Abstract
Most tumors are generated and evolve under high-nutrient conditions, yet therapy does not include dietary changes generating a hostile environment for cancer cells. Because fasting promotes the most drastic change in the levels of plasma macro- and micro-nutrients, and consequently in glucose and growth factors, it has the potential to maximize cancer cell sensitization.
Keywords: Differential Stress Sensitization (DSS), Fasting, Fasting Mimicking Diet (FMD), p53, REV1
In organisms ranging from bacteria to humans, complete nutrient deprivation is an intervention associated with major changes in the levels of pro-growth signaling triggered by glucose and other nutrients and leading to a profound metabolic reprogramming and increased investment in protective systems. These changes have been shown to extend longevity and stimulate stem cell-based regeneration in multiple systems including the hematopoietic and nervous systems.1 Fasting, defined as complete food deprivation, has been shown to protect normal cells against a wide variety of stress conditions but either kills cancer cells or sensitizes them to chemotherapy or to novel anticancer drugs such as kinase inhibitors. Increasing efforts are being made to provide alternative fasting regimens or diets that mimic fasting in order to achieve benefits equal to the complete food deprivation while maximizing calorie intake and nourishment.
Highly mutagenic cancer cells evolve from normal cells to become well adapted and fast growing in an environment rich in nutrients. As well established in evolutionary biology, most mutations are deleterious not necessarily in all environments but in the ones that can be encountered and must be overcome in order for the organism to survive and reproduce. This concept could explain how, when placed in a hostile environment such as the lack of nutrients (fasting conditions), highly proliferative cancer cells become weak and more vulnerable to chemotherapy.2
As we have previously shown, circulating insulin-like growth factor 1 (IGF-1) is a key factor affected by fasting and reduction in its levels is crucial for the establishment of differential stress sensitization (DSS), a condition where cancer cells, but not normal cells, are sensitized, particularly in the presence of cytotoxic drugs.3 The mechanistic target of rapamycin (mTOR), a metabolic regulator on which signaling pathways dependent on IGF-1, glucose, and amino acids converge to regulate growth and autophagy4,5 and which has a central role in the growth of many cancers (as shown in Fig. 1), is also down-regulated during fasting.
Figure 1.
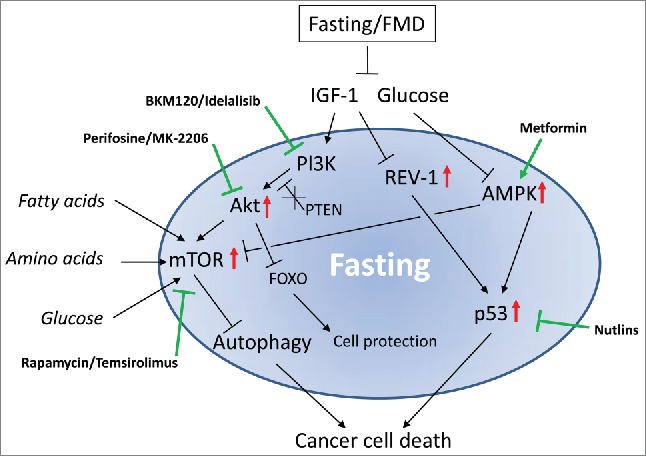
Schematics of key factors modulated by nutrients. The effect of fasting on a broad range of key factors involved in cell metabolism and protection (contained in the blue area) results in cancer cell death. In contrast, the inhibition of single factors by pharmaceutical intervention (e.g., kinase inhibitors) often results in metabolic rearrangements and compensatory mechanisms that allow cancer cells to survive and grow. Drugs used for cancer treatment (indicated in bold) and their effects on each factor (marked in green) are shown. The combination of fasting with chemotherapy or targeted inhibitors gives the best outcome for cancer cell targeted killing (differential stress sensitization or DSS).
As demonstrated in our recent work, protein restriction regimens effectively reduce IGF-1 levels and tumor incidence in mice and possibly humans,6 suggesting that the lack of certain amino acids and the resulting downregulation of mTOR4 could be sufficient to mimic some of the effects of fasting on cancer incidence.7 However, protein restriction can have a major effect on the progression of certain cancers but not others, suggesting that limiting amino acid levels alone may not promote changes that are broad enough to replace fasting.
Other studies have concluded that serum starvation, an in vitro model for protein restriction in animals, causes a selective induction of autophagy in cancer but not normal cells.8 This targeted induction is mediated by the AMP-activated protein kinase (AMPK) and the tumor protein p53 (TP53, best known as p53). We have also recently provided evidence for the role of the DNA repair protein REV1 as a novel modulator of p53 in response to starvation conditions, whereby REV1 sumoylation results in the activation of p53 and elevated expression of p53-dependent pro-apoptotic genes (Puma, Bax, Noxa, Killer, Tigar, and SESTRIN2) and ultimately in cancer cell death.9 These findings in part clarify the poorly understood mechanism of selective targeted killing of cancer cells under starvation conditions (i.e., DSS), which seems to involve both AMPK and REV1 (as shown in Fig. 1). Notably, REV1 mediates DSS but is not responsible for the protection of normal cells during starvation (differential stress resistance or DSR). These data suggest that, although activated by fasting, DSS and DSR could be mediated by the modulation of different pathways.
The understanding of the mechanisms of nutrient sensing5 and the molecular pathways regulated by fasting has led to the development of pharmaceutical interventions aimed at reproducing the beneficial effects of fasting without food deprivation (as shown in Fig. 1). Such intervention would allow longer treatment periods without compromising safety. Although fasting mimicking drugs would be preferred by patients and would lead to a higher compliance compared to dietary interventions, their development will require lengthy clinical trials in order to avoid the side effects that will take many years to obtain FDA approval. In addition, the fact that multiple key pathways such as those regulated by IGF1, the oncogene Ras, REV1, and protein kinase A (PKA) may need to be simultaneously blocked to match the effects of fasting suggests that it will be difficult to identify pharmacological interventions that are both as effective and as safe as fasting, even after long-term chronic use.
The broad acting but highly coordinated differential effect of fasting on normal and cancer cells that is mediated by the levels of various factors including glucose, amino acids, and growth factors makes this approach not only highly effective in animal studies but also a candidate for successful translation into clinical applications (as shown in Fig. 1). Thus, fasting mimicking diets (FMDs), which are as effective as fasting in promoting the protection of normal cells, and cancer cell death, are candidates for clinical application in the near future. Eventually, these diets will be accompanied by drugs that enhance their fasting mimicking effects.
Disclosure of potential conflicts of interest
VDL has equity interest in L-Nutra, a company developing fasting mimicking diets.
References
- 1.Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB, et al.. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell stem cell 2014; 14:810-23; PMID:24905167; http://dx.doi.org/ 10.1016/j.stem.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raffaghello L, Safdie F, Bianchi G, Dorff T, Fontana L, Longo VD. Fasting and differential chemotherapy protection in patients. Cell Cycle 2010; 9:4474-6; PMID:21088487; http://dx.doi.org/ 10.4161/cc.9.22.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al.. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Science Translational Medicine 2012; 4:124ra27; PMID:22323820; http://dx.doi.org/ 10.1126/scitranslmed.3003293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, et al.. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015; 347:188-94; PMID:25567906; http://dx.doi.org/ 10.1126/science.1257132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature 2015; 517:302-10; PMID:25592535; http://dx.doi.org/ 10.1038/nature14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al.. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell metabolism 2014; 19:407-17; PMID:24606898; http://dx.doi.org/ 10.1016/j.cmet.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al.. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell metabolism 2014; 19:418-30; PMID:24606899; http://dx.doi.org/ 10.1016/j.cmet.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC cancer 2012; 12:571; PMID:23211021; http://dx.doi.org/ 10.1186/1471-2407-12-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shim HS, Wei M, Brandhorst S, Longo VD. Starvation promotes REV1 SUMOylation and p53-dependent sensitization of melanoma and breast cancer cells. Cancer Res 2015; 75(6):1056-67; PMID: 25614517; http://dx.doi.org/10.1158/0008-5472.CAN-14-2249 [DOI] [PMC free article] [PubMed] [Google Scholar]


