ABSTRACT
In a recent study we reported that mammalian cells exposed to stress such as ionizing radiation can survive with activation of caspase-3 or caspase-7. We found that sublethal activation of the executioner caspases promotes chemical- and radiation-induced genetic instability and carcinogenesis, in contrast to their perceived roles as tumor suppressors.
KEYWORDS: Caspase-3, carcinogenesis, endonuclease G, genetic instability
Apoptosis and carcinogenesis, the prevailing view
Major physiologic functions of apoptosis are the removal of damaged or unwanted cells during development and maintenance of somatic tissue homeostasis. As such, it is generally assumed that apoptosis is an anticarcinogenic process. Consistent with this point of view, many oncogenes that are frequency overexpressed in cancer cells possess antiapoptotic functions. Examples of these include BCL21 and Akt/PKB.2
However, there is increasing recognition that the relationship between apoptosis and carcinogenesis may not be so straightforward. For example, MYC, a well-established powerful oncogene, has long been known to promote caspase activation and apoptosis.3 More recently, it was established that Fas ligand (CD95), a major factor known to initiate the extrinsic pathway of cellular apoptosis, promotes carcinogenesis in mice by activating downstream c-JUN and JNK pathways.4
Sublethal activation of caspases and its unexpected consequences on genetic instability and carcinogenesis
In a recently published study,5 we set out to examine the roles of apoptotic caspases-3 and -7 in radiation- and chemical-induced genetic instability and carcinogenesis. In an initial series of experiments, we determined the fate of cells with caspase-3/7 activation. We conducted these experiments because much of the current paradigm surrounding the roles of caspases is based the premise that once a cell initiates the apoptotic process it will die, and thus DNA damage in the apoptotic cells will not matter. Using a non-invasive reporter that allowed us to sort cells with different levels of caspase-3/7 activation, we found that many cells exposed to ionizing radiation can survive with a robust level of caspase 3 activation. More importantly, the surviving cells with enhanced levels of caspase-3 activation showed significantly higher levels of DNA damage as measured by γH2AX foci formation, comet assays, or chromosome aberration analysis. A causative role for caspase-3 in mediating DNA damage was confirmed by the use of cells expressing a dominant negative version of caspase-3. Those cells showed significantly reduced radiation-induced genetic instability. The results were also confirmed in mice with caspase-3 deficiency. Those mice showed significantly reduced radiation-induced chromosome aberrations. Consistently, MCF10A cells expressing a dominant negative version of caspase-3 showed a significantly reduced rate of radiation-induced oncogenic transformation compared with wild-type control cells. In addition, mice with caspase-3 deficiency showed a significant reduction in skin carcinogenesis induced by DMBA (7,12-dimethylbez[a]anthracene) + TPA(12-O-tetradecanoylphobol-13-acetate) treatment. Mechanistically, we show that endonuclease G, a mitochondrial nuclease that migrates to the nucleus during apoptosis to fragment host cell DNA, is a key downstream effector of caspase-3/7 in the generation of genetic instability. Our study therefore provides definitive evidence that caspases 3 and 7, key players in apoptosis, promote rather than prevent carcinogenesis (Fig. 1).
Figure 1.
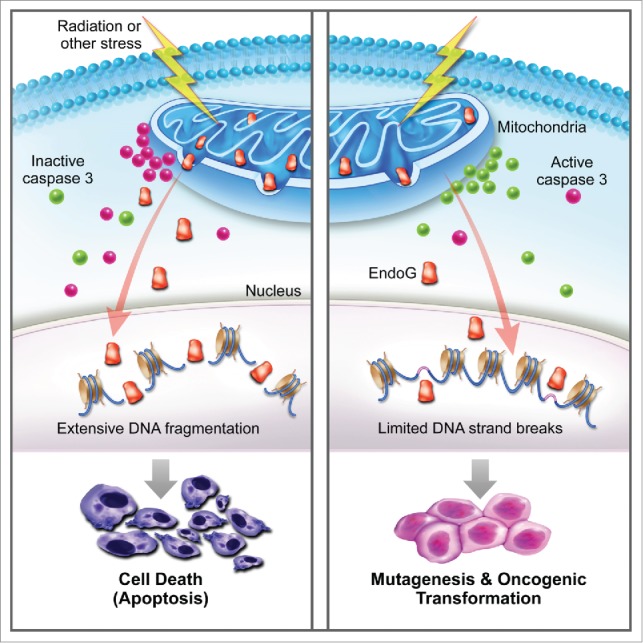
Genetic instability and carcinogenesis induced by abortive apoptosis. The prevailing view of apoptosis (depicted on the left) is that exposure to stress leads to changes in the mitochondria such as leakage of cytochrome c that activate caspase 3, which in turn activates downstream nucleases such as endonuclease G. Activated nucleases then cause extensive DNA fragmentation and cell death. However, our recent study5 shows that many cells exposed to radiation and chemical stress can survive with caspase activation (depicted on the right). The cells with sublethal caspase activation exhibit increased genetic instability and carcinogenesis.
Our results are in agreement with several recent studies. One report showed that treatment of glioma or mouse embryonic fibroblast (MEF) cells with TRAIL or FasL, 2 apoptosis-inducing factors, caused increased DNA damage and mutagenesis that was caspase 8 dependent.6 Another study showed that stress-exposed cells could reverse the apoptotic process and survive with genetic instability7 and yet another showed that low level mitochondrial leakage and caspase 3 activation were responsible for increased genetic instability and oncogenic transformation in mouse MEF cells.8
Making sense of the newly revealed roles of apoptotic caspases
How do we make sense of the fact that eukaryotic cells possess mechanisms that appear to amplify the genotoxic effects of environmental stress such as radiation exposure? Mechanisms such as those described in our study are certainly detrimental at the organismal level by significantly increasing the chances of genomic instability and cancer when the organism is exposed to stress. Although not clearly understood at present, we speculate that while caspase-mediated genetic instability is not advantageous at the whole organism level, at the individual cellular level it is reminiscent of the SOS response in E. coli in which bacterial cells utilize an error-prone system to increase the cellular mutation rate to adapt to environmental pressure such as exposure to antibiotics.9 Such a system allows individual cells to genetically adapt to environmental stress exposure at a higher rate. In this respect it is especially interesting that activated caspase 3 has been shown to be involved in cleaving and inactivating key DNA repair genes such as DNA-PKcs10 in apoptotic cells. One can imagine that cells with temporarily deactivated DNA repair factors would exhibit increased genetic instability that provides an advantage at the individual cell level from an evolutionary point of view; this may be a fundamental property of all eukaryotic cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported in part by grants CA131408, CA136748, CA155270, ES024015 from the National Institutes of Health (to C-Y Li), and grant NNX12AB88G (to C-Y Li) from NASA Space Radiation Biology Research Program; and grants 30428015, 30325043 from the National Science Foundation of China, grant 2004CB518804 from Ministry of Science of China “973 project” to Q. Huang.
References
- 1.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2:647-56; PMID:12209154; http://dx.doi.org/ 10.1038/nrc883 [DOI] [PubMed] [Google Scholar]
- 2.Sabbatini P, McCormick F. Phosphoinositide 3-OH kinase (PI3K) and PKB/Akt delay the onset of p53-mediated, transcriptionally dependent apoptosis. J Biol Chem 1999; 274:24263-9; PMID:10446202; http://dx.doi.org/ 10.1074/jbc.274.34.24263 [DOI] [PubMed] [Google Scholar]
- 3.Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell 1992; 69:119-28; PMID:1555236; http://dx.doi.org/ 10.1016/0092-8674(92)90123-T [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E, Peter ME. CD95 promotes tumour growth. Nature 2010; 465:492-6; PMID:20505730; http://dx.doi.org/ 10.1038/nature09075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, He Y, Li F, Huang Q, Kato TA, Hall RP, Li CY. Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell 2015; 58:284-96; PMID:25866249; http://dx.doi.org/ 10.1016/j.molcel.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovric MM, Hawkins CJ. TRAIL treatment provokes mutations in surviving cells. Oncogene 2010; 29:5048-60; PMID:20639907; http://dx.doi.org/ 10.1038/onc.2010.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang HL, Tang HM, Mak KH, Hu S, Wang SS, Wong KM, Wong CS, Wu HY, Law HT, Liu K, et al.. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol Biol Cell 2012; 23:2240-52; PMID:22535522; http://dx.doi.org/ 10.1091/mbc.E11-11-0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, Haller M, Riley JS, Mason SM, Athineos D, et al.. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol Cell 2015; 57:860-872; PMID:25702873; http://dx.doi.org/ 10.1016/j.molcel.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci 1975; 5A:355-67; PMID:1103845 [DOI] [PubMed] [Google Scholar]
- 10.Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med 1995; 182:1625-1634; PMID:7500007; http://dx.doi.org/ 10.1084/jem.182.6.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]


