Abstract
The entire double-stranded DNA genome of the Actinobacillus actinomycetemcomitans bacteriophage AaΦ23 was sequenced. Linear DNA contained in the phage particles is circularly permuted and terminally redundant. Therefore, the physical map of the phage genome is circular. Its size is 43,033 bp with an overall molar G+C content of 42.5 mol%. Sixty-six potential open reading frames (ORFs) were identified, including an ORF resulting from a translational frameshift. A putative function could be assigned to 23 of them. Twenty-three other ORFs share homologies only with hypothetical proteins present in several bacteria or bacteriophages, and 20 ORFs seem to be specific for phage AaΦ23. The organization of the phage genome and several genetic functions share extensive similarities to that of the lambdoid phages. However, AaΦ23 encodes a DNA adenine methylase, and the DNA packaging strategy is more closely related to the P22 system. The attachment sites of AaΦ23 (attP) and several A. actinomycetemcomitans hosts (attB) are 49 bp long.
Actinobacillus actinomycetemcomitans is a capnophilic, nonmotile gram-negative bacterium which has been strongly implicated in the etiology of several forms of periodontitis and may play a role in extraoral infection. Several putative virulence factors, including a leukotoxin, have been described previously (13).
Lysogeny is widespread in A. actinomycetemcomitans. Lysogenic A. actinomycetemcomitans isolates have been isolated from periodontal pockets (11, 15, 30, 33) as well as from periodontally healthy individuals (38). The role of phages in the etiology of periodontal diseases is not yet clear. Specifically, members of the AaΦ23 family of phages have been found in about 40% of the A. actinomycetemcomitans isolates (11, 15, 25, 38), and AaΦ23 was shown to transduce antibiotic resistance markers in vitro (39). In several bacterial species, such as Vibrio cholerae (17, 36) and Escherichia coli (24), phage-encoded genes modulate virulence.
In previous work, it was shown that phages released from several A. actinomycetemcomitans lysogens possess an isometric icosahedral head of 65 nm, a contractile tail of 110 nm, and a baseplate with up to four fibers and are genetically related (40). Phage particles contain approximately 45 kb of double-stranded linear DNA that is circularly permuted and terminally redundant for 1.6 kb (37). To further our understanding of the biology of A. actinomycetemcomitans bacteriophages, we decided to sequence the entire genome of the bacteriophage AaΦ23 naturally carried by A. actinomycetemcomitans strain ZIB1023 (40).
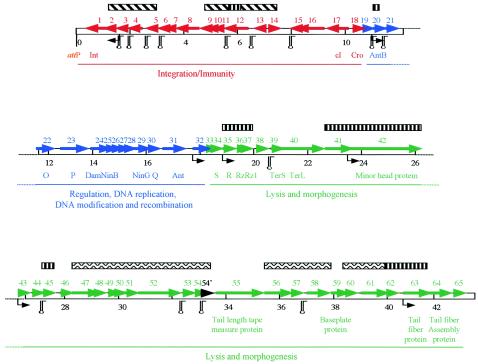
Four shotgun subclone libraries were prepared from purified phage DNA. After sequencing of 289,652 bp, representing coverage of the AaΦ23 genome 6.7 times, the final consensus sequence obtained with phred/phrap/consed (GCG Wisconsin package; Genetics Computer Group, Accelrys, Inc., Munich, Germany) was circular. For convenience, the phage genome was linearized at the first nucleotide of the attP core sequence (Fig. 1). The sequence of the AaΦ23 genome is composed of 43,033 bp. Virtual restriction maps of this sequence are in good agreement with the restriction maps previously obtained (40), suggesting correct assembly of the sequences. The average G+C content of AaΦ23 is 42.5 mol%, which is similar to the 42.7 mol% reported for the A. actinomycetemcomitans genome (16). Sixty-six potential genes were predicted by analyses of the AaΦ23 genome sequence with the heuristic approaches of version 2.4 of GeneMark and version 2.0 of GeneMarkHMM (http://opal.biology.gatech.edu/GeneMark/heuristic_hmm2.cgi) and confirmed with a modified version of the GeneMarkS program specifically developed for viral genomes (21). The genome of AaΦ23 is very condensed in terms of coding sequences, as 93.8% are covered by open reading frames (ORFs). No tRNA genes are present. Fifteen stem-loop-like structures that may represent rho-independent transcription termination signals have been identified (Fig. 1). The 66 potential genes and the corresponding protein sequences were compared, by using PSI-BLAST (http://www.ncbi.nlm.nih.gov/BLAST), to the GenBank and Swissprot databases. Characteristics of the gene products and the significant homologies are listed in Table 1, and some are detailed below. Twenty ORFs may be specific for AaΦ23, as no homology to other sequences was found. When analyzing putative gene functions, three distinct regions can be delimited on the genome: (i) integration and immunity, (ii) regulation, DNA replication, DNA modification, and recombination, and (iii) lysis and morphogenesis (Fig. 1). The same genetic organization has also been observed with other bacteriophages such as lambda, P22, L, and LP-7 (5).
FIG. 1.
Schematic representation of the AaΦ23 genome. ORFs are numbered consecutively from left to right and indicated by arrows pointing in the direction of transcription. Putative functions are indicated. Filled boxes with diagonal, vertical, and zigzag lines indicate regions of homology to the genome of A. actinomycetemcomitans strain HK1651, the H. influenzae φflu prophage, and the X. fastidiosa Xfp3/Xfp4 prophages, respectively. Rho-independent terminators and promoters are highlighted by hairpins and small black arrows, respectively. The −1 ORF (orf54′) is represented by a black arrow.
TABLE 1.
Description of bacteriophage AaΦ23 ORFs, gene products, and functional assignments
| Gene | Start position | Stop position | Size (aa)a | Potential function (gene name) | Significant matches (source, accession no., e value) |
|---|---|---|---|---|---|
| orf1 | 1115 | 69 | 348 | Integrase (int) | Integrase (phage P22, P04890, 9e−66) |
| orf2 | 1336 | 989 | 115 | ||
| orf3 | 1666 | 1352 | 104 | Putative prophage protein (A. actinomycetemcomitans strain HK1651, 2e−55) | |
| orf4 | 2377 | 1721 | 218 | Putative prophage protein (A. actinomycetemcomitans strain HK1651, e−113) | |
| orf5 | 2929 | 2444 | 161 | Putative prophage protein (A. actinomycetemcomitans strain HK1651, 6e−48) | |
| orf6 | 3144 | 2962 | 60 | ||
| orf7 | 3760 | 3137 | 207 | ||
| orf8 | 4650 | 3757 | 297 | ||
| orf9 | 4961 | 4662 | 99 | ||
| orf10 | 5218 | 4964 | 84 | ||
| orf11 | 5483 | 5208 | 91 | ||
| orf12 | 6226 | 5570 | 218 | Putative prophage protein HI1422 (prophage φflu, P44193, 8e−10) | |
| orf13 | 6670 | 6587 | 83 | ||
| orf14 | 7138 | 7662 | 174 | Putative prophage protein (A. actinomycetemcomitans strain HK1651, 6e−78) | |
| orf15 | 8491 | 8018 | 157 | ||
| orf16 | 9347 | 8460 | 295 | ||
| orf17 | 10121 | 9405 | 250 | Repressor (cl) | Repressor protein C1 (phage phi-80, P14819, e−44) |
| orf18 | 10231 | 10419 | 68 | Repressor (cro) | Hypothetical protein YdaS (E. coli strain K12, P76063, 7e−07); Cro protein (phage P22; P09964, 1.2) |
| orf19 | 10410 | 10736 | 108 | Putative prophage protein (A. actinomycetemcomitans strain HK1651, 3e−08) | |
| orf20 | 10788 | 11507 | 239 | Antirepressor (antB) | Putative prophage protein HI1422 (prophage φflu, E64029, 6e−24); AntB (phage N15, AAM53132.1, e−22) |
| orf21 | 11507 | 11665 | 52 | ||
| orf22 | 11662 | 12444 | 260 | DNA replication protein (o) | Protein GP18 (phage P22, P03687, 6e−19) |
| orf23 | 12444 | 13901 | 485 | DNA Helicase (p) | Replicative DNA helicase (phage P22, P03006, 2e−45) |
| orf24 | 13904 | 14398 | 163 | DNA adenine methylase | Putative adenine-specific methylase (phage HP1, P51715, e−19) |
| orf25 | 14405 | 14902 | 165 | Recombination protein (ninB) | Protein NinB (phage lambda, P03765, e−13) |
| orf26 | 14881 | 15126 | 81 | ||
| orf27 | 15126 | 15305 | 59 | EF-Tu (Photorhabdus luminescens subsp. lumondii TTOI, NP_931892, 2e−07) | |
| orf28 | 15280 | 15495 | 71 | ||
| orf29 | 15495 | 16064 | 189 | Recombination endonuclease (ninG) | Protein NinG (phage lambda, P03770, 3e−18) |
| orf30 | 16064 | 16435 | 123 | Antitermination protein (q) | Probable phage antitermination protein Q (Yersina pestis strain KIM, CAC90068.1, 7e−02) |
| orf31 | 16725 | 17621 | 298 | Antirepressor (ant) | Antirepressor protein Ant (phage P22, P03037, 3e−26) |
| orf32 | 17785 | 18231 | 148 | ||
| orf33 | 18228 | 18596 | 122 | Antiholin, holin 1?b | |
| orf34 | 18505 | 18870 | 121 | Holin S, holin 2?b | |
| orf35 | 18939 | 19520 | 193 | Lysin (R) | Putative prophage protein HI1415 (prophage φflu, H64028, 2e−56) |
| Endolysin (bacteriophage epsilon15, NP_848233, 5e−29) | |||||
| orf36 | 19523 | 19855 | 110 | Rz lytic protein (rz) | Putative prophage protein HI1414 (prophage φflu, P44186, 7e−12) |
| Putative Rz lytic protein (phage SfV, AAL89453.1, e−3) | |||||
| orf37 | 19767 | 20048 | 93 | Rz1 lytic protein (rz1) | Putative prophage protein HI1413 (prophage φflu, P44185, 3e−33) |
| orf38 | 20052 | 20318 | 88 | ||
| orf39 | 20475 | 21035 | 186 | Terminase small subunit (terS) | Terminase small subunit (phage SF6, Q57374, 3e−11) |
| orf40 | 20980 | 22443 | 487 | Terminase large subunit (terL) | Terminase large subunit (phage Beep781, AAN38019.1, e−50) |
| orf41 | 22445 | 23758 | 437 | Putative prophage protein HI1409 (prophage φflu, P44183, 0.0) | |
| orf42 | 23679 | 26081 | 800 | Head protein | Putative prophage protein HI1407 (prophage φflu, B64122, e−109) |
| Minor prohead-head protein (bacteriophage SPP1, NP_690662, 3.5e−02) | |||||
| orf43 | 26078 | 26296 | 72 | ||
| orf44 | 26430 | 26630 | 66 | ||
| orf45 | 26713 | 27825 | 370 | Putative prophage protein HI1405 (prophage φflu, AAC23055.1, e−171) | |
| orf46 | 27839 | 28288 | 149 | ||
| orf47 | 28300 | 29214 | 304 | Putative prophage proteins XF1577 and XF1682 (prophages XfP3 and XfP4, H82650, 7e−11) | |
| orf48 | 29225 | 29584 | 119 | Putative prophage proteins XF1579 and XF1684 (prophages XfP3 and XfP4, A82665, 2e−14) | |
| orf49 | 29585 | 30031 | 148 | Putative prophage proteins XF1580 and XF1685 (prophages XfP3 and XfP4, C82651, 7e−11) | |
| orf50 | 30028 | 30399 | 123 | Putative prophage proteins XF1581 and XF1686 (prophages XfP3 and XfP4, C82665, e−17) | |
| orf51 | 30185 | 30826 | 213 | Putative prophage proteins XF1582 and XF1687 (prophages XfP3 and XfP4, E82651, 9e−12) | |
| orf52 | 30831 | 32339 | 502 | Putative prophage proteins XF1583 and XF1688 (prophages XfP3 and XfP4, F82651, e−123) | |
| orf53 | 32389 | 32820 | 143 | Putative prophage proteins XF1584 and XF1689 (prophages XfP3 and XfP4, F82665, 9e−46) | |
| orf54 | 32820 | 33239 | 139 | Putative prophage proteins XF1585 and XF1690 (prophages XfP3 and XfP4, H82651, 5e−20) | |
| orf54-54′ | 32820 | 33403 | 194 | Putative prophage proteins XF1585 and XF1690 (prophages XfP3 and XfP4, H82651, 1e−19) | |
| orf55 | 33390 | 35498 | 702 | Tail-length tape measure protein | Tail-length tape measure protein (Clostridium tetani strain E88, AAO35681, 8e−09) |
| orf56 | 35502 | 36269 | 255 | Putative prophage protein XF1591 (prophage XfP3, B82661, e−37) | |
| orf57 | 36280 | 36594 | 104 | Hypothetical protein XF1628 (X. fastidiosa strain 9a5c, C82648, 2e−20) | |
| orf58 | 36881 | 37735 | 284 | Putative prophage protein XF1593 (prophage XfP3, D82661, 4e−78) | |
| orf59 | 37732 | 38376 | 214 | Baseplate protein | Putative baseplate protein (phage phi-CTX, BAA36243.1, 3e−06) |
| orf60 | 38373 | 38732 | 119 | Putative prophage protein XF1701 (prophage XfP4, F82648, 9e−26) | |
| orf61 | 38763 | 39905 | 380 | Putative prophage protein XF1704 (prophage XfP4, A82649, 5e−96) | |
| orf62 | 39905 | 40486 | 193 | Putative prophage protein HI1404 (prophage φflu, P44179, 5e−08) | |
| orf63 | 40497 | 41981 | 494 | Tail fiber protein | Putative prophage protein HI1403 (prophage φflu, P44178, e−07) |
| Putative tail fiber protein H (phage 186, AAC34164.1, 2e−04) | |||||
| orf64 | 41972 | 42592 | 206 | Tail fiber assembly protein | Tail fiber assembly protein P37 (phage SfV, O22005.1, 2e−04) |
| orf65 | 42573 | 42833 | 86 | Hypothetical protein NMB1120 (Neisseria meningitidis strain MC58, B81120, 4e−24) |
aa, amino acids.
?, assignment is speculative (see the text for a discussion).
ORF1 may represent the integrase of phage AaΦ23 (Table 1 and data not shown). The InterProScan software (http://www.ebi.ac.uk/InterProScan) identified a domain conserved among phage integrases from amino acid 145 to 324 (PFAM accession number PF00589, e value of 1.7e−38). Thus, this protein may be a new member of the large family of Int/recombinases (23) which catalyzes the site-specific integration and excision of the AaΦ23 genome into and out of the host chromosome. As for phage lambda, the putative AaΦ23 integrase gene is located in a cluster of genes transcribed leftwards on the genetic map, i.e., in the opposite orientation to the majority of the phage genes (Fig. 1). No gene coding for an excisionase has been identified on the AaΦ23 genome. This situation is also encountered in other phages, e.g., Pseudomonas aeruginosa phage D3 (18). By semirandom PCR, a 49-bp-long direct repeat was identified at the junctions of prophage and bacterial DNA in strain ZIB1023. Moreover, a single copy of this repeat was found in the chromosome of the nonlysogenic strains ZIB1001 and ZIB1015 and was also evident on the AaΦ23 DNA sequence. When strain ZIB1001 was lysogenized to result in ZIB1515, the prophage was flanked by direct copies of the repeat, representing the att site (Fig. 2). Analysis also revealed a single copy of this repeat in the genome of A. actinomycetemcomitans strain HK1651 and Haemophilus influenzae Rd strain KW20. In AaΦ23, the attP site is located 19 bp downstream from the putative integrase gene. The attB homologous sequence is located intergenically in the genomes of A. actinomycetemcomitans and H. influenzae.
FIG. 2.
attP, attL, attR, and attB sites on the bacteriophage AaΦ23 genome and on several bacterial genomes. The att core sequence is boxed. The C-terminal end of the putative integrase is underlined. The asterisk represents the stop codon of the integrase gene.
ORF17 presents strong homologies to the repressor protein cI of the lambda-like phage phi-80 (9). Moreover, this protein contains a helix-turn-helix motif from amino acid 12 to 66 (PFAM accession number PF013081, e value of 1.1e−08), which is a signature of DNA binding proteins. The product of orf18 is a 68-amino-acid protein that shares significant homologies with hypothetical proteins and weak homologies to the transcription regulatory protein Cro of several bacteriophages (Table 1 and data not shown). Moreover, three regions of inverted repeats, which may be analogues of the three sequences that compose the OR operator of phage lambda, and two putative promoter sequences, which may correspond to the promoters for repressor maintenance (PRM) and to PR′, are evident in the cI and cro intergenic region of AaΦ23 (data not shown). Thus, AaΦ23 is thought to use a lysogeny control system similar to that of phage lambda (26).
The deduced gene product of orf20 may be a antirepressor protein similar to the one named AntB in E. coli phage N15, thought to play a role in anti-immunity (28). orf20 is also closely related to gene HI1422 of the prophage φflu (Table 1). orf31 codes for a second putative antirepressor belonging to the protein family of Ant that is expressed in several phages and has been extensively characterized for P22 (31). The gene product of orf30 shares significant homology with a probable phage antitermination protein Q (Table 1). Finally, no homologies to other regulatory proteins, such as cII, cIII, or antitermination protein N, have been identified on the AaΦ23 genome.
The DNA replication machinery of AaΦ23 (including ORFs 22 and 23, encoding phage P22 DNA replication protein and P22 DNA helicase homologues, respectively) seems to be closely related to that of the lambdoid bacteriophages (35).
ORFs 25 and 29 show homologies to the NinB and NinG proteins, respectively, of several phages (Table 1). In lambda, these two genes are known as orf (ninB) and rap (ninG), two recombination genes located in the ninR region. A recent study showed that they participate in Red recombination, the primary pathway operating when wild-type lambda grows lytically in Rec+ cells (34). Additional experiments are needed to determine whether AaΦ23 induces a similar pathway when it grows lytically in A. actinomycetemcomitans.
In contrast to lambda, AaΦ23 codes for a DNA adenine methylase (ORF24) (Table 1). When orf24 was introduced into the Dam− E. coli strain GM48, the genomic DNA was resistant to digestion by MboI but not by Sau3AI, indicating that it was methylated on the adenine residue of specific GATC sequences (data not shown). Also, a host-encoded A. actinomycetemcomitans DNA adenine methylase has been shown to be active in vivo (8). DNA methylases participate in regulatory events of DNA replication, methyl-directed mismatch repair, and transposition (20). These enzymes are also associated with bacterial DNA restriction-modification systems that are responsible for the degradation of foreign DNA such as conjugative plasmids, transposons, or bacteriophage DNA (29). It has been speculated that some bacteriophages express their own DNA adenine methylase to overcome this bacterial protection. Some DNA adenine methylases also play a role in regulating bacterial virulence (12). Nevertheless, the involvement of these enzymes in the regulation of A. actinomycetemcomitans virulence genes remains to be studied.
AaΦ23 probably uses the same lysis strategy as described for many double-stranded DNA phages (41). The genetic organization (SRRzRz1) of the putative lysis cassette, coded by orf34 to orf37, is closely related to the one generally described for lambdoid phages. None of the AaΦ23 ORFs share homologies with a holin (S), but ORF34 may be a good candidate because (i) it is topologically similar to the T4 holin (27; data not shown), (ii) it is localized upstream of R, and (iii) ORF34 may correspond to HI1416 of φflu (14). In fact, the RRzRz1 part of the AaΦ23 putative lysis cassette is closely related to the H. influenzae prophage Φflu (Table 1). Interestingly, when scanned for protein domains (http://hits.isb-sib.ch/cgi-bin/PFSCAN), HI1416 was found to match the phage holin 3 family (4e−66), which also includes the lambda holin S.
Nevertheless, no dual start motif (4) is present on the sequence of orf34, suggesting that an antiholin function, if present, may be coded by a separate gene. It would not be surprising that ORF33 plays a role in lysis, as it also represents a putative membrane protein sharing the same organization and charge distribution as ORF34 (data not shown). Additional experiments are required to characterize the AaΦ23 holin system. orf35 probably codes for the lysin (R), which could represent a new lysozyme (glycosylase), as this protein shares strong homologies with several bacteriophage lytic enzymes, putative lysozymes, and chitinases (Table 1 and data not shown). Moreover, this ORF shares homologies with the glycoside hydrolase 19 protein family (PFAM accession number PF00182, 3.7e−2), which groups proteins with chitinase activity. Some chitinases have been shown to display a lysozyme activity (22). Nevertheless, it is not clear whether ORF35 is a true lysozyme or whether it is capable of transglycosylation, as reported for the endolysin gpR of phage lambda (2). orf36, which is located directly downstream of orf35, presents weak homologies to the putative Rz lytic protein expressed by the Shigella flexneri bacteriophage SfV (Table 1). Moreover, orf36 overlaps with orf37, the 93-amino-acid gene product of which harbors a prokaryotic lipoprotein motif (Prosite accession number PS00013) between residues 17 and 27 and may, therefore, represent the AaΦ23 homologue of the Rz1 prolipoprotein. In many phages of gram-negative hosts, Rz and Rz1 play a role as auxiliary lytic proteins that are thought to interact with the outer membrane or with its links to the peptidoglycans (41).
orf39 and orf40 are possibly coding for the small and large subunits of the terminase enzyme, respectively (Table 1). This holoenzyme is required for packaging of the phage genomic DNA into the empty capsid shells (6). In AaΦ23, the phage DNA has been shown to be circularly permuted and terminally redundant for approximately 1.6 kb (37), and it may therefore be packaged by the headful mechanism. Thus, the AaΦ23 terminase complex may cut concatemeric DNA molecules, resulting from rolling circle replication, at a unique pac site to start the first round of packaging. Taking into consideration the 1.6-kb terminal redundancy, about 104% of the genomic DNA molecule is packaged into phage heads on subsequent rounds of packaging.
Few genes coding for structural components were identified on the AaΦ23 genome. The gene products of orf42, orf55, orf63, orf64, and orf59 seem to be elements of the head, tail, tail fibers, and baseplate, respectively, of the AaΦ23 phage particle (Table 1). ORF42 contains a phage Mu protein F-like domain, from amino acid 156 to 262, found in members of a family representing possible minor phage head proteins (PFAM accession number PF04233, e value of 2.7e−30). It shows significant amino acid sequence homologies to one member of this family, the gene 7 protein of bacteriophage SPP1, which has been previously described as being required for viral head morphogenesis (1). Programmed translational frameshifts occur in the tail gene operon of several bacteriophages, usually in the gene preceding the tape measure protein (7, 19). Indeed, a small additional orf (orf54′) is present upstream of orf55 on the AaΦ23 genome (Fig. 1). This 99-amino-acid ORF starts with a cysteine in the −1 reading frame at 135 nucleotides before the end of orf54 and extends 12 nucleotides into orf55 (data not shown). Seventy-two nucleotides downstream of the first codon of orf54′, a stretch of seven T is found, which may be related to sites used for the −1 translational frameshift (10). Thus, orf54′ may be translated as an extension of orf54 following a −1 translational frameshift. The gene product of orf54-orf54′ is predicted to be composed of 194 amino acids. Both, ORF54 and ORF54-ORF54′ have unknown functions.
BLAST analysis of the phage genomic sequence revealed two segments of strong homology over 8.7% of the AaΦ23 genome (average of 97% homology over 3,795 bp) with the A. actinomycetemcomitans strain HK1651 genomic sequence (ftp://ftp.genome.ou.edu/pub/act) (Fig. 1). To determine whether these homologies are part of a degenerate prophage or some other modularly related prophage, 40-kb genomic sequences flanking the region of homology on either side were analyzed. Only four additional phage-related ORFs were identified among 80 potential ORFs detected. This indicates that this genomic region of A. actinomycetemcomitans strain HK1651 contains an AaΦ23-related prophage remnant rather than an intact prophage. Therefore, it was not surprising that we were not able to induce phage production from A. actinomycetemcomitans strain HK1651 by mitomycin C treatment.
A. actinomycetemcomitans is most closely related to Haemophilus aphrophilus and other members of the family Pasteurellaceae (3) while the plant pathogen Xylella fastidiosa is also a gammaproteobacterium (family Xanthomonadaceae) more distantly related to H. influenzae (32). Ten AaΦ23 ORFs share homologies with ORFs of φflu (14) and are present in a very similar gene order on both genomes. 36% of the AaΦ23 ORFs (24 of 66) share homology with hypothetical proteins from X. fastidiosa (Table 1 and data not shown). Interestingly, 14 of these ORFs share homology and show a similar gene order to ORFs contained in X. fastidiosa strain 9a5c prophages XfP3 and/or XfP4 (Table 1 and data not shown). These observations suggest that AaΦ23 is related to φflu and XfP3/XfP4 and that they could have had common ancestor(s).
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been assigned accession number AJ560763 in the EMBL database.
Acknowledgments
We are very grateful to Peter Philippsen for providing the sequencing facilities and to Tom Bickle for very fruitful discussions and comments on an earlier version of the manuscript. We also thank Eric Kofoid and Sophie Lemire-Brachat for advice concerning the semirandom PCR protocol and the annotation and submission of the AaΦ23 genomic sequence, respectively. We are grateful to Mark Borodovsky and Ryan Mills for help with gene annotation improvement. We thank Mogens Kilian for A. actinomycetemcomitans strain HK1651 and the Actinobacillus Genome Sequencing Project, Bruce Roe, Fares Najar, Allison Gillaspy, Sandra Clifton, Tom Ducey, Lisa Lewis, and David Dyer for the A. actinomycetemcomitans sequencing data. Finally, we thank Sylvia Voegeli and Anita Lerch for technical assistance.
This work was supported by the Swiss Dental Association (SSO, grant no. 196).
Footnotes
This paper is dedicated to Werner Arber on the occasion of his 75th birthday.
REFERENCES
- 1.Becker, B., N. de la Fuente, M. Gassel, D. Günther, P. Tavares, R. Lurz, T. A. Trautner, and J. C. Alonso. 1997. Head morphogenesis genes of the Bacillus subtilis bacteriophage SPP1. J. Mol. Biol. 268:822-839. [DOI] [PubMed] [Google Scholar]
- 2.Bienkowska-Szewczyk, K., B. Lipinska, and A. Taylor. 1981. The R gene product of bacteriophage lambda is the murein transglycosylase. Mol. Gen. Genet. 184:111-114. [DOI] [PubMed] [Google Scholar]
- 3.Bisgaard, M. 1995. Taxonomy of the family Pasteurellaceae Pohl 1981, p. 1-7. In W. Donachie, F. A. Lainson, and J. C. Hodgson (ed.), Haemophilus, Actinobacillus, and Pasteurella. Plenum Press, New York, N.Y.
- 4.Bläsi, U., and R. Young. 1996. Two beginnings for a single purpose: the dual-start holins in the regulation of phage lysis. Mol. Microbiol. 21:675-682. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, A. 1994. Comparative molecular biology of lambdoid phages. Annu. Rev. Microbiol. 48:193-222. [DOI] [PubMed] [Google Scholar]
- 6.Catalano, C. E. 2000. The terminase enzyme from bacteriophage lambda: a DNA-packaging machine. Cell. Mol. Life Sci. 57:128-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie, G. E., L. M. Temple, B. A. Barlett, and T. S. Goodwin. 2002. Programmed translational frameshift in the bacteriophage P2 FETUD tail gene operon. J. Bacteriol. 184:6522-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhard, J., J. Oza, and N. O. Reich. 2001. Cloning, sequence analysis and heterologous expression of the DNA adenine-(N6) methyltransferase from the human pathogen Actinobacillus actinomycetemcomitans. FEMS Microbiol. Lett. 195:223-229. [DOI] [PubMed] [Google Scholar]
- 9.Eguchi, Y., T. Ogawa, and H. Ogawa. 1988. Cleavage of bacteriophage phi 80 CI repressor by RecA protein. J. Mol. Biol. 202:565-573. [DOI] [PubMed] [Google Scholar]
- 10.Gestland, R. F., and J. F. Atkins. 1996. Recoding: dynamic reprogramming of translation. Annu. Rev. Biochem. 65:741-768. [DOI] [PubMed] [Google Scholar]
- 11.Haubek, D., K. Willi, K. Poulsen, J. Meyer, and M. Kilian. 1997. Presence of bacteriophage AaΦ23 correlates with the population genetic structure of Actinobacillus actinomycetemcomitans. Eur. J. Oral Sci. 105:2-8. [DOI] [PubMed] [Google Scholar]
- 12.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967-970. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, B., S. P. Nair, J. M. Ward, and M. Wilson. 2003. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu. Rev. Microbiol. 57:29-55. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix, R. W., M. C. M. Smith, R. N. Burns, M. E. Ford, and G. F. Hatfull. 1999. Evolutionary relationship among diverse bacteriophages and prophages: all the world's a phage. Proc. Natl. Acad. Sci. USA 96:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iff, M., K. Willi, J. Guindy, U. Zappa, and J. Meyer. 1997. Prevalence and clinical significance of a temperate bacteriophage in Actinobacillus actinomycetemcomitans. Acta Med. Dent. Helv. 2:33-38. [Google Scholar]
- 16.Kaplan, J. B., and D. H. Fine. 1998. Codon usage in Actinobacillus actinomycetemcomitans. FEMS Microbiol. Lett. 163:31-36. [DOI] [PubMed] [Google Scholar]
- 17.Karaolis, D. K. R., S. Somara, D. R. Maneval, J. A. Johnson, and J. B. Kaper. 1999. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature 399:375-379. [DOI] [PubMed] [Google Scholar]
- 18.Kropinski, A. M. 2000. Sequence of the genome of the temperate, serotype-converting, Pseudomonas aeruginosa bacteriophage D3. J. Bacteriol. 182:6066-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin, M. E., and R. W. Hendrix. 1993. A programmed translational frameshift is required for the synthesis of a bacteriophage λ tail assembly protein. J. Mol. Biol. 234:124-139. [DOI] [PubMed] [Google Scholar]
- 20.Marinus, M. G. 1996. Methylation of DNA, p. 782-791. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 21.Mills, R., M. Rozanov, A. Lomsadze, T. Tatusova, and M. Borodovsky. 2003. Improving gene annotation of complete viral genomes. Nucleic Acids Res. 31:7041-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minic, Z., S. Brown, Y. De Kouchkovsky, M. Schultze, and C. Staehelin. 1998. Purification and characterization of a novel chitinase-lysozyme, of another chitinase, both hydrolysing Rhizobium meliloti Nod factors, and of a pathogenesis-related protein from Medicago sativa roots. Biochem. J. 332:329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes-Düby, S. E., H. J. Kwon, R. S. Tirumalai, T. Ellenberger, and A. Landy. 1998. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 26:391-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plunkett, G., III, D. J. Rose, T. J. Durfee, and F. R. Blattner. 1999. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J. Bacteriol. 181:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preus, H. R., I. Olsen, and P. Gjermo. 1987. Bacteriophage infection-a possible mechanism for increased virulence of bacteria associated with rapidly destructive periodontitis. Acta Odontol. Scand. 45:49-54. [DOI] [PubMed] [Google Scholar]
- 26.Ptashne, M. 1992. A genetic switch, 2nd ed. Cell Press & Blackwell Scientific Publications, Cambridge, Mass.
- 27.Ramanculov, E., and R. Young. 2001. Genetic analysis of the T4 holin: timing and topology. Gene 265:25-36. [DOI] [PubMed] [Google Scholar]
- 28.Ravin, N. V., A. N. Svarchevsky, and G. Deho. 1999. The anti-immunity system of phage-plasmid N15: identification of the antirepressor gene and its control by a small processed RNA. Mol. Microbiol. 34:980-994. [DOI] [PubMed] [Google Scholar]
- 29.Redaschi, N., and T. A. Bickle. 1996. DNA restriction and modification systems, p. 773-781. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C.
- 30.Sandmeier, H., A. J. van Winkelhoff, K. Bär, E. Ankli, M. Maeder, and J. Meyer. 1995. Temperate bacteriophages are common among Actinobacillus actinomycetemcomitans isolates from periodontal pockets. J. Periodontal Res. 30:418-425. [DOI] [PubMed] [Google Scholar]
- 31.Sauer, R. T., H. C. Nelson, K. Hehir, M. H. Hecht, F. S. Gimble, J. DeAnda, and A. R. Poteete. 1983. The lambda and P22 phage repressors. J. Biomol. Struct. Dyn. 1:1011-1022. [DOI] [PubMed] [Google Scholar]
- 32.Simpson, A. J., F. C. Reinach, P. Arruda, F. A. Abreu, M. Acencio, R. Alvarenga, L. M. Alves, J. E. Araya, G. S. Baia, C. S. Baptista, M. H. Barros, E. D. Bonaccorsi, S. Bordin, J. M. Bove, M. R. Briones, M. R. Bueno, A. A. Camargo, L. E. Camargo, D. M. Carraro, H. Carrer, N. B. Colauto, C. Colombo, F. F. Costa, M. C. Costa, C. M. Costa Neto, L. L. Coutinho, M. Cristofani, E. Dias-Neto, C. Docena, H. El-Dorry, A. P. Facincani, A. J. S. Ferreira, V. C. A. Ferreira, J. A. Ferro, J. S. Fraga, S. C. França, M. C. Franco, M. Frohme, L. R. Furlan, M. Garnier, G. H. Goldman, M. H. S. Goldman, S. L. Gomes, A. Gruber, P. L. Ho, J. D. Hoheisel, M. L. Junqueira, E. L. Kemper, J. P. Kitajima, J. E. Krieger, E. E. Kuramae, F. Laigret, M. R. Lambais, L. C. C. Leite, E. G. M. Lemos, M. V. F. Lemos, S. A. Lopes, C. R. Lopes, J. A. Machado, M. A. Machado, A. M. B. N. Madeira, H. M. F. Madeira, C. L. Marino, M. V. Marques, E. A. L. Martins, E. M. F. Martins, A. Y. Matsukuma, C. F. M. Menck, E. C. Miracca, C. Y. Miyaki, C. B. Monteriro-Vitorello, D. H. Moon, M. A. Nagai, A. L. T. O. Nascimento, L. E. S. Netto, A. Nhani, Jr., F. G. Nobrega, L. R. Nunes, M. A. Oliveira, M. C. de Oliveira, R. C. de Oliveira, D. A. Palmieri, A. Paris, B. R. Peixoto, G. A. G. Pereira, H. A. Pereira, Jr., J. B. Pesquero, R. B. Quaggio, P. G. Roberto, V. Rodrigues, A. J. de M. Rosa, V. E. de Rosa, Jr., R. G. de Sá, R. V. Santelli, H. E. Sawasaki, A. C. R. da Silva, A. M. da Silva, F. R. da Silva, W. A. da Silva, Jr., J. F. da Silveira, et al. 2000. The genome sequence of the plant pathogen Xylella fastidiosa. Nature 406:151-157. [DOI] [PubMed] [Google Scholar]
- 33.Stevens, R. H., B. F. Hammond, and C. H. Lai. 1982. Characterization of an inducible bacteriophage from a leukotoxic strain of Actinobacillus actinomycetemcomitans. Infect. Immun. 35:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarkowski, T. A., D. Mooney, L. C. Thomason, and F. W. Stahl. 2002. Gene products encoded in the ninR region of phage lambda participate in Red-mediated recombination. Genes Cells 7:351-363. [DOI] [PubMed] [Google Scholar]
- 35.Taylor, K., and G. Wegrzyn. 1995. Replication of coliphage lambda DNA. FEMS Microbiol. Lett. 17:109-119. [DOI] [PubMed] [Google Scholar]
- 36.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 37.Willi, K., and J. Meyer. 1998. DNA analysis of temperate bacteriophage AaΦ23 isolated from Actinobacillus actinomycetemcomitans. Mol. Gen. Genet. 258:323-325. [DOI] [PubMed] [Google Scholar]
- 38.Willi, K., H. Sandmeier, S. Asikainen, M. Saarela, and J. Meyer. 1997. Occurrence of temperate bacteriophages in different Actinobacillus actinomycetemcomitans serotypes isolated from periodontally healthy individuals. Oral Microbiol. Immunol. 12:40-46. [DOI] [PubMed] [Google Scholar]
- 39.Willi, K., H. Sandmeier, E. M. Kulik, and J. Meyer. 1997. Transduction of antibiotic resistance markers among Actinobacillus actinomycetemcomitans strains by temperate bacteriophages Aaφ23. Cell. Mol. Life Sci. 53:904-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willi, K., H. Sandmeier, and J. Meyer. 1993. Temperate bacteriophages of Actinobacillus actinomycetemcomitans associated with periodontal disease are genetically related. Med. Microbiol. Lett. 2:419-426. [Google Scholar]
- 41.Young, R., I.-N. Wang, and W. D. Roof. 2000. Phages will out: strategies of host cell lysis. Trends Microbiol. 8:120-128. [DOI] [PubMed] [Google Scholar]