Abstract
Lipoteichoic acids (LTAs) were purified from Lactobacillus delbrueckii subsp. lactis ATCC 15808 and its LL-H adsorption-resistant mutant, Ads-5, by hydrophobic interaction chromatography. L. delbrueckii phages (LL-H, the LL-H host range mutant, and JCL1032) were inactivated by these poly(glycerophosphate) type of LTAs in vitro in accordance to their adsorption to intact ATCC 15808 and Ads-5 cells.
Cell walls of gram-positive eubacteria consist mainly of a thick peptidoglycan layer and various compositions of proteins and accessory polymers, such as polysaccharides and teichoic acids. Lactococcal bacteriophages have been reported to use cell wall carbohydrates as (primary) receptors for adsorption, and for some a requirement of certain membrane proteins has been demonstrated (18, 28). Glycosylated teichoic acids are essential at least for the adsorption of some Bacillus subtilis, Staphylococcus aureus, and Lactobacillus plantarum phages (9, 11, 19, 30). Even a peptidoglycan layer has been reported to serve as a receptor substance in phage adsorption (29). Although (lipoteichoic acids) LTAs are widely distributed in gram-positive eubacteria, no reports of their role as a receptor substance have been published so far. In Lactococcus lactis subsp. cremoris SK110, modified LTAs have been suggested to prevent phage adsorption by masking the actual receptor site (26, 27).
The isometric-headed Lactobacillus delbrueckii subsp. lactis phage LL-H is the only L. delbrueckii phage that is completely sequenced (2, 22). The LL-H genome exhibits a limited homology to the genome of the prolate-headed L. delbrueckii subsp. lactis phage JCL1032 (17). Both phages have noncontractile tails, and their genetic determinants involved in host recognition have been characterized. Gp71 and its homolog ORF474 determine the adsorption specificities of LL-H and JCL1032, respectively (23). Ads-5, one of the LL-H-resistant mutants of L. delbrueckii subsp. lactis ATCC 15808, is able to block the adsorption of LL-H but not the adsorption of the LL-H host range mutant LL-H-a21 or JCL1032 (23). In this study, we investigated if purified LTAs isolated from ATCC 15808 and Ads-5 could serve as a receptor substance in the early stage of L. delbrueckii phage infections.
Bacterial strains and bacteriophages.
L. delbrueckii subsp. lactis strains were grown at 37°C in MRS broth (Difco Laboratories), and for phage propagation, MRS broth was supplemented with 10 mM CaCl2. Bacterial strains and bacteriophages used in this study are listed in Table 1. For the isolation of LTAs, strains were grown in 1-liter batch cultures (supplemented with 10 mM CaCl2) to an optical density at 600 nm of 0.5 to 0.6.
TABLE 1.
Bacterial strains and bacteriophages used in this study
| Strain or phage | Description | Reference or source |
|---|---|---|
| L. delbrueckii subsp. lactis | ||
| ATCC 15808 | Host strain for the phages LL-H, JCL1032, and mv4 | ATCCa |
| Ads-5 | LL-H-resistant mutant of ATCC 15808; blocks phage LLH adsorption; host strain for the phage LL-H-a21 | This laboratory (23) |
| Bacteriophage (Siphoviridae) | ||
| LL-H | Small isometric-headed phage of L. delbrueckii subsp. lactis, virulent | Valio, Hauho, Finland (1, 16) |
| LL-H-a21 | Spontaneous phage LL-H host range mutant isolated from Ads-5 | This laboratory (23) |
| JCL1032 | Small prolate-headed phage of L. delbrueckii subsp. lactis | This laboratory (17) |
| mv4 | Small isometric-headed phage of L. delbrueckii subsp. bulgaricus, temperate | 21 |
ATCC, American Type Culture Collection.
Extraction and purification of LTAs by HIC.
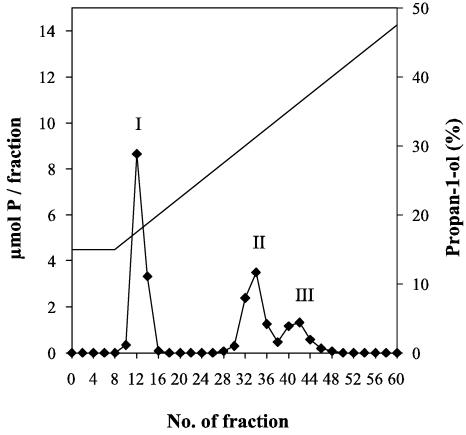
LTAs were extracted from lipid-free bacterial cells by hot 80% (wt/vol) aqueous phenol (20). For purification by hydrophobic interaction chromatography (HIC), LTAs extracted from ATCC 15808 cells were dissolved in 50 mM sodium acetate (pH 4.0) containing 15% propan-1-ol and applied to a column of octyl-Sepharose CL 4B (2 by 22 cm). The column was eluted with a linear gradient (15 to 60% [vol/vol]) of propan-1-ol in 50 mM sodium acetate (pH 4.0) at a flow rate of 20 ml h−1. Every second fraction (5 ml) was analyzed for nucleic acids (A260) and for phosphorus (4). Three pools containing phosphorus, shown in Fig. 1, were detected. According to its UV absorbance spectrum, pool I contained nucleic acids (data not shown). Pools II and III, which eluted between propan-1-ol concentrations of 28 and 39%, were practically free of nucleic acids and proteins. Based on the general elution profile, pools II and III were considered as possible fractions of LTAs. They were dialyzed against distilled water.
FIG. 1.
Elution profile of hot-phenol-extracted LTAs from L. delbrueckii ATCC 15808 after HIC on octyl-Sepharose CL 4B. I, nucleic acids; II (fractions 28 to 37) and III (fractions 38 to 46), LTAs. For technical details, see the text.
In the faster purification procedure, dissolved LTAs were applied to a column (2.6 by 13 cm) of octyl-Sepharose 4 Fast Flow (Pharmacia Biotech AB, Uppsala, Sweden). The column was eluted with a linear gradient (15 to 70% [vol/vol]) of propan-1-ol in 100 mM sodium acetate (pH 5.0) at a flow rate of 1.8 ml min−1. Collected fractions (3 ml) were analyzed for nucleic acids and for phosphorus as previously described. LTAs eluted as a single peak between propan-1-ol concentrations of 30 and 41% (data not shown).
Chemical composition analysis of LTAs purified from ATCC 15808.
Pools II and III were investigated for d-alanine, sugars, and polyols (Table 2). Amino acids were quantitatively determined as previously described (25). Glycerol, ribitol, and sugars were analyzed as peracetylated or reduced peracetylated derivatives by gas-liquid chromatography (GLC) after hydrolysis in 60% (wt/vol) hydrofluoric acid (3, 6, 25). For the analysis of sugars, hydrofluoric acid-hydrolyzed material was further hydrolyzed in 2 M HCl (100°C for 3 h). Both the pools contained almost equal molar amounts of glycerol and phosphorus (Table 2). They eluted separately most likely due to the different number of fatty acids (12, 15). On the basis of our composition analysis and survey of the literature, LTAs from L. delbrueckii subsp. lactis ATCC 15808 probably belong to the most widespread type of LTAs, 1-3-linked poly(glycerophosphate)s (13, 14).
TABLE 2.
Chemical composition of HIC-purified LTAs (pool II and III; see Fig. 1) extracted from L. delbrueckii subsp. lactis strain ATCC 15808
| LTA component | HIC-purified LTAsa
|
|
|---|---|---|
| Pool II (nmol/ratio) | Pool III (nmol/ratio) | |
| Glycerol | 392.09/0.98 | 356.36/0.89 |
| Ribitol | 24.75/0.06 | 20.46/0.05 |
| Glucose | 34.56 | 39.16 |
| d-Alanine | 42.96/0.11 | 77.24/0.19 |
Each acid-hydrolyzed sample for GLC contained 400 nmol of total phosphorus. The calculated molar ratios between LTA components and total phosphorus are presented.
Besides glycerol, a small amount of ribitol was also detected by GLC. The presence of ribitol in LTAs is rarely observed. Only the so-called Forsman antigen from Streptococcus pneumoniae has been described to contain ribitol phosphate and choline phosphate repeating units (8). More often the occurrence of ribitol has been described in wall teichoic acids of some bacilli, staphylococci, and lactobacilli (5, 7, 10). However, we do not believe the observed ribitol is due to a contamination with teichoic acids. HIC has been shown to effectively separate LTAs from polyanionic contaminants (12).
Poly(glycerophosphate)s are often substituted by glucosyl and d-alanine residues (13). In ATCC 15808, glucose was exclusively found in the glycolipid anchors, since no glycosylglycerol was detected in GLC. Only 10 to 20% of the glycerol residues were substituted with d-alanine, which is considerably less than with LTAs from Lactobacillus casei or Lactobacillus helveticus (13).
LTAs and phage inactivation assays.
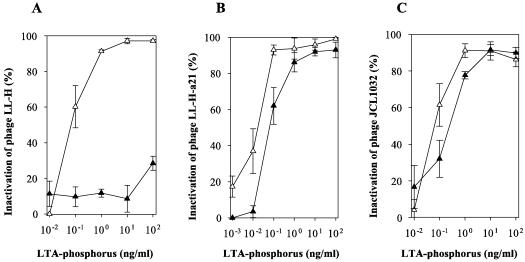
LTAs were tested for their ability to inactivate the L. delbrueckii phages listed in Table 1. The observation that both the LTA pools (II and III) of ATCC 15808 were able to inactivate the phage LL-H allowed us to use the LTAs obtained with the faster purification procedure. Usually more than 90% of phages (106 PFU/ml) were inactivated during the first 5 to 10 min of incubation at 37°C when a concentration of 1 ng of LTA-phosphorus per ml was used (data not shown). Minimum concentrations of LTAs needed for significant inactivation (≥50%) of different L. delbrueckii phages were determined by incubating phages (106 PFU/ml) with various concentrations of the LTAs in 20-min incubations (Fig. 2).
FIG. 2.
Inactivation of L. delbrueckii subsp. lactis phages with purified LTAs derived from L. delbrueckii subsp. lactis strain ATCC 15808 (▵) and Ads-5 (▴). The data represent the means ± the standard errors from three independent experiments. Phage (106 PFU) was incubated with 100 to 1 pg of LTA-phosphorus in 1 ml of 10 mM Tris-HCl [pH 7.0] supplemented with 10 mM MgCl2 at 37°C for 20 min before plaque assay (24).
Minimum dosages for inactivation of LL-H and JCL1032 were estimated as 100 pg of LTA-phosphorus of ATCC 15808 per ml (Fig. 2A and C). For LL-H-a21, the minimum dosage was closer to 10 than to 100 pg per ml (Fig. 2B). Approximately fivefold more LTA-phosphorus of Ads-5 was needed to significantly reduce the plaque formation of LL-H-a21 or JCL1032 (Fig. 2B and C). The phage LL-H, instead, was not inactivated even by 103-fold dosages of LTA-phosphorus of Ads-5 (Fig. 2A). Despite the overall genome homology between LL-H and mv4 (2), mv4 was not inactivated by any of the tested LTAs (data not shown).
The most prominent feature in these assays was the lack of LL-H inactivation with the LTAs of Ads-5 contrary to efficient inactivation of LL-H-a21. As far as we know, LL-H-a21 differs from LL-H only by a single amino acid in the receptor binding protein Gp71, thus suggesting that the specificity of inactivation reactions resides in the Gp71-LTA interaction (23). Like LL-H-a21, JCL1032 reacted with the LTAs examined in this study, although more LTAs were needed for significant inactivation. This could be an indication of JCL1032 interaction with a different kind of structural feature of LTAs. Comparative chemical and physical analyses on the LTA structures are required to reveal the crucial structural feature(s) of LTAs responsible for the specific interactions with L. delbrueckii subsp. lactis phages.
We have previously suggested that there are at least three types of receptors for L. delbrueckii phages: two specific for LL-H and its host range mutant LL-H-a21 and one specific for the phage JCL1032 (23). In this study, we demonstrate that these L. delbrueckii phages are inactivated by purified LTAs from L. delbrueckii subsp. lactis in a manner that is consistent with their behavior with intact cells.
Acknowledgments
We thank Franz Fiedler (Munich, Germany) for the opportunity to analyze our LTA samples in his laboratory.
This study was supported by the grants from the Academy of Finland (SA 46921), EC Biotech II program (BIO4-CT98-0406), the National Technology Agency in Finland (Tekes 70062/01), and by a personal grant from the University of Oulu to L.R.
REFERENCES
- 1.Alatossava, T., and M. J. Pyhtilä. 1980. Characterization of a new Lactobacillus lactis bacteriophage. IRCS Med. Sci. 8:297-298. [Google Scholar]
- 2.Alatossava, T., P. Forsman, M. Mikkonen, L. Räisänen, and A. Vasala. 1998. Molecular genetics and evolution of Lactobacillus phage LL-H and its related phages. Recent Res. Dev. Agric. Biol. Chem. 2:345-360. [Google Scholar]
- 3.Albersheim, J. W., D. J. Nevins, P. D. English, and A. Karr. 1967. A method for the analysis of sugars in plant cell wall polysaccharide by gas liquid chromatography. Carbohydr. Res. 5:340-345. [Google Scholar]
- 4.Ames, B. N. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8:115-118. [Google Scholar]
- 5.Amstrong, J. J., J. Baddiley, J. G. Buchanan, A. L. Davison, M. V. Kelemen, and F. C. Neuhaus. 1959. Teichoic acids from bacterial walls. Composition of teichoic acids from a number of bacterial cell walls. Nature 184:247-248. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, A. J., R. S. Green, and A. R. Archibald. 1977. Specific determination of ribitol teichoic acid in whole bacteria and isolated walls of Bacillus subtilis W 23. Carbohydr. Res. 57:c7-c10. [DOI] [PubMed] [Google Scholar]
- 7.Baddiley, J., and A. L. Davison. 1961. The occurrence and location of teichoic acids in lactobacilli. J. Gen. Microbiol. 24:295-299. [DOI] [PubMed] [Google Scholar]
- 8.Behr, T., W. Fischer, J. Peter-Katalinić, and H. Egge. 1992. The structure of pneumococcal lipoteichoic acid. Improved preparation, chemical and mass spectrometric studies. Eur. J. Biochem. 207:1063-1075. [DOI] [PubMed] [Google Scholar]
- 9.Coyette, J., and J.-M. Ghuysen. 1968. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. IX. Teichoic acid and phage adsorption. Biochemistry 7:2385-2389. [DOI] [PubMed] [Google Scholar]
- 10.Davison, A. L., and J. Baddiley. 1963. The distribution of teichoic acids in staphylococci. J. Gen. Microbiol. 32:271-276. [DOI] [PubMed] [Google Scholar]
- 11.Douglas, L. J., and M. J. Wolin. 1971. Cell wall polymers and phage lysis of Lactobacillus plantarum. Biochemistry 10:1551-1555. [DOI] [PubMed] [Google Scholar]
- 12.Fischer, W., H. U. Koch, and R. Haas. 1983. Improved preparation of lipoteichoic acids. Eur. J. Biochem. 133:523-530. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, W. 1988. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29:233-301. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, W., T. Mannsfeld, and G. Hagen. 1990. On the basic structure of poly(glycerophosphate) lipoteichoic acids. Biochem. Cell Biol. 68:33-43. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, W. 1993. Molecular analysis of lipid macroamphiphiles by hydrophobic interaction chromatography, exemplified with lipoteichoic acids. Anal. Biochem. 208:49-56. [DOI] [PubMed] [Google Scholar]
- 16.Forsman, P., and T. Alatossava. 1991. Genetic variation of Lactobacillus delbrueckii subsp. lactis bacteriophages isolated from cheese processing plants in Finland. Appl. Environ. Microbiol. 57:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsman, P. 1993. Characterization of a prolate-headed bacteriophage of Lactobacillus delbrueckii subsp. lactis, and its DNA homology with isometric-headed phages. Arch. Virol. 132:321-330. [DOI] [PubMed] [Google Scholar]
- 18.Garbutt, K. C., J. Kraus, and B. L. Geller. 1997. Bacteriophage resistance in Lactococcus lactis engineered by replacement of a gene for a bacteriophage receptor. J. Dairy Sci. 80:1512-1519. [Google Scholar]
- 19.Glaser, L., H. Ionesco, and P. Schaeffer. 1966. Teichoic acids as components of a specific phage receptor in Bacillus subtilis. Biochim. Biophys. Acta 124:415-417. [DOI] [PubMed] [Google Scholar]
- 20.Heckles, J. E., and M. Virji. 1988. Separation and purification of surface components, p. 67-133. In I. Hancock and I. Poxton (ed.), Bacterial cell surface techniques. John Wiley & Sons, Bath, Great Britain.
- 21.Lahbib-Mansais, Y., B. Boizet, L. Dupont, M. Mata, and P. Ritzenthaler. 1992. Characterization of a temperate bacteriophage of Lactobacillus delbrueckii subsp. bulgaricus and its interactions with the host cell chromosome. J. Gen. Microbiol. 138:1139-1146. [Google Scholar]
- 22.Mikkonen, M., L. Räisänen, and T. Alatossava. 1996. The early gene region completes the nucleotide sequence of Lactobacillus delbrueckii subsp. lactis phage LL-H. Gene 175:49-57. [DOI] [PubMed] [Google Scholar]
- 23.Ravin, V., L. Räisänen, and T. Alatossava. 2002. A conserved C-terminal region in Gp71 of the small isometric-head phage LL-H and ORF474 of the prolate-head phage JCL1032 is implicated in specificity of adsorption of phage to its host, Lactobacillus delbrueckii. J. Bacteriol. 184:2455-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarimo, S. S., M. Hartiala, and L. Aaltonen. 1976. Preparation and partial characterization of a Lactobacillus lactis bacteriophage. Arch. Microbiol. 107:193-197. [DOI] [PubMed] [Google Scholar]
- 25.Schubert, K., D. Reiml, J.-P. Accolas, and F. Fiedler. 1993. A novel type of meso-diaminopimelic acid-based peptidoglycan and novel poly(erythritol phosphate) teichoic acids in cell walls of two coryneform isolates from the surface flora of French cooked cheeses. Arch. Microbiol. 160:222-228. [DOI] [PubMed] [Google Scholar]
- 26.Sijtsma, L., J. T. M. Wouters, and K. J. Hellingwerf. 1990. Isolation and characterization of lipoteichoic acid, a cell envelope component involved in preventing phage adsorption, from Lactococcus lactis subsp. cremoris SK110. J. Bacteriol. 172:7126-7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sijtsma, L., A. Sterkenburg, and J. T. M. Wouters. 1988. Properties of the cell walls of Lactococcus lactis subsp. cremoris SK110 and SK112 and their relation to bacteriophage resistance. Appl. Environ. Microbiol. 54:2808-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valyasevi, R., W. E. Sandine, and B. L. Geller. 1990. The bacteriophage kh receptor of Lactococcus lactis subsp. cremoris KH is the rhamnose of the extracellular wall polysaccharide. Appl. Environ. Microbiol. 56:1882-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendlinger, G., M. J. Loessner, and S. Scherer. 1996. Bacteriophage receptor on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142:985-992. [DOI] [PubMed] [Google Scholar]
- 30.Young, F. E. 1967. Requirement of glycosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc. Natl. Acad. Sci. USA 58:2377-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]