabolites from IC/BPS and control urines shows the distinct differences in the urinary sulfometabolome of IC/BPS subjects. (d) The distribution of values from the loading plot arising from shifts in multiple sulfoconjugated molecules. These results were confirmed in three independent experiments.
3.6. IC/BPS-associated Sulfometabolite Shifts are Longitudinally Sustained
To determine whether IC/BPS -associated sulfometabolomic shifts are transient or persistent, we compared IC/BPS urinary sulfometabolomes for the female ‘discovery’ cohort at enrollment to their follow-up visits. Referred to here as the ‘longitudinal’ dataset, this group consisted of 6- and 12-month time points for IC/BPS participants only. The distinctive sulfometabolomic shift in IC/BPS subjects was sustained upon follow up, with subgroup 2 subjects remaining distinctive from subgroup 1 (Fig. 5a and b). Etio-S levels similarly distinguished subgroup 1 and 2 subjects at longitudinal timepoints, suggesting that metabolite levels are broadly representative of sulfometabolome differences (Supplemental Fig. 2). Together, these findings are consistent with Etio-S as a readily measured urinary correlate of multiple biochemical changes in highly symptomatic IC/BPS subjects.
Fig. 5.
IC/BPS associated sulfometabolomic shifts are temporally sustained. (a) Profiling sulfoconjugated urinary molecules reveals persistent long-term IC/BPS-associated metabolic shifts. The PCA-DA plot indicates that the metabolic shifts in the IC/BPS sulfometabolome are sustained at longitudinal time points. (b) Values from the D1 loadings plot show that metabolic shifts in the urinary sulfometabolome can stratify subjects into subgroups, indicating its correlative capacity for IC/BPS. Each data point represents the average of triplicate runs.
4. Discussion
Here we have used a metabolomics-based analytical platform to seek molecular correlates of IC/BPS from urine derived from female participants. This metabolomic analysis resolved a phenotypic subgroup of high symptom IC/BPS subjects with distinctive urinary levels of etiocholan-3α-ol-17-one sulfate (Etio-S), a steroid metabolite. In longitudinal studies, this IC/BPS-associated molecular signature persisted through twelve months of participant follow up. Urinary Etio-S and related sulfometabolites may be diagnostically useful and may also serve as a useful insight into pathophysiologic processes that contribute to IC/BPS symptoms.
Etio-S is a 5-β reduced androstane metabolite of testosterone (Slaunwhite and Sandberg, 1958), not previously reported as an IC/BPS biomarker. Its unexpected association with a IC/BPS patient subgroup exhibiting high extra-urinary symptoms (Table S1) is consistent with, though not proof of, a connection to the disease process. Etio-S appears in the urine following hepatic sulfoconjugation of etiocholan-3α-ol-17-one (Falany, 1991). Longitudinal metabolite differences between IC/BPS subgroups may reflect differences in diet, lifestyle, or hepatic activity. It is unclear whether etiocholan-3α-ol-17-one and its associated metabolites directly affect IC/BPS symptoms or are indirect markers of another disease process. Further investigation of these findings in an experimental study design may address these questions.
Although the mechanistic relationship between Etio-S and IC/BPS remains to be defined, the implication of a bioactive steroid in IC/BPS symptoms is notable and suggests a possible causative mechanism. High local etiocholan-3α-ol-17-one concentrations can stimulate a variety of acute phase responses (Wilmore, 1986), including inflammation, fever, leukocytosis, increased serum C-reactive protein, and increased plasma interleukin-1 activity. In addition to these inflammatory effects, Etio-S also acts as a positive allosteric modulator of the GABAA receptor (Li et al., 2007). As GABAA receptors are ubiquitously expressed throughout the central nervous system, changes in Etio-S levels may physiologically manifest as the acute stress (Reddy, 2003), depression (Fujii and Mellon, 2001) and changes in nociception (Drouet et al., 2015)that are often reported by IC/BPS patients. Intriguingly, sex-matched murine experiments indicate that sulfation activity is higher in females and exhibits a different range of substrates (Singer et al., 1976, Mulder, 1986). Combined with the observation that etiocholanolone accumulates in higher levels in female urine (Zumoff and Bradlow, 1980), these metabolic shifts could contribute to higher IC/BPS incidence in females.
As a proof of concept pilot study, this report is inherently limited by the sample sizes associated with the discovery and validation groups. The sample sizes are further constrained by the discovery of sub-groups, which indicate that all IC/BPS samples cannot be uniformly assessed by phenotypic criteria alone. The correlation between Etio-S levels was strongest in samples with high symptoms scores, which may limit the molecule's utility as a diagnostic tool for patients who report lower scores in the metrics examined in this study. The results reported here warrant larger validation studies, and should be expanded to determine whether similar biochemical shifts can be linked to the UCPPS phenotypes in males.
There is a longstanding need to identify biochemical correlates of IC/BPS. Despite its high prevalence and debilitating burden, no organ-specific disease, pathognomonic findings, definitive disease phenotypes, or objective biomarkers have been conclusively identified. Clinical diagnosis is based primarily on subjective symptom reporting by patients and exclusion of other conditions. There has been no objective way to definitively distinguish the disease from other overlapping conditions, or to identify meaningful IC/BPS patient subgroups (phenotyping), frustrating both clinical management and therapeutic development. By combining metabolomics-based discovery with intensive longitudinal clinical phenotyping analysis of a representative patient population, we have identified candidate biochemical markers linked to symptom-based subgroups. The metabolites identified in this study may complement ongoing attempts to identify IC/BPS biomarkers and underlying etiology through by proteomic methods (Dimitrakov and Guthrie, 2009), brain imaging (Bagarinao et al., 2014), and inflammatory marker quantification (Schrepf et al., 2015, Schrepf et al., 2014). By combining results from these approaches, we may map the early biochemical changes associated with heterogeneous IC/BPS symptoms and develop predictive biomarkers that can trigger earlier interventions.
Funding sources
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, DK82316, DK103260, DK103277, and DK103271). In addition, this work was supported in part by a Career Award for Medical Scientists from the Burroughs-Wellcome Fund awarded to J.P.H.
Conflict of interest statement
The authors have no conflict of interest to declare.
Author contributions
Metabolomic analysis and interpretation: Parker, Crowley, Lai, and Henderson. Statistical analysis of patient data: Stephens-Shields. Patient cohort selection, defined clinical significance, conceived and directed the study, wrote the manuscript: Hooton, Mullins, Lai, van Bokhoven, Andriole, Lucia, Parker, Henderson.
IC—Interstitial Cystitis; SYM-Q—Symptom and Health Care Utilization Questionnaire—the non-urological questions rate the severity of pain symptoms that are not urologic or pelvic pain symptoms, such as back pain and headaches; GUPI—Genitourinary Pain Index; BPI—Brief Pain Inventory; AUASI—American Urological Association Symptom Index; HADS—Hospital Anxiety and Depression Scale.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.03.040.
Appendix A. Supplementary data
Supplementary material.
References
- Argade S., Shaw T., Su Y., Parsons C.L. Tamm–Horsfall protein-associated nucleotides in patients with interstitial cystitis. BJU Int. 2013;111:811–819. doi: 10.1111/j.1464-410X.2012.11691.x. [DOI] [PubMed] [Google Scholar]
- Bagarinao E., Johnson K.A., Martucci K.T., Ichesco E., Farmer M.A., Labus J., Ness T.J., Harris R., Deutsch G., Apkarian A.V., Mayer E.A., Clauw D.J., Mackey S. Preliminary structural MRI based brain classification of chronic pelvic pain: a MAPP network study. Pain. 2014;155:2502–2509. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S.H., Elliott M.N., Suttorp M., Bogart L.M., Stoto M.A., Eggers P., Nyberg L., Clemens J.Q. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J. Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J.Q. Male and female pelvic pain disorders—is it all in their heads? J. Urol. 2008;179:813–814. doi: 10.1016/j.juro.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Clemens J.Q., Calhoun E.A., Litwin M.S., Mcnaughton-Collins M., Kusek J.W., Crowley E.M., Landis J.R., Urologic Pelvic Pain Collaborative Research, N. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74:983–987. doi: 10.1016/j.urology.2009.06.078. quiz 987 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J.Q., Mullins C., Kusek J.W., Kirkali Z., Mayer E.A., Rodriguez L.V., Klumpp D.J., Schaeffer A.J., Kreder K.J., Buchwald D., Andriole G.L., Lucia M.S., Landis J.R., Clauw D.J., GROUP, M. R. N. S The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrakov J., Guthrie D. Genetics and phenotyping of urological chronic pelvic pain syndrome. J. Urol. 2009;181:1550–1557. doi: 10.1016/j.juro.2008.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet J.B., Fauvelle F., Maunoir-Regimbal S., Fidier N., Maury R., Peinnequin A., Denis J., Buguet A., Canini F. Differences in prefrontal cortex GABA/glutamate ratio after acute restraint stress in rats are associated with specific behavioral and neurobiological patterns. Neuroscience. 2015;285:155–165. doi: 10.1016/j.neuroscience.2014.10.058. [DOI] [PubMed] [Google Scholar]
- Erickson D.R., Xie S.X., Bhavanandan V.P., Wheeler M.A., Hurst R.E., Demers L.M., Kushner L., Keay S.K. A comparison of multiple urine markers for interstitial cystitis. J. Urol. 2002;167:2461–2469. [PubMed] [Google Scholar]
- Falany C.N. Molecular enzymology of human liver cytosolic sulfotransferases. Trends Pharmacol. Sci. 1991;12:255–259. doi: 10.1016/0165-6147(91)90566-b. [DOI] [PubMed] [Google Scholar]
- Fujii E., Mellon S.H. Regulation of uterine gamma-aminobutyric acid(A) receptor subunit expression throughout pregnancy. Endocrinology. 2001;142:1770–1777. doi: 10.1210/endo.142.5.8153. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Kato M., Inoue Y., Matsubara A., Itoh K. A metabonomic approach identifies human urinary phenylacetylglutamine as a novel marker of interstitial cystitis. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3806–3812. doi: 10.1016/j.jchromb.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Hanno P.M., Erickson D., Moldwin R., Faraday M.M., American Urological, A. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J. Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Im Y.J., Choi H.C., Kang H.J., Kim J.Y., Kim J.H. Urinary nerve growth factor correlates with the severity of urgency and pain. Int. Urogynecol. J. 2014;25:1561–1567. doi: 10.1007/s00192-014-2424-8. [DOI] [PubMed] [Google Scholar]
- Lamale L.M., Lutgendorf S.K., Zimmerman M.B., Kreder K.J. Interleukin-6, histamine, and methylhistamine as diagnostic markers for interstitial cystitis. Urology. 2006;68:702–706. doi: 10.1016/j.urology.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Landis J.R., Williams D.A., Lucia M.S., Clauw D.J., Naliboff B.D., Robinson N.A., Van Bokhoven A., Sutcliffe S., Schaeffer A.J., Rodriguez L.V., Mayer E.A., Lai H.H., Krieger J.N., Kreder K.J., Afari N., Andriole G.L., Bradley C.S., Griffith J.W., Klumpp D.J., Hong B.A., Lutgendorf S.K., Buchwald D., Yang C.C., Mackey S., Pontari M.A., Hanno P., Kusek J.W., Mullins C., Clemens J.Q., Group M.R.N.S. The MAPP research network: design, patient characterization and operations. Bmc Urol. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Bracamontes J., Katona B.W., Covey D.F., Steinbach J.H., Akk G. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat alpha1beta2gamma2l GABAA receptor. Mol. Pharmacol. 2007;71:1582–1590. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- Lv H., Hung C.S., Chaturvedi K.S., Hooton T.M., Henderson J.P. Development of an integrated metabolomic profiling approach for infectious diseases research. Analyst. 2011;136:4752–4763. doi: 10.1039/c1an15590c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder G.J. Sex differences in drug conjugation and their consequences for drug toxicity. Sulfation, glucuronidation and glutathione conjugation. Chem. Biol. Interact. 1986;57:1–15. doi: 10.1016/0009-2797(86)90044-x. [DOI] [PubMed] [Google Scholar]
- O'leary M.P., Sant G.R., Fowler F.J., Jr., Whitmore K.E., Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58–63. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- Reddy D.S. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol. Sci. 2003;24:103–106. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Schrepf A., O'donnell M., Luo Y., Bradley C.S., Kreder K., Lutgendorf S., Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research, N Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: associations with painful symptoms. Pain. 2014;155:1755–1761. doi: 10.1016/j.pain.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepf A., Bradley C.S., O'donnell M., Luo Y., Harte S.E., Kreder K., Lutgendorf S., Multidisciplinary Approach To The Study Of Chronic Pelvic Pain Research, N. Toll-like receptor 4 and comorbid pain in interstitial cystitis/bladder pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun. 2015;49:66–74. doi: 10.1016/j.bbi.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields-Cutler R.R., Crowley J.R., Hung C.S., Stapleton A.E., Aldrich C.C., Marschall J., Henderson J.P. Human urinary composition controls antibacterial activity of siderocalin. J. Biol. Chem. 2015;290:15949–15960. doi: 10.1074/jbc.M115.645812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S.S., Giera D., Johnson J., Sylvester S. Enzymatic sulfation of steroids: I. The enzymatic basis for the sex difference in cortisol sulfation by rat liver preparations. Endocrinology. 1976;98:963–974. doi: 10.1210/endo-98-4-963. [DOI] [PubMed] [Google Scholar]
- Slaunwhite W.R., Jr., Sandberg A.A. Metabolism of 4-C14-testosterone in human subjects III. Fate of androsterone and etiocholanolone. J Clin Endocrinol Metab. 1958;18:1056–1066. doi: 10.1210/jcem-18-10-1056. [DOI] [PubMed] [Google Scholar]
- Snaith R.P. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Merwe J.P., Nordling J., Bouchelouche P., Bouchelouche K., Cervigni M., Daha L.K., Elneil S., Fall M., Hohlbrugger G., Irwin P., Mortensen S., Van Ophoven A., Osborne J.L., Peeker R., Richter B., Riedl C., Sairanen J., Tinzl M., Wyndaele J.J. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur. Urol. 2008;53:60–67. doi: 10.1016/j.eururo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Wen H., Lee T., You S., Park S.H., Song H., Eilber K.S., Anger J.T., Freeman M.R., Park S., Kim J. Urinary metabolite profiling combined with computational analysis predicts interstitial cystitis-associated candidate biomarkers. J. Proteome Res. 2015;14:541–548. doi: 10.1021/pr5007729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D.W. Are the metabolic alterations associated with critical illness related to the hormonal environment? Clin. Nutr. 1986;5:9–19. doi: 10.1016/0261-5614(86)90037-3. [DOI] [PubMed] [Google Scholar]
- Yi L., Dratter J., Wang C., Tunge J.A., Desaire H. Identification of sulfation sites of metabolites and prediction of the compounds' biological effects. Anal. Bioanal. Chem. 2006;386:666–674. doi: 10.1007/s00216-006-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumoff B.V., Bradlow H.L. Sex difference in the metabolism of dehydroisoandrosterone sulfate. J. Clin. Endocrinol. Metab. 1980;51:334–336. doi: 10.1210/jcem-51-2-334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.