ABSTRACT
Unc-51 like kinase-1 (Ulk1) is essential for autophagy induction. We recently reported that p32/C1QBP is crucial for the induction of autophagic flux upon starvation and the clearance of damaged mitochondria through the regulation of Ulk1 stability and kinase activity. Our results underscore the importance of p32 in fine-tuning the stress response and mitochondrial homeostasis via Ulk1.
Keywords: Autophagy; p32; ULK1
Autophagy is an evolutionarily conserved catabolic process in eukaryotic cells and represents one of the major intracellular degradation pathways for protein and organelle turnover. There are 3 major types of autophagy in eukaryotic cells: macroautophagy, microautophagy, and chaperone-mediated autophagy. The most remarkable event in macroautophagy is the formation of cytosolic double-membraned vesicles that sequester portions of the cytoplasm to form autophagosomes, which subsequently fuse with lysosomes for degradation. Mitophagy is a selective form of autophagy by which dysfunctional mitochondria are cleared in lytic compartments. Mitophagy has important roles in mitochondrial quality control and homeostasis under normal growth conditions and in the response to cellular stress.
In the last decade, genetic studies in yeast have characterized a number of autophagy-related (atg) genes involved in all three types of autophagy, and mammalian homologs of many of these genes have subsequently been identified. Extensive studies have shown that Unc-51 like kinase-1 (Ulk1), a serine/threonine kinase and the mammalian functional homolog of yeast Atg1, has a crucial role in the initiation step of autophagy and also functions in mitophagy induction. Post-translational modifications on Ulk1, including K63-linked ubiquitylation1 and phosphorylation,2,3 have been reported to modulate the rates of Ulk1 turnover and kinase activity in different cellular contexts, providing insights into how the cell modulates autophagy.
Previous studies have showed that p32 (C1QBP/gC1qR/HABP1, best known as p32) is a biologically important, widely distributed, multiligand-binding, and multifunctional protein. Maintenance of mitochondrial membrane potential and oxidative phosphorylation is one of the fundamental functions of p32 in eukaryotic cells. Depletion of p32 in human cancer cells strongly shifts their metabolism from oxidative phosphorylation toward glycolysis and reduces their tumorigenicity.4 Although oxidative phosphorylation has been reported to enhance mitophagy to promote mitochondrial renewal,5 little is known about how the bioenergetics of mitochondria are linked to mitophagy. In addition, the relationship between p32 and mitochondrial homeostasis has not been addressed.
We identified p32 and Ulk1 from a stable protein complex independent of Ulk1 kinase activity and nutrient conditions (Fig. 1).6 Previous reports have claimed that p32 functions as chaperone protein, required for the localization of ARF to mitochondria.7 Interestingly, Ulk1 was found to translocate to mitochondria that were damaged by either hypoxia or mitochondrial uncoupler to induce mitophagy.8 We showed that a subset of ectopic Ulk1 was distributed to mitochondria in cells expressing p32, suggesting that Ulk1 may translocate to mitochondria upon binding to p32. Whether this translocation leads to initiation of mitophagy needs further investigation.
Figure 1.
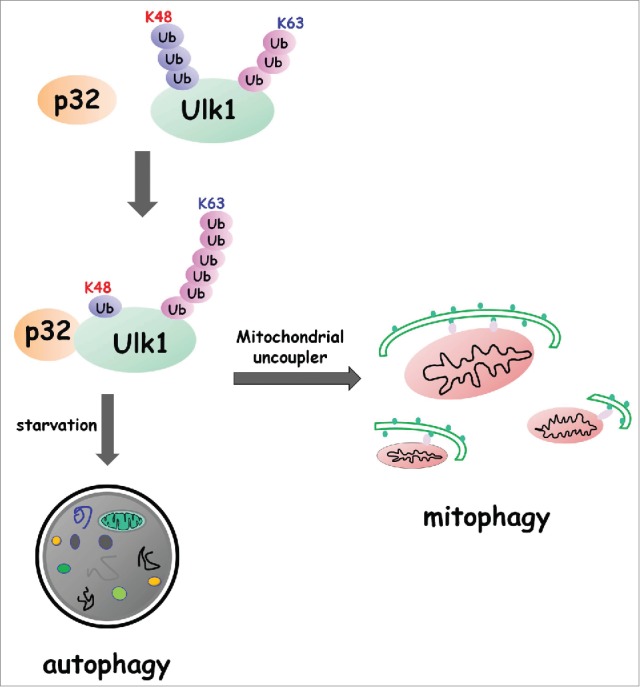
p32 regulates autophagy by interfering with the polyubiquitylation of Ulk1. By forming a complex with Ulk1, p32 prevents K48-linked polyubiquitylation, but facilitates K63-linked polyubiquitylation, of Ulk1. Therefore, p32 depletion results in increased degradation of Ulk1. p32-mediated Ulk1 stabilization and subsequent activation is essential for starvation-induced autophagy and the clearance of damaged mitochondria caused by mitochondrial uncoupler.
Ulk1 is a relatively stable protein with a half-life close to 24 h. We found that p32 is crucial for maintaining the steady-state levels and activity of Ulk1 by blocking its proteasomal degradation. In the absence of p32, the turnover rate of Ulk1 is profoundly increased, which in turn compromises its kinase activity toward Atg13. Previous studies reported that Ulk1 is a K63-linked, but not K48-linked, ubiquitylated protein.1 Unlike previous studies, we found that Ulk1 is subject to both K48-linked and K63-linked ubiquitylation in our experimental settings. Furthermore, we found that p32 depletion potentiated K48-linked, but impaired K63-linked, polyubiquitination of Ulk1. These data indicate that p32 may prevent the proteasomal degradation of Ulk1 by interfering with its polyubiquitylation. We excluded the possibility that p32 works in concert with TRAF6 signaling to regulate K63-linked ubiquitylation of Ulk1. Currently, the E3 ubiquitin ligase for Ulk1, which is capable of generating K48-linked ubiquitin chains, remains unknown. We propose that p32 exerts a positive effect on Ulk1 stability and activity through 2 possible mechanisms. First, p32 may crosstalk with the unidentified ubiquitylation/deubiquitylation processes targeting Ulk1. Alternatively, p32 has been proposed to act as a multifunctional chaperone protein, as evidenced by its participation in different biological processes. It is likely that the chaperone-like activity of p32 is essential for Ulk1 regulation, through a process similar to Hsp90-Cdc37 complex-dependent regulation of Ulk1.9
Ulk1 is essential for starvation-induced autophagy and mitochondrial uncoupler-induced mitophagy. In line with the crucial role of p32 in regulating Ulk1 steady-state levels and activity, we found that silencing p32 resulted in autophagy defects. Autophagy is a prosurvival mechanism that is engaged by metabolic stress. Cells defective for autophagy tend to undergo apoptosis after stress stimuli that normally would activate autophagy to promote cell survival.10 We found that p32 ablation augmented cell death following treatment with Earle's balanced salt solution (EBSS), which could be partially but significantly rescued by Ulk1 reconstitution. This suggests that p32 exerts a protective effect under starvation conditions by both inducing Ulk1-dependent autophagy and maintaining oxidative phosphorylation. p32 is often aberrantly overexpressed in human tumor tissues. Consistent with this, we found that p32 protein levels were upregulated in human colon cancer tissues, and that Ulk1 proteins levels significantly correlated with p32 status. On the basis of our data, it is reasonable to speculate that the p32-Ulk1-autophagy axis may provide a survival advantage when nutrients are scarce that ultimately promotes tumorigenesis.
Previous studies showed that p32 has a crucial role in maintaining mitochondrial membrane potential and oxidative phosphorylation. However, the molecular mechanism underlying this p32-mediated metabolic regulation remains completely unknown. Our findings that damaged mitochondria induced by treatment with the protonophore CCCP failed to be cleared in p32-deficient HeLa cells stably expressing parkin unveiled an unexpected role of p32 in mitophagy. Reconstitution with WT p32, but not the Ulk1-binding defective mutant, rescued the mitochondrial degradation defect associated with p32 depletion, indicating that the ability of p32 to regulate CCCP-induced mitophagy is mediated through its interaction with Ulk1. Consistent with this notion, introduction of ectopic Ulk1 effectively restored mitochondrial clearance in p32-ablated cells treated with CCCP. Thus, p32 could act upstream of Ulk1 in mitochondrial homeostasis. It has been reported that stimulation of mitochondrial oxidative phosphorylation enhances mitochondrial renewal by increasing the degradation rate. This indicates that mitochondrial homeostasis and oxidative phosphorylation might be mutually beneficial and mitochondrial quality control may serve as an important mechanism for maintaining cellular energy homeostasis. Thus, our results provide a plausible explanation for the previously reported p32-mediated regulation of mitochondrial bioenergetics. Collectively, our findings underscore the importance of p32 in fine-tuning mitochondrial quality control and mitochondrial metabolism via Ulk1 under physiologic conditions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, et al.. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol 2013; 15:406-16; PMID:23524951; http://dx.doi.org/ 10.1038/ncb2708 [DOI] [PubMed] [Google Scholar]
- 2.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al.. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011; 331:456-61; PMID:21205641; http://dx.doi.org/ 10.1126/science.1196371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011; 13:132-41; PMID:21258367; http://dx.doi.org/ 10.1038/ncb2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fogal V, Richardson AD, Karmali PP, Scheffler IE, Smith JW, Ruoslahti E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol Cell Biol 2010; 30:1303-18; PMID:20100866; http://dx.doi.org/ 10.1128/MCB.01101-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrighton KH. Metabolism: Putting energy into mitophagy. Nat Rev Mol Cell Biol 2013; 14:324; PMID:23657497; http://dx.doi.org/ 10.1038/nrm3586 [DOI] [PubMed] [Google Scholar]
- 6.Jiao H, Su GQ, Dong W, Zhang L, Xie W, Yao LM, Chen P, Wang ZX, Liou YC, You H. Chaperone-like protein p32 regulates ULK1 stability and autophagy. Cell Death Differ 2015; PMID:25909887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itahana K, Zhang Y. Mitochondrial p32 is a critical mediator of ARF-induced apoptosis. Cancer Cell 2008; 13:542-53; PMID:18538737; http://dx.doi.org/ 10.1016/j.ccr.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W, Tian W, Hu Z, Chen G, Huang L, Li W, Zhang X, Xue P, Zhou C, Liu L, et al.. ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep 2014; 15:566-75; PMID:24671035; http://dx.doi.org/ 10.1002/embr.201438501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joo JH, Dorsey FC, Joshi A, Hennessy-Walters KM, Rose KL, McCastlain K, Zhang J, Iyengar R, Jung CH, Suen DF, et al.. Hsp90-Cdc37 chaperone complex regulates Ulk1- and Atg13-mediated mitophagy. Mol Cell 2011; 43:572-85; PMID:21855797; http://dx.doi.org/ 10.1016/j.molcel.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132:27-42; PMID:18191218; http://dx.doi.org/ 10.1016/j.cell.2007.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]


