ABSTRACT
Exosomes are secreted vesicles involved in signaling processes. The biogenesis of a class of these vesicles depends on syntenin and syndecans. Heparanase acts as a regulator of the syndecan-syntenin-exosome biogenesis pathway. The upregulation of syntenin and heparanase in cancers may support the suspected roles of exosomes in tumor biology.
KEYWORDS: ALIX, ESCRT, exosome, exosomal cargo, heparan sulfate, heparanase, PDZ domains, syndecan, syntenin, tumor progression
Abbreviations
- ALIX
ALG-2-interacting protein X
- CD
cluster of differentiation
- CTF
C-terminal fragment
- ILV
intraluminal vesicle
- ESCRT
endosomal-sorting complex required for transport
- MVB
multivesicular body
- PDZ
postsynaptic density 95/disc-large/zona occludens
Exosomes are nanovesicles of endosomal origin that are secreted by cells. They contain various membrane and cytoplasmic components, commonly designated as cargo, with a composition that reflects the state of the cell of origin. Exosomes may play an important role in intercellular communication;1 for example, tumor-derived exosomes stimulate pre-metastatic niche formation,2 and exosomal communication between cancer-associated fibroblasts and the primary tumor stimulates breast cancer cell motility and metastasis.3
The mechanisms that control the biogenesis of exosomes and the sorting of specific cargo into these vesicles are only partially understood. Intraluminal budding of the limiting membrane of endosomes creates intraluminal vesicles (ILVs). Late endosomes that contain multiple (up to 30) ILVs are called multivesicular bodies (MVBs). When MVBs fuse with lysosomes, their cargo, including their ILVs, is degraded. However, late endosomes and MVBs can also fuse with the plasma membrane, releasing their ILVs into the extracellular space as exosomes1 (Fig. 1). Several mechanisms for the control of intraluminal budding have been suggested. The endosomal-sorting complex required for transport (ESCRT) sorts ubiquitinylated membrane proteins into specific membrane domains, induces inward budding, and mediates membrane abscission to form ILVs.4 Other studies have pointed to ceramide and possibly other lipids as mediators of ILV and exosome biogenesis.5,6
Figure 1.
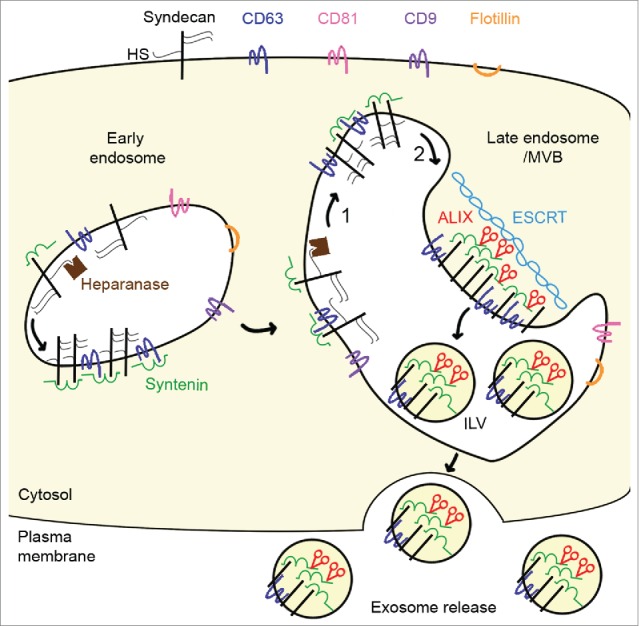
Heparanase tailors syndecan for exosome production. Syndecan and heparan sulfate (HS) cargo (not shown) are internalized. In endosomes, syntenin directly interacts with syndecans and the tetraspanin cluster of differentiation 63 (CD63) via its tandem postsynaptic density 95/disc-large/zona occludens (PDZ) domains. During endosome maturation into late endosomes, syndecans are trimmed by heparanase (1) and undergo proteolytic cleavage of their extracellular part (2) to generate a membrane-associated syndecan C-terminal fragment. These cleavages allow syndecan to cluster, and stimulate endosomal budding into intraluminal vesicles (ILV) and exosome release. The endosomal budding of syndecan-syntenin-CD63 also depends on the direct interaction of the N-terminal domain of syntenin with the endosomal-sorting complex required for transport (ESCRT) accessory component ALG-2-interacting protein X (ALIX), and on several ESCRT proteins. Heparanase does not stimulate all types of exosomes; it stimulates the release of syndecan C-terminal fragment, syntenin, ALIX and CD63, but has no effect on the release of exosomal flotillin, CD9, or CD81.
Recently, syndecan heparan sulfate proteoglycans and their cytoplasmic adaptor syntenin have been implicated in the biogenesis of exosomes. Syntenin interacts directly with ALG-2-interacting protein X (ALIX), an auxiliary component of the ESCRT machinery, through 3 LYPXnL motifs located in its N-terminus, and with the conserved cytoplasmic domains of the syndecans via its postsynaptic density 95/disc-large/zona occludens (PDZ) domains. Since ALIX binds several ESCRT proteins, the syntenin–ALIX complex links syndecans and syndecan cargo to the ESCRT budding machinery at the MVBs. Heparan sulfate is essential for this mechanism of exosome formation. Syndecan cargo, e.g., growth factor–receptor complexes bound to the heparan sulfate of syndecan, is thought to cluster syndecans and create syndecan assemblies that recruit syntenin and ALIX and subsequently support membrane budding. This pathway allows recruitment of heparan sulfate-binding cargo, such as fibroblast growth factor receptor 1, in exosomes and might thus select which cargo is targeted to exosomes.7
The importance of heparan sulfate for exosome production implies that heparanase might influence this process. Heparanase is the only mammalian enzyme able to cleave heparan sulfate internally, generating short fragments (of 10 to 20 residues) endowed with biological activity. Elevated heparanase expression by tumor cells correlates with increased tumor angiogenesis, invasiveness, and metastasis. Recently, Thompson et al. reported that heparanase stimulates exosome production and affects the composition of exosomes.8 Now, Roucourt et al. provide evidence that heparanase activates the syndecan-syntenin-ALIX pathway that supports the biogenesis of exosomes, affecting specific exosomal cargo.9
When pro-heparanase is added to cells, the enzyme precursor is rapidly internalized and processed into active heparanase. This conversion occurs in endosomes, where the enzyme normally remains localized. An increase in heparanase results in extensive trimming of the heparan sulfate on syndecan and also accelerates the endocytosis of syndecan. Exosomal levels of syntenin and cluster of differentiation 63 (CD63) increase markedly, but most striking is the increase in exosomal syndecan. Of note, most of that syndecan consists of C-terminal fragments (CTFs) that span the membrane but are devoid of any heparan sulfate (or chondroitin sulfate). Conversely, in cells that express high levels of heparanase, stable shRNA-mediated knockdown of the enzyme reduces the amounts of syntenin, CD63, and syndecan-CTFs present in exosomal fractions.
Importantly, the catalytic activity of heparanase is required and heparan sulfate must be provided by syndecan. Indeed, glypicans, heparan sulfate proteoglycans that are linked to the cell surface via glycosylphosphatidylinositol (and thus cannot directly interact with syntenin), cannot substitute for syndecan and restore the effect of heparanase on exosomes. Knockdown of the small GTPase RAB7 abolishes the heparanase-mediated increase in exosomal syntenin, syndecan CTFs, and CD63, confirming that heparanase affects the production of vesicles that are of endosomal origin, the operational definition of exosomes. Furthermore, heparanase was shown to stimulate the endosomal budding of syntenin and syndecan and to require ALIX for these effects (Fig. 1). Thus, heparanase is an activator of the syndecan-syntenin-ALIX pathway of exosome biogenesis.
How might heparanase work? Heparanase leaves syndecan substituted with small heparan sulfate chains (possibly restricted to a single ligand) and thus more likely free to ‘move around’. There is also a simple physical aspect to the action of heparanase. The length of an extended native heparan sulfate chain (with a molecular weight of 40 kDa on average) is close to 50 nm. With three chains of heparan sulfate per syndecan, likely projecting outwards and pointing away from each other, the diameter of a syndecan may be close to 100 nm, which is approximately the diameter of an ILV and exosome. Trimming of the heparan sulfate on syndecan may thus substantially increase the number of syndecan molecules that can be packed in defined membrane domains and thereby help create the density of bait (syndecan cytosolic domains) that is required to recruit syntenin, and along with the syntenin also ALIX and ESCRTs.
Apart from clarifying the role of heparanase in exosome biogenesis, Roucourt et al. also reveal that heparanase stimulates the targeting of specific cargo to exosomes or stimulates specific exosomes. Indeed, exosomal flotillin-1 and exosomal levels of CD9 and CD81, 2 tetraspanins commonly used as exosomal markers, are not affected by heparanase. The specificity of the heparanase effect underpins the hypothesis of multiple different exosomal populations formed through specific biogenesis pathways, one of which would be the syndecan-syntenin-ALIX pathway. Yet, the really important question that should be resolved in the immediate future is whether the cargo directed by heparanase-modified syndecans to exosomes accounts for the biological effects of these vesicles and sustains tumor growth, invasiveness, and dissemination; for example, exosomal components such as hepatocyte growth factor receptor,2 amphiregulin,10 Wnt proteins3 and likely others. If this is the case, syntenin and heparanase, both of which are often upregulated in cancer, represent interesting targets for modulating exosome effects in cancer therapies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Fund for Scientific Research-Flanders (FWO, G.0683.09N), the Concerted Actions Program of the KU Leuven (GOA/12/016 and GOA/2006/13), the VIB, the Belgian Federation against Cancer (Stichting tegen Kanker, SCIE2006–36 and 214–2008) and the Interuniversity Attraction Poles of the Prime Ministers Services (IUAP).
References
- 1.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200:373-83; PMID:23420871; http://dx.doi.org/ 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al.. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883-91; PMID:22635005; http://dx.doi.org/ 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luga V. Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151:2012; 1542-56; PMID:23260141; http://dx.doi.org/ 10.1016/j.cell.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 4.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 2010; 464:864-9; PMID:20305637; http://dx.doi.org/ 10.1038/nature08849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, Slavík J, Machala M, Zimmermann P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun 2014; 5:3477; PMID:24637612; http://dx.doi.org/ 10.1038/ncomms4477 [DOI] [PubMed] [Google Scholar]
- 6.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319:1244-7; PMID:18309083; http://dx.doi.org/ 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- 7.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al.. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 2012; 14:677-85; PMID:22660413; http://dx.doi.org/ 10.1038/ncb2502 [DOI] [PubMed] [Google Scholar]
- 8.Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem 2013; 288:10093-9; PMID:23430739; http://dx.doi.org/ 10.1074/jbc.C112.444562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res 2015; 25:412-28; PMID:25732677; http://dx.doi.org/ 10.1038/cr.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higginbotham JN. Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, et al.. Amphiregulin exosomes increase cancer cell invasion. Curr Biol 2011; 21:779-86; PMID:21514161; http://dx.doi.org/ 10.1016/j.cub.2011.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]


