ABSTRACT
The Hippo signaling effector Yes-associated protein (Yap) is known for its potent control of tissue growth. Our recent work now shows that Yap promotes regeneration in the intestine by reprogramming intestinal stem cells and blocking their terminal differentiation. Similarly, in tumor-initiating cells Yap regenerative signaling synergizes with Wnt activation to drive adenoma formation.
KEYWORDS: Egfr, hippo, intestinal stem cells, Paneth cells, Wnt, Yap
Maintaining the integrity and function of the gut epithelium in the face of constant microbial and environmental threats is a major challenge. One of the key defense mechanisms triggered upon injury to the gut involves the ability of intestinal stem cells (ISCs) to rapidly replenish damaged cells. The discovery of the Hippo pathway has provided new insight into this process. Numerous studies using chemical injury models showed that Yes-associated protein (Yap), a transcriptional activator of Hippo signaling, has a key role in promoting epithelial regeneration.1-3 However, in 2013 this view was challenged by Barry et al., who suggested that in the context of injury induced by ionizing radiation Yap acts as a suppressor of ISC expansion via suppression of Wnt signaling.4 We were performing similar experiments, but had arrived at an alternative conclusion: loss of Yap caused severe crypt depletion upon irradiation. To address this discrepancy we performed a time course of crypt regeneration in irradiated Yap-deficient mice. Our data showed that at early stages there was a profound loss of regenerative crypts upon loss of Yap whereas, surprisingly, at later stages the few surviving Yap-deficient crypts restored the epithelial lining and appeared hyperproliferative relative to the temporally matched controls. Analysis of mosaic mice further suggested that this later phenomenon may be caused by delayed regeneration in Yap KO mice, whereas genetic labeling of ISCs showed that maintenance of Yap-deficient ISCs was significantly compromised following irradiation. Our studies definitively demonstrate that, as for chemically induced injury, Yap is important in the promotion of intestinal regeneration in response to damage from ionizing radiation. However, whether loss of Yap accelerates repopulation of the denuded intestine from the few surviving crypts remains unclear.
To explore Yap function during crypt regeneration we performed transcriptional profiling of intestinal organoids. Similar to the conclusions of Barry et al. and our own earlier work,5 we observed that Yap suppresses Wnt/Beta-catenin signaling. These observations begged the question: Why does Yap antagonize a major mitotic signal during crypt regeneration? A recent study suggested that high Wnt activity in ISCs predisposes them to DNA damage and apoptosis following irradiation.6 Indeed, we observed increased apoptosis in early Yap mutant crypts but resolution of DNA damage foci was unperturbed by the loss of Yap (data not shown). As an alternative notion, we were intrigued by our finding that Yap also suppresses Paneth cell differentiation in regenerating crypts, in particular given that Wnt signaling, in addition to promoting ISC self renewal, also drives differentiation into Paneth cells.7 Yap inactivation during regeneration thus decouples the promitotic and prodifferentiation effects of Wnt signaling to cause aberrant Paneth cell differentiation and exhaustion of the ISC pool.
Strikingly, the role of Yap in regeneration does not end there. Yap accumulates in the nucleus of regenerating crypts, where it activates numerous genes implicated in wound healing, cancer, and inflammation. Interestingly, some of these target genes were epidermal growth factor receptor (Egfr) ligands including Amphiregulin (Areg) and Epiregulin (Ereg), known regulators of intestinal regeneration. Moreover, other studies have shown that Egfr signaling and Yap are functionally linked. In Drosophila, the Yap ortholog Yorkie activates Egfr ligands to promote gut regeneration.2 In cancer cells, Yap drives proliferation by inducing expression of Areg, and resistance to Raf and Mek inhibitor therapy is promoted by Yap.8,9 We therefore tested whether Yap stimulates expression of Egfr ligands to promote regeneration. Indeed, by treating Yap mutant organoids with recombinant Ereg we rescued de novo crypt formation whereas our in vivo data suggested that stromal Ereg may drive non-cell autonomous growth of Yap mutant crypts. Together, these findings imply a central role for the Yap/Egfr axis in promoting crypt formation.
Causative links between wound healing and cancer development have been recognized for well over a century. To explore whether the Yap regenerative program contributes to intestinal cancer initiation, we used several mouse models of intestinal tumorigenesis based on mutations in the tumor suppressor gene adenomatous polyposis coli (Apc). We observed that loss of Yap completely abolished polyp formation in the Apcmin tumor mouse model, as well as the formation of Apc-/- organoids that otherwise grow as undifferentiated spheroids. We also found that Yap prevented Paneth cell differentiation and boosted the proregenerative signature identified in damaged crypts that included activation of Egfr signaling. Of note, by employing a mosaic model of Yap and Apc deletion in ISCs, we discovered that although Yap is not required for initiation of early Apc-/- microlesions, it is critical for their progression to adenomas. What distinguishes a regenerative versus tumorigenic response is thus the constitutive activation of Wnt signaling by Apc mutation, which serves to by-pass Yap-dependent suppression of the Wnt/Beta-catenin pathway. These results highlight the commonalities between crypt regeneration and tumor initiation and suggest a 2-signal model for adenoma formation in which tumor-initiating cells, having acquired activating mutations in the Wnt pathway, must undergo a secondary event that drives activation of the Yap regenerative program. Our results indicate that Yap activation is not directly dependent on disruption of the Wnt signaling machinery, but may rather reflect changes in the tissue environment as a whole. Like normal stem cells, tumor-initiating cells are exposed to various environmental and intracellular stresses and their survival in this context depends on Yap. Thus, local tissue damage and concomitant Yap activation may establish the necessary conditions for progression of tumor-initiating cells into adenomas. One possible signal underlying this process may be inflammatory cytokines, as the laboratories of Karin and Guan recently showed that the gp130-Src module is an important stimulator of Yap in the gut epithelium.10 Cell tension, shape, and polarity are also key modulators of Yap activity. Therefore, local inflammation and/or alterations in tissue architecture may provide key environmental cues that drive Yap transcriptional activity and tumor progression (Fig. 1).
Figure 1.
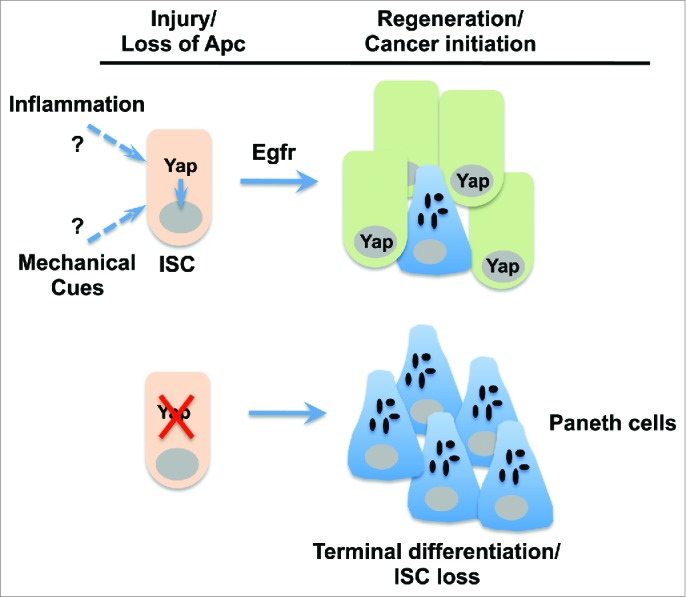
Functions of Yap in intestinal stem cells during tissue regeneration and tumorigenesis. Upon injury or loss of the tumor suppressor Apc (adenomatous polyposis coli), Yap (Yes-associated protein) promotes epidermal growth factor receptor (Egfr) signaling and maintains proper coupling of intestinal stem cell (ISC) self renewal and Paneth cell differentiation. In the absence of Yap, ISCs preferentially differentiate into Paneth cells and are ultimately lost. The precise signal leading to Yap nuclear activation is unknown but may involve inflammatory cytokines and/or mechanical stress imposed on ISCs as a result of tissue damage.
In summary, regenerating ISCs are faced with the difficult task of maintaining a proper balance between self-renewal and differentiation. Yap controls this process by driving Egfr-dependent signaling and preventing the excessive differentiation into Paneth cells that is inherent to high Wnt activity. Thus, the dual function of Yap in regeneration is hijacked by tumor-initiating cells to promote adenoma formation (Fig. 1). In future work it will be important to elucidate the molecular mechanisms by which Yap drives tumor initiation; in particular, it is unclear how Yap suppresses Paneth cell differentiation in tumor cells with constitutively high levels of Wnt signaling. Furthermore, analysis of the downstream functions of Yap target genes will keep researchers busy for several years to come.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 2010; 24:2383-8; PMID:21041407; http://dx.doi.org/ 10.1101/gad.1978810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A 2010; 107:21064-9; PMID:21078993; http://dx.doi.org/ 10.1073/pnas.1012759107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol 2010; 20:1580-7; PMID:20727758; http://dx.doi.org/ 10.1016/j.cub.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, et al.. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 2013; 493:106-10; PMID:23178811; http://dx.doi.org/ 10.1038/nature11693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, et al.. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell 2010; 18:579-91; PMID:20412773; http://dx.doi.org/ 10.1016/j.devcel.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 6.Tao S, Tang D, Morita Y, Sperka T, Omrani O, Lechel A, Sakk V, Kraus J, Kestler HA, Kühl M, et al.. Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. Embo J 2015; 34:624-40; PMID:25609789; http://dx.doi.org/ 10.15252/embj.201490700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, et al.. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol 2005; 7:381-6; PMID:15778706; http://dx.doi.org/ 10.1038/ncb1240 [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 2009; 11:1444-50; PMID:19935651; http://dx.doi.org/ 10.1038/ncb1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al.. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet 2015; 47:250-6; PMID:25665005; http://dx.doi.org/ 10.1038/ng.3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al.. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015; 519:57-62; PMID:25731159; http://dx.doi.org/ 10.1038/nature14228 [DOI] [PMC free article] [PubMed] [Google Scholar]


