Abstract
In a recent article in Cell Stem Cell, we showed that mesenchymal stem cells (MSCs), progenitor cells that populate the breast tumor stroma, induce microRNA-mediated FOXP2 repression in breast cancer cells (BCCs), thus promoting cancer stem cell (CSC) and metastatic traits. Here, we discuss the implications of these findings for understanding metastatic CSC genesis.
Keywords: breast, cancer stem, forkhead, FOXP2, metastasis, microRNA, tumor initiation, tumor progression
The root causes underlying the progression of local neoplasia to metastatic disease remain largely undetermined. Recent advances suggest that only a subset of cancer cells is able to complete the metastasis cascade and successfully initiate secondary tumors. Such unique and rare cells, called “cancer stem cells” (CSCs), are described to possess self-renewing and differentiation capabilities that approximate those of normal stem cells, and to exhibit enhanced malignant features in addition to metastasis, such as therapy resistance.1 Mounting evidence suggests that CSCs emerge from highly dynamic cellular differentiation states that can be critically regulated by the tumor microenvironment (TME). Indeed, the interactions of cancer cells with components of the tumor stroma have been shown to robustly influence tumor initiation and progression.2 At this time, however, how the TME regulates metastatic breast CSC phenotypes remains poorly understood.
We have previously demonstrated that tumor-associated mesenchymal stem cells (MSCs),3,4 a group of ambulatory multipotent fibroblastic progenitors involved in tissue maintenance and wound healing,5 populate the TME and cause robust propagation of CSC traits in breast cancer cells (BCCs), concomitant with profound induction of overall tumor metastasis. To elucidate the heterotypic interactions that MSCs establish with BCCs in the context of CSC generation and metastasis, we performed molecular analysis of MSC-stimulated BCCs with special emphasis on changes in their microRNA (miR/miRNA) landscape. We found that MSCs induced microRNAs from the 199a–214 cluster, which enhanced CSC features such as tumor initiation using limiting dilution analyses, as well as metastatic properties. Interestingly, we further found that miR-199a–214 led a network of 4 other microRNAs (miR-762, miR-1915, let-7b, and miR-34a), that converged on and repressed the expression of FOXP2, a forkhead transcription factor associated with differentiation in development, as well as vocalization and speech.6 FOXP2 knockdown in BCCs was sufficient to promote CSC propagation, tumor initiation, and metastasis. Notably, we found that suppression of FOXP2 and elevation of miR-199a expression characterized malignant clinical breast cancer, associating significantly with poor patient outcome.7
Our observations describe the involvement of FOXP2 and its upstream regulatory network in the microenvironmental regulation of metastatic breast CSCs and evoke multiple conceptual advances that are highly pertinent to our basic understanding of breast cancer pathogenesis:
Metastatic CSCs can be created. Although the contributions of breast CSCs to the growth and differentiation of neoplastic cells have received much attention, the molecular mechanisms underlying the genesis and maintenance of metastatic CSCs have not been fully explored. Our ability to induce, propagate, and maintain CSCs from non-CSC populations by (a) exposing BCCs to MSCs, (b) up-regulating miR-199a, or (c) silencing FOXP2, suggests that metastatic CSCs are not necessarily born from the tumorigenic transformation of tissue stem cells, but are made through contextual signals received by more differentiated BCCs.
Reversible epigenetics at the root of metastasis. Our studies indicate that metastatic BCCs that have contacted MSCs retain a ‘molecular memory’ of their experience for a finite period of time before presumably reverting back to their original state(s). Indeed, 2 of the major metastatic phenotypes imparted to MSC-stimulated BCCs—EMT8 and CSC-ness7—would need to be reversed (via MET and/or differentiation) to allow regrowth of cells as secondary colonies. Such temporal and plastic processes work at the epigenetic level in developmental contexts, and BCCs are likely utilizing these same programs.9 These notions are in agreement with DNA sequencing and barcoding experiments, which indicate that primary tumors and their distant metastases are often genetically identical and implicate that reversible epigenetic changes are central to navigating metastasis.10 Indeed, the metastatic cascade is fraught with dissimilar obstacles that must be overcome by disseminated tumor cells (DTCs), and it becomes difficult to imagine that accumulation of permanent genetic events would provide the flexibility necessary for such cells to successfully navigate invasion, dissemination, survival, and colonization.
TWIST(s) in metastasis. TWIST is critical in the regulation of MSC-induced and miR-mediated FOXP2 silencing. Indeed, TWIST activation, which is triggered by MSCs, was sufficient to induce miR-199a expression, whereas its inhibition crippled MSC-triggered miR-199a expression or FOXP2 downregulation. These activities of TWIST are reminiscent of its similar roles in regulating MSC-stimulated and lysyl-oxidase (LOX)-mediated EMT in BCCs.8 In this regard, TWIST caused upregulation of LOX, and MSCs were unable to promote LOX expression or EMT in BCCs devoid of TWIST. Despite this, we found that LOX was unable to enhance CSC markers or induce the miR-FOXP2 pathway, suggesting that LOX must enhance metastasis by conveying motility/invasion (EMT) properties to CSC populations. This leads to a model in which a molecular bifurcation of EMT- and CSC-regulating pathways exists downstream of TWIST, and the interplay of seemingly separate pathways leads to the functional propagation of metastatic traits in MSC-activated BCCs (Fig. 1).
FOXP2 as a potential novel tumor/metastasis suppressor. FOXP2 functions as a transcriptional repressor that regulates speech and learning in humans. The observation that it is the focal target of a converging prometastatic MSC-regulated miR network suggests that FOXP2 performs important cellular functions that are heavily regulated by redundant mechanisms. Moreover, the findings that downregulation of FOXP2 triggers tumor initiation, and that it represents a feature of both tumorigenic versus benign, as well as malignant versus in situ breast cancers, underscore the newly discovered functions of FOXP2 as a putative tumor/metastasis suppressor in breast cancer.
Figure 1.
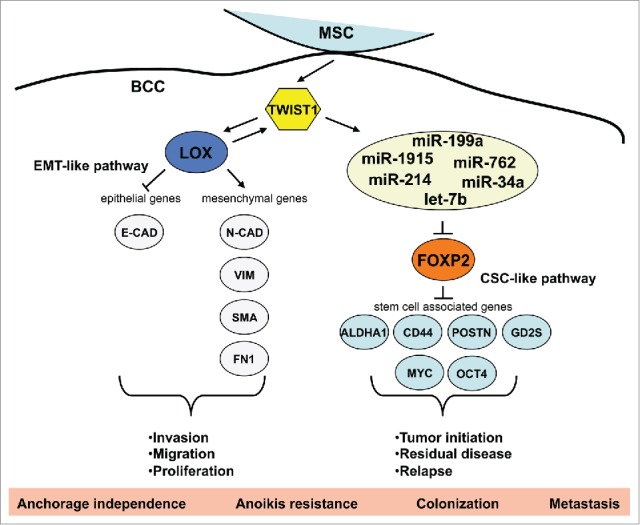
Divergent pathways in MSC-supported metastasis. Tumor-associated mesenchymal stem/stromal cells (MSCs) trigger TWIST-dependent pathways in breast cancer cells (BCCs). These pathways, mediated by lysyl oxidase (LOX) or the indicated microRNA (miR) network, may alternate temporally in BCC-contacted cells or may represent pathways that are triggered in separate BCC populations as they react differentially to MSC influences. The collaboration of such epithelial-mesenchymal transition (EMT)-like and cancer-stem-cell (CSC)-like events leads to overall enhancement of tumor metastasis. ALDHA1, aldehyde dehydrogenase A1; E-CAD, E-cadherin; FN1, fibronectin; GD2S, GD2 synthase; N-CAD, N-cadherin; OCT4, octamer-binding transcription factor 4; POSTN, periostin; SMA, smooth muscle actin; VIM, vimentin.
Much remains to be elucidated regarding the MSC-miRNA-FOXP2 pathway. For example, the initiating event(s) upstream of TWIST1 activation leading to miR-199a upregulation remain unknown. It is possible that such mechanisms involve ECM- or membrane-initiated cascades operating at the MSC:BCC interface, akin to the HA–CD44 interactions that we described previously.8 Such cell:cell interactions, together with the aforementioned miRs, would represent tractable therapeutic targets in the management of malignant breast cancers. Certainly, there is much to learn about the newly described miR-FOXP2 pathway in the pathogenesis of breast cancer, knowledge that will likely yield to new advances toward the goal of eradicating relapse-promoting CSCs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work in the Karnoub laboratory is supported by grants from the Sidney Kimmel Cancer Research Foundation (AEK) and the Susan Komen For The Cure (AEK), start-up funds from Beth Israel Deaconess Medical Center (AEK), and by a postdoctoral fellowship from the American Cancer Society (BGC).
References
- 1.Kreso A, Dick JE.. Evolution of the cancer stem cell model. Cell Stem Cell 2014; 14(3):275-91; PMID:24607403; http://dx.doi.org/ 10.1016/j.stem.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 2.Quail DF, Joyce JA.. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19(11):1423-37; PMID:24202395; http://dx.doi.org/ 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449(7162):557-63; PMID:17914389; http://dx.doi.org/ 10.1038/nature06188 [DOI] [PubMed] [Google Scholar]
- 4.Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh Migr 2012; 6(3):220-30; PMID:22863739; http://dx.doi.org/ 10.4161/cam.20875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prockop DJ, Oh JY.. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther 2012; 20(1):14-20; PMID:22008910; http://dx.doi.org/ 10.1038/mt.2011.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam EW, Brosens JJ, Gomes AR, Koo CY.. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev. Cancer 2013; 13(7):482-95; PMID:23792361; http://dx.doi.org/ 10.1038/nrc3539 [DOI] [PubMed] [Google Scholar]
- 7.Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, Lien EC, Bhasin MK, Raimo M, Hanson SE, Marusyk A, et al.. MSC-Regulated MicroRNAs Converge on the Transcription Factor FOXP2 and Promote Breast Cancer Metastasis. Cell Stem Cell 2014; 15(6):762-74; PMID:25515522; http://dx.doi.org/ 10.1016/j.stem.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 8.El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM, Csizmadia E, Mariani O, Zhu C, Campagne A, Toner M, et al.. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci U S A 2012; 109(43):17460-5; PMID:23033492; http://dx.doi.org/ 10.1073/pnas.1206653109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brabletz T. To differentiate or not-routes towards metastasis. Nat Rev Cancer 2012; 12(6):425-36; PMID:22576165; http://dx.doi.org/ 10.1038/nrc3265 [DOI] [PubMed] [Google Scholar]
- 10.Vanharanta S, Massague J.. Origins of metastatic traits. Cancer Cell 2013; 24(4):410-21; PMID:24135279; http://dx.doi.org/ 10.1016/j.ccr.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]


