Abstract
Chronic exposure of epithelial cells to high levels of bone morphogenetic protein 2 (BMP2) has recently been demonstrated to initiate stem cell transformation toward a luminal tumor-like phenotype through a BMP2–BMPR1B-dependent mechanism. Carcinogen-driven deregulation of the stem cell niche could therefore represent a driving force to promote transformation and dictate the ultimate breast tumor subtype.
Keywords: bisphenol, BMP, BMPR1B, carcinogen, epithelial stem cell, breast tumor, niche, pollutant
Although current data implicate bone morphogenetic proteins (BMPs) in late stages of tumorigenesis and metastasis, we demonstrated that BMP2 also plays a role in promoting the transformation process of immature cells at very early stages.1 Using a model of immature cells (MCF10-A cells), we have shown both in vitro and in vivo that chronic exposure of epithelial cells to BMP2 promotes their malignant transformation in an inflammatory context that is mimicked by interleukin 6 (IL6). Using an Affymetrix dataset together with transcript analysis of freshly isolated tumor cells, we established that BMP2/IL6-transformed cells display a luminal breast tumor-like phenotype. We identified bone morphogenetic protein receptor type 1B (BMPR1B) as the main BMP receptor expressed by transformed MCF10-A cells and by human luminal tumors. Although the role of BMPR1B in breast tumors remains unclear, we confirmed that it has a function specific to luminal tumors extending from the earliest stages up to established tumors and might constitute a potent biological marker of tumor progression. We identified small mothers against decapentaplegic 5 (SMAD5), GATA binding protein 3 (GATA3), and the forkhead box A1 and C1 (FOXA1/FOXC1) balance as potential BMP2 targets involved in the luminal epithelial cell transformation process. Our data suggest that upon binding of BMP2 to the BMPR1B receptor, epithelial stem cells upregulate SMAD5. Interestingly, BMP/SMAD5 signals negatively regulate the NODAL pathway that controls the expression of FOXH1, an activator of FOXC1 transcription.2 SMAD5 could then replace SMAD1 in SMAD4 complexes, leading to repression of the FOXH1 promoter and a rapid decrease in FOXC1 transcription. Synergistically, SMAD5 complexes could directly induce GATA3 expression,3 also repressing FOXC1. Expression of estrogen-dependent genes would then commence and promote luminal epithelial cell comitment.
We showed that the BMPR1B ligands BMP2 and BMP4 are present in the normal mammary gland, where they function differentially and regulate stem/progenitor cell fate.1 Using primary human breast stem cells, we confirmed that only BMP2 expression is able to commit cells to differentiation and expansion of the luminal progenitor compartment, according to the role of BMPs in the development of the luminal lineage of murine mammary gland (summarized in Fig. 1 upper panel).4 Interestingly, we previously reported that, despite their strong homology and similar functions in mice, BMP2 and BMP4 regulate the human hematopoietic system differently, with BMP2 being involved in erythroid lineage and BMP4 in stem cell amplification and megakaryocyte lineage.5,6 Our data in both systems thus reveal that BMP2 seems to preferentially affect lineage-committed progenitors, whereas BMP4 has broader effects on stem cells and phenotypically related cells (megakaryocytic or myoepithelial progenitors). However, it is not known how these molecules distinctly signal in immature human cells to determine cell fate decisions.
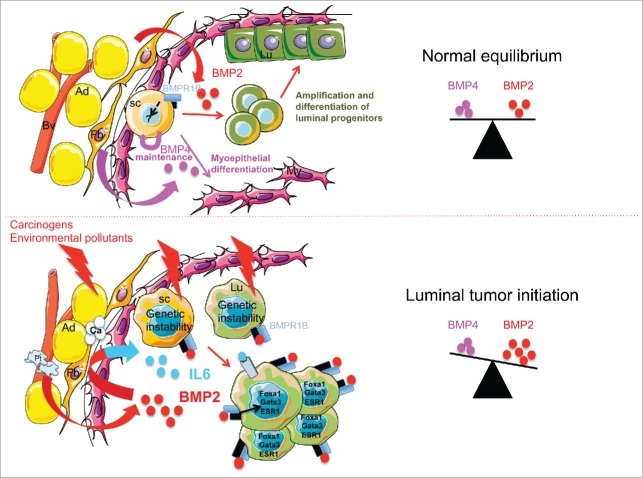
Figure 1.
Proposed mechanism for bone morphogenetic protein (BMP) function to maintain normal equilibrium and how this this equilibrium is disrupted by environmental pollutants. In normal breast, stem cells (SC) reside in a niche composed of adipocytes (Ad), fibroblasts (Fb), and blood vessels (Bv), that provide cytokines such as BMP2 and BMP4 to maintain normal equilibrium and tissue homeostasis. By binding to bone morphogenetic protein receptor 1B (BMPR1B), BMP2 regulates the amplification and differentiation of luminal progenitors (Lu) whereas BMP4 seems to maintain stem cells and regulate the myoepithelial lineage (My). Downstream intracellular pathways involved in these mechanisms remain to be deciphered. Over the course of a lifetime, the tissue is exposed to a number of pollutants affecting the cellular components of the niche and the different mammary cell types. This can lead to an intrinsic genetic instability among stem cells and progenitors but also to altered extrinsic signals from the niche. BMP2 secretion is increased mainly around blood vessels (Bv) by endothelial and recruited platelets (Pt). A local high concentration of BMP2 leads to increased inflammation and IL6 production from the niche and affects differentiation of stem cells and progenitors via activation of intrinsic signaling pathways. This process contributes to the emergence of cells with luminal features such as high levels of forkhead box A1 (FOXA1), GATA binding protein (GATA3), and estrogen-related genes (estrogen receptor 1 ESR1), and high levels of BMPR1B. Disequilibrium of BMP2 levels might also lead to changes in the niche structure such as local microcalcification (Ca), another early step in the emergence of breast luminal tumors.
The luminal progenitors compartment has been reported to have enhanced susceptibility to oncogenic events that might confer more genetically unstable features and increase BMP-specific targets such as BMPR1B to control stem cell fate.7 The evolution toward luminal tumors might then be driven by specific microenvironmental disruption of available amounts of soluble BMP2. Binding of BMP2 to BMPR1B rapidly induces sustained signaling involving GATA3 and a change in the FOXA1/FOXC1 balance, leading to expansion of the luminal immature progenitors compartment that is further transformed through BMPR1B-dependent signaling.1 Therefore, it is possible that upon BMP2 signaling, transformation arises either from a stem/basal cell that first engages toward the luminal lineage before proliferation and further progress, or directly emerges from an already genetically altered committed luminal progenitor. Although based on our findings we cannot rule out either of these 2 hypotheses, we revealed the key active role that BMP2 can play in early phases of epithelial cell transformation.
Irrespective of the impact of BMP2 on luminal lineage commitment, analysis of normal and tumor tissue indicated that tumor cells themselves are the target rather than the origin of BMP2 overproduction.1 The transformation process may either lead to the loss of BMP2 expression in mammary epithelial cells or may occur in an epithelial cell that does not produce BMP2. We suspected that transforming agents that perturb signaling by niche components lead to local accumulation of BMP2 and IL6. We showed that radiation or environmental pollutants such as bisphenol A (BPA), a contaminant of food and drink through the use of plastic containers, and its substitute bisphenol S (BPS) are able to shift the balance of secreted BMP molecules in favor of BMP2. This seems to happen more frequently in individuals susceptible to developing breast cancer.1 Furthermore, IL6 secretion by stromal cells can be induced by BPA8 and BMP2,9 suggesting a possible feedback loop that maintains transforming conditions. Accordingly, the high BMP2 staining detected in tumor endothelial cells revealed increased angiogenesis that resulted in a continuous influx of BMP-laden platelets, thereby sustaining local high concentrations of BMPs. Indeed, human platelets are a main source of BMPs5 and could contribute to an early local increase in the concentration of soluble BMP molecules within the pretumoral niche. In addition, a local increase in BMP2, which is directly involved in breast microcalcification,10 could explain the clinical observation that microcalcification, one of the earliest mammographic signs, is associated early on with an increased risk of breast cancer. Taking all these observations into account, it is tempting to speculate that many other environmental factors could promote the development of breast cancer through stromal BMP2 release as proposed in Figure 1 (lower panel). This makes environmental chemicals an important part of cancer causation, and an area that needs to be fully understood if we hope to take steps to reduce the incidence of cancer.
Consistent with the fact that distinct pathways often share a number of different regulators or targets to ensure the same biological or oncogenic function to induce malignant transformation, our results illustrate the power of the stem cell niche to deliver exogenous cues that promote transformation and dictate the ultimate breast tumor type. Clinically, it might therefore be more efficient to develop new strategies that simultaneously and synergistically affect exogenous and endogenous target pathways rather than to concentrate on single drug targets.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Sarah Kabani (CRCL) for excellent English proof reading assistance and Servier for providing images.
Funding
This study was funded by INSERM, Canceropôle Rhone-Auvergne (CLARA), La Ligue Nationale Contre le Cancer (Ain, Rhône), ARC (SFI20111203500) and partly by ANR (ANR-10-LABX-0061 and 2011 ANR-CESA-018-04) and Region Rhône-Alpes (CMIRA-COOPERA-12-004945-01, CMIRA-COOPERA 2014 OTP431681), INCA grants to V.M-S. PhD-fellowships for M.C were from the French government and ARC.
References
- 1.Chapellier M, Bachelard-Cascales E, Schmidt X, Clément F, Treilleux I, Delay E, Jammot A, Ménétrier-Caux C, Pochon G, Besançon R, et al. Disequilibrium of BMP2 Levels in the Breast Stem Cell Niche Launches Epithelial Transformation by Overamplifying BMPR1B Cell Response. Stem Cell Reports 2015; 4(2):239-54; PMID:25601208; http://dx.doi.org/ 10.1016/j.stemcr.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massague J, Xi Q. TGF-beta control of stem cell differentiation genes. FEBS Lett 2012; 586(14):1953-8; PMID:22710171; http://dx.doi.org/ 10.1016/j.febslet.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonilla-Claudio M, Wang J, Bai Y, Klysik E, Selever J, Martin JF. Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development 2012; 139(4):709-19; PMID:22219353; http://dx.doi.org/ 10.1242/dev.073197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsman CL, Ng BC, Heinze RK, Kuo C, Sergi C, Gopalakrishnan R, Yee D, Graf D, Schwertfeger KL, Petryk A. BMP-binding protein twisted gastrulation is required in mammary gland epithelium for normal ductal elongation and myoepithelial compartmentalization. Dev Biol 2013; 373(1):95-106; PMID:23103586; http://dx.doi.org/ 10.1016/j.ydbio.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeanpierre S, Nicolini FE, Kaniewski B, Dumontet C, Rimokh R, Puisieux A, Maguer-Satta V. BMP4 regulation of human megakaryocytic differentiation is involved in thrombopoietin signaling. Blood 2008; 112(8):3154-3163; PMID:18664625; http://dx.doi.org/ 10.1182/blood-2008-03-145326 [DOI] [PubMed] [Google Scholar]
- 6.Maguer-Satta V, Bartholin L, Jeanpierre S, Ffrench M, Martel S, Magaud JP, Rimokh R. Regulation of human erythropoiesis by activin A, BMP2, and BMP4, members of the TGFbeta family. Exp Cell Res 2003; 282(2):110-20; PMID:12531697; http://dx.doi.org/ 10.1016/S0014-4827(02)00013-7 [DOI] [PubMed] [Google Scholar]
- 7.Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, Mackay A, Grigoriadis A, Tutt A, Ashworth A, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 2010; 7(3):403-17; PMID:20804975; http://dx.doi.org/ 10.1016/j.stem.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 8.Ben Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. MolCell Endocrinol 2009; 304(1-2):49-54; PMID:19433247; http://dx.doi.org/ 10.1016/j.mce.2009.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyzy SL, Olivares-Navarrete R, Hutton DL, Tan C, Boyan BD, Schwartz Z. Microstructured titanium regulates interleukin production by osteoblasts, an effect modulated by exogenous BMP-2. Acta Biomater 2013; 9(3):5821-9; PMID:23123301; http://dx.doi.org/ 10.1016/j.actbio.2012.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Bloch N, Bhushan KR, De Grand AM, Tanaka E, Solazzo S, Mertyna PM, Goldberg N, Frangioni JV, Lenkinski RE. Humoral bone morphogenetic protein 2 is sufficient for inducing breast cancer microcalcification. MolImaging 2008; 7(4):175-86; PMID:19123988 [PMC free article] [PubMed] [Google Scholar]