ABSTRACT
Tumor stroma is characterized by abnormal accumulation of dense fibrillar collagen, which promotes tumor progression and metastasis. However, the effect of desmoplastic collagen on cells has been unclear. Our recent findings demonstrate that dense fibrillar collagen activates a novel phosphosignaling mechanism for robust induction of invadopodia in tumor cells and normal fibroblasts.
Keywords: Cell-matrix interactions, dense fibrillar collagen, desmoplasia, extracellular matrix, invadopodia, invadosomes, integrin, metastasis
Advanced stages of cancers are characterized by desmoplasia, an extensive deposition of fibrillar collagens in the extracellular matrix (ECM) surrounding the tumor. This dense network of fibrillar collagen, particularly collagen type I, promotes tumor progression and metastasis, and correlates with low survival rates for cancer patients.1 However, a mechanistic link between desmoplastic collagen accumulation and its effect on tumor cell invasive properties is poorly understood.
There is increasing experimental evidence implicating invadosomes, which include both malignant cell invadopodia and normal cell podosomes, in local ECM degradation and invasive migration of cells.2 Invadopodia are dynamic microscopic protrusions of tumor cell membranes that are the sites of cell–ECM adhesion, controlled actin polymerization, and membrane remodeling due to directed endo/exocytosis, as well as localized delivery of proteases required for ECM degradation.3,4 The molecular mechanisms of invadopodia formation and function in ECM degradation have been extensively studied under conditions of tumor cell interaction using artificial ECMs, such as gelatin or polyacrylamide coated with globular fibronectin. Recently, invadopodia have been investigated with respect to tumor cell interactions with naturally occurring loose fibrillar collagen and isolated intact basement membrane. Invadopodia assemble upon adhesion of tumor cells to the ECM. Intravital imaging of mouse mammary tumors detected invadopodia formation in cancer cells contacting single collagen fibers.5 Loose fibrillar collagen was also shown to induce linear arrays of invadopodia by engaging the collagen receptor DDR1 independently from integrin collagen receptors.6 In addition, studies with gelatin and polyacrylamide matrices demonstrated that matrix rigidity can have stimulatory effects on invadopodia and its degrading activity via a myosin II-FAK-Cas pathway.7 However, there has been a major gap in our understanding of how desmoplastic collagen promotes metastasis or what role it may play in invadopodia induction.
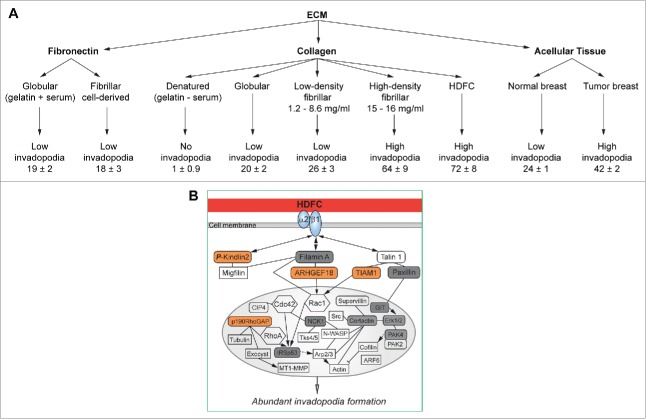
We have isolated desmoplastic collagen by decellularizing human tumor tissues and demonstrated that it stimulates high numbers of invadopodia in cancer cells.8 We have also developed an in vitro high-density fibrillary collagen (HDFC) matrix consisting of a densely packed network of fibrillar collagen type I compacted by centrifugation to closely mimic desmoplastic collagen.8 Direct comparisons of invadopodial response to a variety of ECMs revealed that dense fibrillar collagen is a superior inducer of invadopodia in a variety of tumor cell lines (Fig. 1, A).8 Interestingly, the induction of abundant invadopodia by HDFC was found to be independent of growth factor stimulation.8 Moreover, HDFC not only stimulated invadopodia formation in transformed cancer cells, but also elicited high numbers of functional invadopodia in normal human fibroblasts.8 Thus, we established that desmoplastic collagen can activate both tumor cells and stromal fibroblasts to proteolytically degrade and remodel the surrounding ECM, thus contributing to local invasion.
Figure 1.
High density fibrillar collagen (HDFC) is a potent inducer of invadopodia. (A) Effect of different extracellular matrices (ECMs) on invadopodia in MDA-MB-231 carcinoma cells. Invadopodia are quantified as mean number of invadopodia per cell ± standard error of the mean. (B) Schematic representation of the signaling network sustaining the abundant-invadopodia phenotype in MDA-MB-231 cells on HDFC. Key levels of the signaling network are highlighted: α2β1 integrin, an HDFC receptor; serine-phosphorylated kindlin2, filamin A, and talin1 in the integrin regulatory module; ARHGEF18/Rho/Rac guanine nucleotide exchange factor 18 and TIAM1/T-cell lymphoma invasion and metastasis 1, regulators of Rac1 activity; and invadopodial proteins regulating actin polymerization and MT1-MMP protease trafficking at invadopodia. All phosphoproteins identified in comparative phosphoproteomics studies are depicted in round-cornered boxes: orange boxes = HDFC-unique phosphoproteins; gray boxes = phosphoprotein phospho-sites differing in HDFC vs. gelatin; and open boxes = phosphoprotein phospho-sites similar in HDFC and gelatin. Rac1, Cdc42, RhoA, tubulin, exocyst, MT1-MMP, Src, N-WASP (Wiskott–Aldrich Syndrome protein), Tks4/5, actin, cofilin, and ARF6 (ADP-ribosylation factor 6) are shown to clarify the signaling context of the proteins identified by phosphoproteomics. Abbreviations: CIP4, Cdc42-interacting protein 4; Erk1/2, mitogen-activated protein kinase 1/2; GIT, G protein-coupled receptor kinase interacting ArfGAP 1; IRSp53, BAI1-associated protein 2; NCK1, NCK adaptor protein 1; p190RhoGAP, Rho GTPase activating proteins 5 and 35; PAK2/4, p21 protein (Cdc42/Rac)-activated kinase 2/4.
The effect of HDFC on invadopodia upregulation cannot be simply explained by increases in stiffness of the collagen matrix resulting from a high collagen concentration. We found that the stiffness of HDFC is close to that of rigid “gelatin cushions”7 and much lower than that of conventional thin 2-dimensional gelatin matrices coated on glass.8 However, HDFC greatly outperforms both gelatin cushions and matrices in inducing an invadopodial response.8 Although fibronectin is another ECM ligand that is highly upregulated in cancers, we found that fibrillar fibronectin fails to elicit abundant invadopodia compared to HDFC.8 The density of the fibrillar collagen is an important regulator of the invadopodia induction response because only thin 3-dimensional collagen matrices polymerized at high concentrations of collagen (15–16 mg/mL) were able to recapitulate abundant invadopodia formation similar to that induced by HDFC or acellular tumor collagen extracts.8
We found that functional α2β1 integrin, a fibrillar collagen receptor,9 was required for abundant invadopodia induction by HDFC.8 Tumor cells required no changes in gene or protein expression to switch between 2 modes of interaction with the ECM: cell adhesion to gelatin under conditions of suppressed invadopodia formation and adhesion to HDFC with extensive invadopodia-mediated collagen degradation.8 We identified a complex integrin signaling network regulated by phosphorylation that governs the potent invadopodial response induced by HDFC (Fig. 1, B).8 Phosphoproteomics analysis identified unique HDFC regulators of downstream integrin signaling.8 One such novel regulator of invadopodia specific to dense fibrillar collagen was kindlin2, also known as mitogen-inducible gene 2 (MIG-2) or fermitin family homolog 2 (FERMT2). Kindlin2 is known to be an essential activator of integrin function.10 We found that kindlin2 preferentially localized to invadopodia induced by HDFC, but not by gelatin/fibronectin, and was required for invadopodia formation and function in the degradation of dense collagen in multiple cell lines.8 Moreover, we established that kindlin2 function was regulated by phosphorylation; expression of kindlin2 dominant-negative phospho-mutants suppressed invadopodia formation, whereas expression of phosphomimetic mutants resulted in invadopodia upregulation.8
In summary, our recent findings underscore the importance of ECM in the regulation of invadopodia formation in normal and tumor cells, and identify desmoplastic collagen as a potent inducer of invadopodia and a regulator of cell invasive potential. These findings open new avenues of further investigation, such as: (1) understanding the molecular differences in the structures of invadopodia and the molecular mechanisms driving invadopodia on different ECMs that could provide common therapeutic targets and approaches to control invadopodia formation; (2) identifying molecular switches controlling invadopodia formation in normal fibroblasts with the goal of altering and controlling tumor stroma; and (3) establishing molecular mechanisms of kindlin2 activation by phosphorylation and its role in integrin activation, as well as testing the possibility of targeting kindlin2 to control tumor metastasis. In addition, our comparative studies on invadopodia using HDFC versus gelatin provide compelling evidence for the need to use in vitro models that accurately mimic desmoplastic collagen when developing and testing pharmaceutical inhibitors of tumor invasion and metastasis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Pathway to Independence Award K99CA129205 from NCI with American Recovery and Reinvestment Act of 2009 award to V.V.A. and NIH/NIDCR Intramural project ZIA DE 000719 to Kenneth M. Yamada.
References
- 1.Conklin MW, Keely PJ. Why the stroma matters in breast cancer: insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adh Migr 2012 May-Jun; 6(3):249-60; PMID:22568982; http://dx.doi.org/ 10.4161/cam.20567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol 2011; 27:185-211; PMID:21801014; http://dx.doi.org/ 10.1146/annurev-cellbio-092910-154216 [DOI] [PubMed] [Google Scholar]
- 3.Jacob A, Prekeris R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol 2015 Feb 2; 3:4; PMID:25699257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res 2006 Mar 15; 66(6):3034-43; PMID:16540652; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2177 [DOI] [PubMed] [Google Scholar]
- 5.Gligorijevic B, Bergman A, Condeelis J. Multiparametric classification links tumor microenvironments with tumor cell phenotype. PLoS Biol 2014 Nov 11; 12(11):e1001995; PMID:25386698; http://dx.doi.org/ 10.1371/journal.pbio.1001995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juin A, Di Martino J, Leitinger B, Henriet E, Gary AS, Paysan L, Bomo J, Baffet G, Gauthier-Rouvière C, Rosenbaum J, et al.. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol 2014 Nov 24; 207(4):517-33; PMID:25422375; http://dx.doi.org/ 10.1083/jcb.201404079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol 2008 Sep 9; 18(17):1295-9; PMID:18718759; http://dx.doi.org/ 10.1016/j.cub.2008.07.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artym VV, Swatkoski S, Matsumoto K, Campbell CB, Petrie RJ, Dimitriadis EK, Li X, Mueller SC, Bugge TH, Gucek M, Yamada KM. Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J Cell Biol 2015 Feb 2; 208(3):331-50; PMID:25646088; http://dx.doi.org/ 10.1083/jcb.201405099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sottnik JL, Daignault-Newton S, Zhang X, Morrissey C, Hussain MH, Keller ET, Hall CL. Integrin alpha2beta 1 (α2β1) promotes prostate cancer skeletal metastasis. Clin Exp Metastasis 2013 Jun; 30(5):569-78; PMID:23242739; http://dx.doi.org/ 10.1007/s10585-012-9561-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 2003 Apr 4; 113(1):37-47; PMID:12679033; http://dx.doi.org/ 10.1016/S0092-8674(03)00163-6 [DOI] [PubMed] [Google Scholar]