ABSTRACT
To identify clinically relevant downstream effectors of androgen signaling, the androgen-regulated kinome was defined in prostate cancer (PCa). Within this study, choline kinase α (CHKA) was identified as an androgen receptor chaperone that is both a biomarker of progression and a potential therapeutic target for PCa.
KEYWORDS: Androgen Receptor, chaperones, therapeutic target, prostate cancer
The androgen receptor (AR) is an oncogenic transcription factor implicated in prostate cancer (PCa) progression. Resistance to androgen deprivation therapy occurs in the progression to castration-resistant prostate cancer (CRPC)1 and is accompanied by resurgence of AR signaling. Multiple mechanisms can stimulate AR signaling in CRPC including gain-of-function mutations in the AR signaling pathway2 and upregulation of heat shock proteins (HSPs) that act as chaperones for AR and kinases that can potentiate AR function.3 Consistent with this concept, targeting of HSPs and kinases in preclinical models inhibits AR function and PCa tumor growth.4,5 While these diverse resistance mechanisms highlight the reliance of PCa on AR signaling, there is a critical need to identify therapeutic targets that regulate AR function as well as key downstream effector pathways.
We profiled the AR-regulated kinome in PCa with a view to demonstrating functionally and clinically important signaling events downstream of the AR. We showed that choline kinase α (CHKA) is a chaperone for the AR, promoting its stability and function.6 This is the first report demonstrating that kinases can act as chaperones. During our studies we also identified 49 androgen-regulated kinases, of which 25 were upregulated and 24 were downregulated. We confirmed the in vivo androgen regulation of a subset of these genes (18/25 androgen upregulated kinase genes) in tumor tissue from chemically castrated PCa patients.
CHKA expression was enhanced in localized (primary) and metastatic PCa and was decreased in tissues from castrated men. Protein levels of CHKA were correlated with Gleason grade and stage, and CHKA was an independent predictor of biochemical recurrence. CHKA can exist as 2 isoforms: CHKA2 and CHKA1. Interestingly, the interaction of CHKA2 with the AR decreased within 5–15 min of androgen treatment, consistent with the timing of AR release from cytoplasmic chaperones and its translocation to the nucleus. AR protein levels were reduced by CHKA knockdown, indicating a reciprocal role of CHKA in regulating AR protein levels. A direct physical interaction was mapped between CHKA2 and the LBD of AR (ARLBD), the AR domain that interacts with HSPs. In a protease protection assay, both dihydrotestosterone (DHT) and CHKA2 were required to stabilize AR. In line with this, CHKA knockdown did not decrease the levels of an AR variant (ARv567es) lacking the LBD. Taken together, our data show for the first time that kinases can function as protein chaperones—a feature not previously attributed to any kinase.
CHKA inhibition decreased the activity of full-length AR but failed to inhibit AR variants lacking the LBD, indicating that CHKA regulates AR transcriptional activity by binding to ARLBD. Transcriptome sequencing from CHKA knockdown in C4-2 cells revealed a highly significant bidirectional overlap between siCHKA and androgen-regulated genes, consistent with a global role for CHKA in AR regulation. Pathway analysis identified enrichment for pathways regulating protein folding and cellular protein localization, consistent with the chaperone function of CHKA. These analyses highlight a functional link between AR and CHKA signaling, indicating a cooperative function of CHKA in driving the AR-regulated transcriptome by stabilizing AR protein levels.
CHKA inhibition induced apoptosis in many CRPC cell line models in a manner similar to AR inhibition. The growth-promoting role of CHKA did not depend on the catalytic activity of CHKA since the addition of exogenous phosphocholine or phosphatidylcholine did not rescue growth inhibition by a CHKA inhibitor that reduced AR activity, AR levels, and CHKA levels. In an ex vivo culture assay, treatment of hormone-naïve primary PCa tissue with CHKA inhibitor decreased AR expression in tumor epithelia while increasing levels of cleaved caspase 3, a marker of apoptosis. Migration and invasion phenotypes were also inhibited by CHKA knockdown. Finally, PCa xenograft growth was inhibited using an inducible CHKA knockdown system. Collectively, these experiments indicate that CHKA inhibition opposes androgen action and reduces malignant phenotypes and triggers apoptosis in PCa cells and tissue. The data demonstrate the importance of CHKA in PCa growth and invasion, highlighting the potential future benefits of inhibitors of this kinase in the clinical management of PCa.
In summary, CHKA can act as a chaperone that regulates AR signaling, elucidating a feed-forward AR–CHKA signaling loop that reinforces AR activity, allowing CHKA to maintain its own expression in PCa. The chaperone function of CHKA confers a growth advantage on PCa by stabilizing the AR in addition to its well-known function in fuelling membrane production through the Kennedy pathway7 (Fig. 1), suggesting a de facto role for CHKA as a rate-limiting factor in cancer cell growth.
Figure 1.
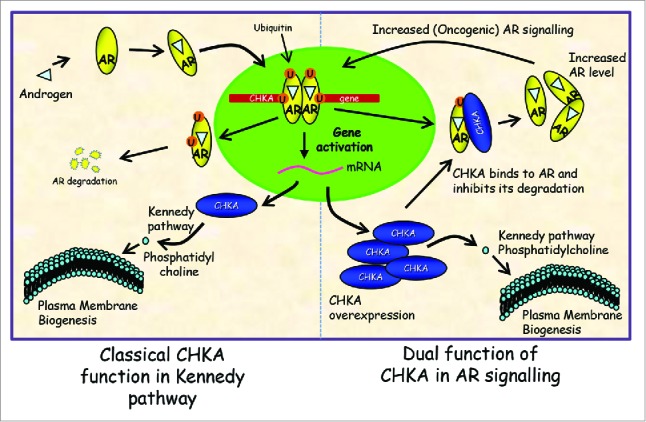
Classical and non-classical functions of CHKA. The classical model involves ubiquitination and activation of AR-dependent transcription that results in upregulation of CHKA, a rate-limiting enzyme in the Kennedy pathway (production of phosphatidylcholine, required for plasma membrane biogenesis). In addition to its role in the Kennedy pathway, CHKA can also interact with the AR in the cytoplasm and promote its stability (non-classical model). This could lead to AR overexpression and increased AR signaling, which in turn may allow more CHKA production. AR, androgen receptor; CHKA, choline kinase α; U, ubiquitin.
This discovery should accelerate the screening of potential CHKA inhibitors that not only inhibit the catalytic function but also decrease the CHKA protein level. We believe that our work will direct efforts in the treatment of PCa in particular, but also other cancers, by stimulating a systematic re-evaluation of CHKA as a therapeutic target in cancers in which it is overexpressed and exhibits analogous effects on other oncogenic proteins.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a Cancer Research UK program grant (to DEN).
References
- 1.Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer 2004; 11:459–76; PMID:15369448; http://dx.doi.org/ 10.1677/erc.1.00525 [DOI] [PubMed] [Google Scholar]
- 2.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X, et al. . A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 2013; 154:1074–84; PMID:23993097; http://dx.doi.org/ 10.1016/j.cell.2013.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asim M., Siddiqui IA, Hafeez BB, Baniahmad A, Mukhtar H. Src kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cells. Oncogene 2008; 27:3596–604; PMID:18223692; http://dx.doi.org/ 10.1038/sj.onc.1211016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asim M, Hafeez BB, Siddiqui IA, Gerlach C, Patz M, Mukhtar H, Baniahmad A. Ligand-dependent corepressor acts as a novel androgen receptor corepressor, inhibits prostate cancer growth, and is functionally inactivated by the Src protein kinase. J Biol Chem 2011; 286:37108–17; PMID:21856747; http://dx.doi.org/ 10.1074/jbc.M111.292771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, Rennie P, Nelson C, Gleave M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res 2007; 67:10455–65; PMID:17974989; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2057 [DOI] [PubMed] [Google Scholar]
- 6.Asim M, Massie CE, Orafidiya F, Pértega-Gomes N, Warren AY, Esmaeili M, Selth LA, Zecchini HI, Luko K, Qureshi A, et al. . Choline kinase alpha as an androgen receptor chaperone and prostate cancer therapeutic target. J Natl Cancer Inst 2016; 108; PMID:26657335; http://dx.doi.org/ 10.1093/jnci/djv371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y, Sreenivas A, Ostrander DB, Carman GM. Phosphorylation of Saccharomyces cerevisiae choline kinase on Ser30 and Ser85 by protein kinase A regulates phosphatidylcholine synthesis by the CDP-choline pathway. J Biol Chem 2002; 277:34978–86; PMID:12105205; http://dx.doi.org/ 10.1074/jbc.M205316200 [DOI] [PubMed] [Google Scholar]


