ABSTRACT
The Forkhead box C1 (FOXC1) transcriptional factor is a critical biomarker for basal-like breast cancer (BLBC). We recently reported that FOXC1 promotes cancer stem cell properties in BLBC by activating Smoothened (SMO)-independent Hedgehog (Hh) signaling, suggesting a FOXC1-mediated mechanism for BLBC cell function and anti-Hh therapy resistance.
KEYWORDS: Basal-like breast cancer, cancer stem cells, FOXC1, GLI2, Hedgehog, Smoothened
Comprehensive gene expression and genomics analysis has classified breast cancer into at least 4 major biologically and clinically distinct subtypes: luminal A, luminal B, human epidermal growth factor receptor 2-overexpressing (HER2+), and basal-like.1 Basal-like breast cancer (BLBC) accounts for 15–20% of all invasive breast cancers and is associated with high histologic grade, more frequent recurrence, younger age of onset, and poor prognosis due to preferential metastasis to the brain and lung.2 Unlike other subtypes of breast cancer, chemotherapy remains the only systemic treatment for BLBC patients. Successful development of new therapies for BLBC depends on further understanding the key regulators and biological basis of this disease.
Compared with other breast cancer subtypes, BLBC exhibits increased cancer stem cell (CSC) properties with an embryonic stem cell-like gene expression signature.3 This observation provides an underlying rationale for the aggressiveness and poor prognosis of BLBC. However, it is not clear how BLBC-specific gene expression or markers relate to the stem-like traits of BLBC. We recently reported that Forkhead box C1 (FOXC1), a key diagnostic biomarker specific for BLBC,4,5 increases CSC properties in BLBC cells through a Glioma-Associated Oncogene Family Zinc Finger 2 (GLI2)-mediated, Smoothened (SMO)-independent Hedgehog (Hh) pathway.6
We initially discovered in our in vitro studies that FOXC1 increases aldehyde dehydrogenase activity and mammosphere formation rate, both of which are indicators of breast CSC. We also found that FOXC1 is essential for tumorigenicity in human breast cancer xenograft models. In line with our findings, FOXC1 has been shown to induce a progenitor-like phenotype in differentiated mammary epithelial cells7 and is a key regulator for development and maintenance of the mesenchymal niches for hematopoietic stem and progenitor cells.8 On the basis of this evidence, we hypothesized that FOXC1-induced CSC properties contribute to the poor prognosis of BLBC.
When examining the mechanism of this pathway we found that FOXC1 activated Hh signaling, which in turn mediates CSC properties in BLBC cells.6 Previous studies have demonstrated that Hh signaling is essential for maintenance of CSC in multiple cancers. In support of this finding in a clinical setting, a positive correlation between FOXC1 levels and the activation of Hh signaling was detected in gene expression analysis of breast tumor samples, and the presence of FOXC1/Hh signaling predicted worse prognosis.
Mechanistic studies demonstrated that FOXC1-induced Hh activation is mediated by a SMO-independent mechanism (Fig. 1). FOXC1 can elicit Hh signaling in 2 BLBC cell lines MDA-MB-231 and HCC1500, neither of which expresses SMO.6 Furthermore, the FOXC1 effect was not affected by SMO knockdown in SMO-expressing BLBC cells.6 Consistent with our results, it has been shown that Hh signaling is highly active in breast CSCs that are characterized by the cell-surface glycoprotein CD44+/CD24− phenotype.9 Because FOXC1 stimulates Hh signaling independently of SMO, we postulated that elevated expression of FOXC1 may render cancer cells refractory to SMO targeting inhibitors. Indeed, by using different SMO-positive BLBC cell lines and xenograft tumors, we confirmed that overexpression of FOXC1 can lead to reduced cellular sensitivity to SMO targeting inhibitors such as vismodegib (GDC-0449). Similarly, FOXC1 levels were upregulated in in vitro-derived SMO antagonist-resistant cell models and FOXC1 knockdown reversed the resistance phenotype. These data uncover a potential mechanism that may explain why anti-Hh inhibitors have not yielded encouraging results in clinical trials of cancer treatment.
Figure 1.
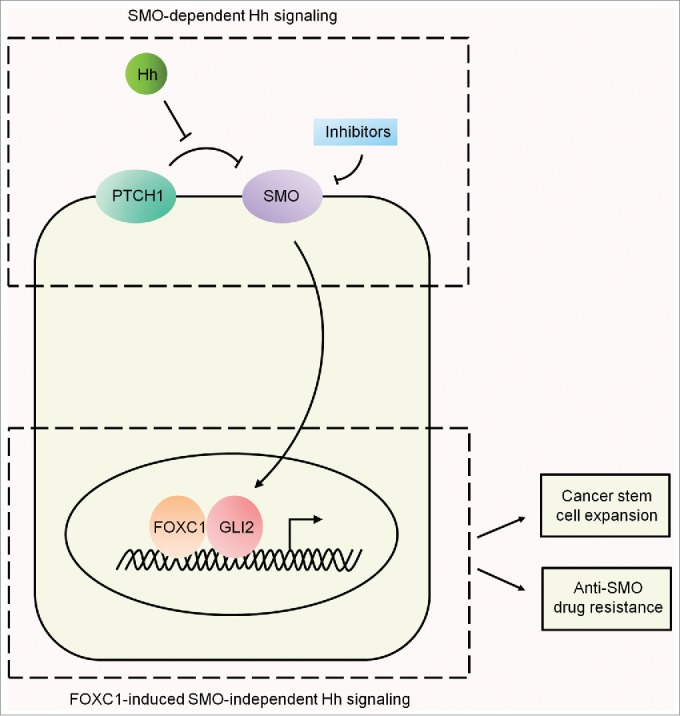
Smoothened-dependent and Forkhead box C1 transcription factor-induced Smoothened-independent Hedgehog signaling. The Smoothened (SMO)-dependent canonical Hedgehog (Hh) pathway is activated upon binding of Hh ligand to the cognate receptor Patched 1 (PTCH1), which enables SMO to activate the Glioma-Associated Oncogene Family Zinc Finger (GLI) proteins. The binding of Forkhead box C1 (FOXC1) to GLI2 activates SMO-independent Hh signaling, which promotes expansion of the cancer stem cell (CSC) population and induces resistance to anti-SMO drugs.
There are 3 ultimate effectors in Hh signaling, GLI1, GLI2, and GLI3. Using siRNA, we demonstrated that only knockdown of GLI2 attenuated the FOXC1-induced Hh activation as well as CSC properties in BLBC cells. These results suggest that GLI2 is the key downstream mediator responsible for the regulation of Hh activity by FOXC1. Unexpectedly, FOXC1 had no effect on the expression level or translocation of GLI2 in BLBC cells.6 Using immunoprecipitation, histidine- and glutathione S transferase-tagged pull down, and fluorescence resonance energy transfer (FRET), we revealed that FOXC1 and GLI2 directly bind to each other through the N-terminal domain of FOXC1 (aa 1–68) and an internal region (aa 898–1168) of GLI2. Functionally, the binding of FOXC1 to GLI2 promotes the DNA-binding capacity of GLI2 in BLBC cells. Mechanistically, this direct interaction is predicted to allosterically open the DNA binding domain of GLI2, providing a structural basis for how FOXC1 promotes the DNA-binding capacity of GLI2.
GLI2 has been shown to be involved in tumorigenesis of various human tumors in SMO-dependent canonical Hh signaling, but also exerts its effect through a SMO-independent manner controlled by changes in expression level.10 Yet, none of these actions were involved in FOXC1-regulated Hh signaling. Instead, our studies uncovered a previously uncharacterized mechanism for Hh signaling regulation through direct transcription factor interaction.
In conclusion, our study showed that the N-terminal of FOXC1 binds directly to GLI2 resulting in the activation of SMO-independent Hh signaling, which in turn promotes CSC properties and resistance to drugs targeting SMO (Fig. 1). These results provide a clinically relevant mechanism for the aggressiveness and poor prognosis of BLBC. Given that SMO inhibitors have been extensively evaluated in clinical trials, we propose that FOXC1 may serve as a marker for selecting cancer patients who may not benefit from anti-SMO therapy and as a therapeutic target for overcoming anti-Hh drug resistance.
Disclosure of potential conflicts of interest
X. Cui is a named inventor on patent applications regarding the role of FOXC1 in cancer. The other authors declare that no conflict of interest exists.
Funding
This work was supported by National Institutes of Health (CA151610), the Avon Foundation (02-2014-063), David Salomon Translational Breast Cancer Research Fund, Eleanor and Glenn Padnick Discovery Fund in Cellular Therapy, the Fashion Footwear Charitable Foundation of New York, Inc., and the Margie and Robert E. Petersen Foundation.
References
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al.. Molecular portraits of human breast tumours. Nature 2000; 406:747-52; PMID:10963602; http://dx.doi.org/ 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 2.Han BC, Audeh W, Jin YL, Bagaria SP, Cui XJ. Biology and treatment of basal-like breast cancer Cell and Molecular Biology of Breast Cancer 2013; Schatten H, ed. (New York, USA: Springer-Humana Press; ), pp. 91-109; http://dx.doi.org/ 10.1007/978-1-62703-634-4_5. [DOI] [Google Scholar]
- 3.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 2008; 40:499-507; PMID:18443585; http://dx.doi.org/ 10.1038/ng.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, et al.. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res 2010; 70:3870-6; PMID:20406990; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-4120 [DOI] [PubMed] [Google Scholar]
- 5.Jensen TW, Ray T, Wang J, Li X, Naritoku WY, Han B, Bellafiore F, Bagaria SP, Qu A, Cui X, et al.. Diagnosis of Basal-Like Breast Cancer Using a FOXC1-Based Assay. J Natl Cancer Inst 2015; 107:djv148; PMID: 26041837; http://dx.doi.org/ 10.1093/jnci/djv148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han BC, Qu Y, Jin YL, Yu Y, Deng N, Wawrowsky K, Zhang X, Li N, Bose S, Liu Z, et al.. FOXC1 Activates Smoothened-Independent Hedgehog Signaling in Basal-like Breast Cancer. Cell Rep 2015; 13:1046-58; PMID:26565916; http://dx.doi.org/ 10.1016/j.celrep.2015.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloushtain-Qimron N, Yao J, Snyder EL, Shipitsin M, Campbell LL, Mani SA, Hu M, Chen H, Ustyansky V, Antosiewicz JE, et al.. Cell type-specific DNA methylation patterns in the human breast. Proc Natl Acad Sci USA 2008; 105:14076-81; PMID:18780791; http://dx.doi.org/ 10.1073/pnas.0805206105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omatsu Y, Seike M, Sugiyama T, Kume T, Nagasawa T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature 2014; 508:536-40; PMID:24590069; http://dx.doi.org/ 10.1038/nature13071 [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006; 66:6063-71; PMID:16778178; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-β: Smad3-dependent activation of GLI2 and GLI1 expression in vitro and in vivo. Cancer Res 2007; 67:6981-6; PMID:17638910; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0491 [DOI] [PubMed] [Google Scholar]


