Abstract
Impaired mobility, such as falls, may be an early biomarker of subsequent cognitive decline and is associated with subclinical alterations in both brain structure and function. In this 12-month prospective study, we examined whether there are volumetric differences in gray matter and subcortical regions, as well as cerebral white matter, between older fallers and non-fallers. In addition, we assessed whether these baseline volumetric differences are associated with changes in cognitive function over 12 months. A total of 66 community-dwelling older adults were recruited and categorized by their falls status. Magnetic resonance imaging occurred at baseline and participants’ physical and cognitive performances were assessed at baseline and 12-months. At baseline, fallers showed significantly lower volumes in gray matter, subcortical regions, and cerebral white matter compared with non-fallers. Notably, fallers had significantly lower left lateral orbitofrontal white matter volume. Moreover, lower left lateral orbitofrontal white matter volume at baseline was associated with greater decline in set-shifting performance over 12 months. Our data suggest that falls may indicate subclinical alterations in regional brain volume that are associated with subsequent decline in executive functions.
Keywords: Mobility, Accidental Falls, Cognitive Function, Older Adults, Structural Magnetic Resonance Imaging
1. INTRODUCTION
Worldwide, the number of individuals aged 60 years or over is expected to more than double, from 841 million people in 2013 to more than 2 billion in 2050 (United Nations 2013). Consequently, geriatric syndromes such as cognitive impairment and impaired mobility will place increasing demand on the public health system. Both geriatric syndromes are associated with institutionalization, reduced quality of life, disability, and death (Scott et al. 2010). Current evidence suggests that cognitive impairment and impaired mobility are associated and often coexist among older adults (Montero-Odasso et al. 2012). Notably, there is growing recognition that clinical gait abnormalities and falls are early biomarkers of cognitive impairment and dementia (Verghese et al. 2002). For example, with data from the Health, Aging and Body Composition Study, Inzitari and colleagues (Inzitari et al. 2007) showed that slower gait speed at baseline was predictive of subsequent cognitive decline over approximately one decade. Others have shown that gait speed begins to decline more precipitously approximately one decade before the diagnosis of mild cognitive impairment (Buracchio et al. 2010). A recent 12-month prospective study of 125 cognitively normal, community-dwelling older adults demonstrated that higher levels of Pittsburgh compound B retention – a biomarker for greater amyloid deposition -and Alzheimer’s disease (AD) related cerebrospinal fluid biomarkers were associated with a faster time to first fall (Stark et al. 2013).
Consistent with these links between mobility and cognition, evidence from neuroimaging studies suggests that slower gait speed or a history of falls is associated with subclinical alterations in both brain structure and function (Rosano et al. 2008; Hsu et al. 2014; Rosso et al. 2014). With respect to structure, in a cross-sectional study of 220 community-dwelling older adults, Rosano and colleagues (Rosano et al. 2008) demonstrated that lower gray matter volume in the sensorimotor regions and frontoparietal regions were associated with impaired gait (i.e., shorter steps and longer double support times). In another cross-sectional study of 112 community-dwelling older adults, Ezzati and colleagues (Ezzati et al. 2015) reported that lower gray matter and hippocampal volumes were associated with slower gait speed. Hippocampal atrophy over 2.5 years was associated with concurrent gait speed decline among 225 older adults between age of 60 to 86 (Callisaya et al. 2013). These brain-mobility associations found in gray matter and subcortical structures are highly relevant within the context of cognitive aging as studies have consistently demonstrated the contribution of total gray matter and hippocampal volumes to cognitive performance in late life (Mungas et al. 2005; Jagust et al. 2006; Persson et al. 2006). Thus, impaired mobility may be an overt biomarker for covert gray and subcortical neural degeneration that predicts subsequent cognitive decline.
In terms of brain function, our own work demonstrated that compared with their non-faller counterparts, older adults with a history of multiple falls (i.e., ≥ 2 non-syncope falls in the previous 12 months) showed aberrant neural network functional connectivity – a measure of synchronous brain activity (Hsu et al. 2014). Notably, the aberrant network connectivity demonstrated by fallers was independently associated with greater decline in both executive functions and mobility over a 12-month period after accounting for relevant covariates (Hsu et al. 2014). Hence, a recent history of multiple falls among older adults without a diagnosis of dementia may indicate sub-clinical changes in brain function and increased risk for subsequent decline.
However, more evidence generated from longitudinal studies is needed to establish falls as a biomarker of covert neural degeneration as well as to evaluate the significance of these changes in relation to subsequent decline in cognitive function among otherwise healthy community-dwelling older adults. Moreover, the contribution of regional cerebral white matter volume in mobility has not been explored extensively to date. Yet, significant age-related reduction in cerebral white matter volume co-occurs with gray matter loss (Bartzokis et al. 2003; Allen et al. 2005), particularly in the frontal and parietal regions (Resnick et al. 2003). Reduced white matter volume is also associated with cognitive impairment and dementia (Salat et al. 2009).
Thus, we conducted a 12-month prospective study to determine whether there are differences in brain structure between otherwise healthy community-dwelling older adults with and without a recent history of multiple falls, and to evaluate the significance of these differences in relation to subsequent changes in cognitive function – with a focus on executive function. Given our emphasis on executive functions, our specific aims were to examine whether: 1) community-dwelling older adults with a recent history of multiple falls have lower regional frontal and parietal gray matter, frontal and parietal cerebral white matter, and subcortical volumes compared with their non-falling counterparts; and 2) whether baseline volumetric differences are independently associated with change in cognitive functions over 12 months. Importantly, this research may lead to the early identification of those at risk for cognitive decline, and thereby facilitate the timely implementation of effective prevention strategies.
2. MATERIAL AND METHODS
2.1 Study design and participants
We conducted a 12-month prospective exploratory study with 66 older adults. Participants were recruited from metropolitan Vancouver via newspaper advertisements. Individuals were eligible if they: 1) were aged 70 to 80 years; 2) scored ≥ 24/30 on the Mini-Mental State Examination (MMSE) (Cockrell and Folstein 1988); 3) were right hand dominant as measured by the Edinburgh Handedness Inventory (Oldfield 1971); 4) were living independently in their own homes; 5) had visual acuity of at least 20/40, with or without corrective lenses; and 6) provided informed consent. We excluded those who: 1) had a neurodegenerative disease, stroke, dementia (of any type), or psychiatric condition; 2) had clinically significant peripheral neuropathy or severe musculoskeletal or joint disease; 3) were taking psychotropic medication; 4) had a history indicative of carotid sinus sensitivity; 5) were living in a nursing home, extended care facility, or assisted-care facility; or 6) were ineligible for MRI scanning.
Based on their falls history in the 12 months prior to study entry, participants were classified as a faller or non-faller (see 2.1.1 and 2.1.2). Ethics approval was obtained from the Vancouver Coastal Research Health Institute and University of British Columbia’s Clinical Research Ethics Board. All participants provided written consent.
2.1.1 Specific inclusion criterion for fallers
An individual must have experienced ≥ 2 minimal displacement non-syncopal falls in the previous 12 months, with one of the falls occurring in the last 6 months (Delbaere et al. 2010). This was determined from two sources: 1) participant recall and 2) participant’s immediate family member or friend recall. Falls were defined as “unintentionally coming to rest on the ground, floor, or lower level” (Hauer et al. 2006).
2.1.2 Specific inclusion criterion for non-fallers
An individual must not have experienced > 1 displacement falls (with or without syncope) in the previous 12 months. This was determined based on two sources: 1) participant recall and 2) participant’s immediate family member or friend recall. We highlight that individuals with one fall (non-injurious) in the previous 12 months resemble the physiological profile of non-fallers (Nevitt et al. 1989; Lord et al. 1991). Specifically, a prospective study found that single fallers had similar physical and mental status compared with non-fallers, while multiple fallers showed significant musculoskeletal and neurological deficits (Nevitt et al. 1989).
2.2 Measurement
All measures, with the exception of neuroimaging, were assessed at baseline and 12 months. All assessors were trained and standardized protocols were used. Neuroimaging data were acquired at baseline only.
2.2.1 Global cognition and current physical activity level
Global cognition was assessed using the MMSE (Cockrell and Folstein 1988) and the Montreal Cognitive Assessment (MoCA) (Nasreddine et al. 2005). The MoCA is a 30-point test that covers multiple cognitive domains. The MoCA has been found to have good internal consistency and test-retest reliability and was able to correctly identify 90% of a large sample of individuals with mild cognitive impairment from two different clinics with a cut-off scores of < 26/30 (Nasreddine et al. 2005). Current level of physical activity (i.e., last 7 days) was determined by the Physical Activities Scale for the Elderly (PASE) self-report questionnaire (Washburn et al. 1999).
2.2.2 Comorbidity and depression
Comorbidities were assessed with the Functional Comorbidity Index (FCI) (Groll et al. 2005), an 18-item questionnaire that calculates the total number of comorbidities associated with physical functioning (Groll et al. 2005). We used the 15-item Geriatric Depression Scale (GDS) (Yesavage et al. 1982; Yesavage 1988) to indicate the presence of depression; a score ≥ 5 indicates depression (van Marwijk et al. 1995).
2.2.3 Physiological falls risk
Physiological falls risk was assessed using the short form of the Physiological Profile Assessment (PPA). The PPA is a valid (Lord et al. 1991; Lord et al. 1994) and reliable (Lord and Castell 1994) measure of falls risk. Based on a participant’s performance in five physiological domains – postural sway, reaction time, strength, proprioception, and vision – the PPA computes a falls risk score (standardized score) that has a 75% predictive accuracy for falls among older people (Lord et al. 1991; Lord et al. 1994). A PPA Z-score ≥ 0.60 indicates high physiological falls risk (Delbaere et al. 2010).
2.2.4 Mobility and balance
Mobility and balance were assessed using the Short Physical Performance Battery (SPPB) (Guralnik et al. 1995), gait speed, and the Timed-Up-and-Go Test (TUG) (Shumway-Cook et al. 2000). For the SPPB, participants were assessed on performances of standing balance, walking (four meters), and sit-to-stand. Each component is rated out of four points, for a maximum of 12 points; a score < 9 predicts subsequent disability (Guralnik et al. 1995). Gait speed was calculated by dividing four meters by the time taken to perform the four-meter walking test in SPPB. The four-meter walking test was performed twice for each individual; therefore gait speed is calculated by dividing four meters by the average time taken from the two walks. For the TUG, participants rose from a standard chair, walked a distance of three meters, turned, walked back to the chair and sat down (Shumway-Cook et al. 2000). We recorded the time (s) to complete the TUG, based on the average of two separate trials.
2.2.5 Executive functions
We used: 1) the Stroop Test (Graf et al. 1995) to assess selective attention and conflict resolution; 2) the Trail Making Tests (Part A & B) to assess set shifting(Spreen O 1998); 3) the Verbal Digits Forward and Backward Tests to index working memory (Wechsler 1981); and 4) Digit Symbol Substitution Test (DSST) (Lezak 1995) to assess information processing, working memory, and psychomotor speed. For the Stroop Test (Graf et al. 1995), participants first read out words printed in black ink (e.g., BLUE). Second, they named the display colour of coloured-X’s (i.e. letter “X” printed in different colours). Finally, they were shown a page with colour-words printed in incongruent coloured inks (e.g., the word “BLUE” printed in red ink). Participants were asked to name the ink colour in which the words were printed (while ignoring the word itself). We recorded the time participants took to read the items in each condition and calculated the time difference between the third condition and the second condition (i.e. Stroop colour-words condition minus Stroop coloured-X’s condition). Smaller time differences indicate better selective attention and conflict resolution performance.
For the Trail Making Tests (Part A & B) (Spreen O 1998), participants were required to draw lines connecting encircled numbers sequentially (Part A) or alternating between numbers and letters (Part B). A standardized score based on performance on Part B and Part A (i.e., (B-A)/A) was calculated, with smaller scores indicating better set shifting performance.
For the Verbal Digits Forward and Backward Tests (Wechsler 1981), participants repeated progressively longer random number sequences in the same order as presented (forward) and the reversed order (backward). Successful performance on the verbal digits span backward test represents a measure of central executive function due to the additional requirement of manipulation of information within temporary storage (Baddeley 1992). Thus, we subtracted the verbal digits backward test score from the verbal digits forward test score to provide an index of working memory with smaller difference scores indicating better working memory.
For the DSST, participants were first presented with a series of numbers (1 to 9) and their corresponding symbols. They were then asked to draw the correct symbol for any digit - placed randomly in pre-defined series - in 90 seconds (Lezak 1995). A higher number score indicates better information processing, working memory, and psychomotor speed.
2.2.6 Structural MRI acquisition and analysis
High resolution structural MRI was performed at the UBC MRI Research Center located at the UBC Hospital on a 3.0 Tesla Intera Achieva MRI Scanner (Phillips Medical Systems Canada, Markham, Ontario) using an 8-channel SENSE neurovascular coil. The high resolution T1 images were acquired using the following parameters: 170 slices (1 mm thick), TR of 7.7 ms, TE of 3.6 ms, FA of 8 degrees, FoV of 256 mm, acquisition matrix of 256x200. Cortical reconstruction and brain volumetric segmentation were performed using the FreeSurfer image analysis suite (Reuter et al. 2012) developed at the Martinos Center for Biomedical Imaging by Laboratory for Computational Neuroimaging (http://surfer.nmr.mgh.harvard.edu/). Data processing included skull-stripping (Segonne et al. 2004), motion correction (Reuter et al. 2010), Talairach transformation (Fischl et al. 2002; Fischl et al. 2004), atlas registration (Fischl et al. 1999) and brain parcellation (Fischl et al. 2004; Desikan et al. 2006). White matter hypointensity labels were determined through a probabilistic process in which total white matter hypointensity volume was calculated per hemisphere and subsequently summed to generate a single white matter hypointensity value for each individual (Fischl et al. 2002). The accuracy of this method was shown to be comparable to previously validated segmentation process (Fischl et al. 2002). All scans underwent manual checking following the automated segmentation (probabilistic process) by one of the co-authors (BKC). No changes resulted from the manual checks. These steps provide quantification of cerebral white matter, gray matter, and subcortical volume. It also provided white matter hypointensity volume and estimated total intracranial volume (to account for difference in head size) which we included as covariates in our analyses (Whitman et al. 2001; Debette and Markus 2010). Specifically, estimated total intracranial volume is determined through Freesurfer via atlas scaling factor – a determinant of linear transformation used to align individual structural image to an atlas (Buckner et al. 2004). These results generated from the Freesurfer processing stream included Jacobian white matter correction to provide better estimation of regional volumes. Based on evidence to date (Mungas et al. 2005; Rosano et al. 2008; Ezzati et al. 2015), we focus specifically on cerebral white matter and gray matter volume within the frontal and parietal regions, as well as subcortical gray matter volume (Table 3).
Table 3.
Regional Gray Matter Volumetric Differences between Fallers and Non-Fallers
| Regions | Non-fallers (N=36) | Fallers (N=30) | ||||
|---|---|---|---|---|---|---|
| Structural Volume (mm^3) | SD | Structural Volume (mm^3) | SD | p-value* | ||
| Frontal Areas | Laterality | |||||
| Rostral anterior cingulate | RH | 1882.83 | 302.69 | 2010.67 | 432.02 | 0.06 |
| Caudal anterior cingulate | LH | 1689.11 | 386.91 | 1763.20 | 524.04 | 0.22 |
| Rostral middle frontal | LH | 13073.47 | 1604.18 | 12827.17 | 1466.79 | 0.82 |
| Caudal middle frontal | RH | 5148.64 | 1036.34 | 5164.20 | 1075.81 | 0.47 |
| Medial orbitofrontal | LH | 4666.78 | 532.87 | 4430.53 | 583.39 | 0.22 |
| Lateral orbitofrontal | LH | 6608.39 | 904.32 | 6223.83 | 597.05 | 0.03 |
| Pars opercularis | RH | 3356.50 | 511.58 | 3368.80 | 491.18 | 0.53 |
| Pars triangularis | LH | 3070.56 | 405.37 | 2865.33 | 451.02 | 0.12 |
| Pars triangularis | RH | 3543.22 | 472.68 | 3335.60 | 400.60 | 0.14 |
| Other Areas | ||||||
| Insula | LH | 6215.03 | 628.92 | 5905.23 | 541.48 | 0.05 |
| Insula | RH | 6363.06 | 733.20 | 5881.10 | 641.23 | 0.01 |
| Subcortical Areas | ||||||
| Total Amygdala | 3010.43 | 419.57 | 2849.39 | 348.07 | 0.14 | |
| Amygdala | LH | 1476.74 | 212.52 | 1391.48 | 220.38 | 0.11 |
| Amygdala | RH | 1533.69 | 248.16 | 1457.91 | 162.17 | 0.30 |
| Total Pallidum | 2801.71 | 351.94 | 2523.99 | 352.38 | 0.01 | |
| Pallidum | LH | 1435.56 | 204.89 | 1264.29 | 204.10 | 0.01 |
| Pallidum | RH | 1366.15 | 194.06 | 1259.70 | 211.19 | 0.04 |
| Total Hippocampus | 7666.96 | 873.03 | 7191.10 | 712.6 | 0.02 | |
| Hippocampus | LH | 3775.46 | 446.57 | 3551.86 | 363.28 | 0.04 |
| Hippocampus | RH | 3891.50 | 460.03 | 3639.23 | 402.26 | 0.03 |
Statistical comparisons were conducted by performing ANCOVA on SPSS.
Note: LH = Left-hemisphere; RH= Right-hemisphere.
All comparisons were adjusted for baseline age, total white matter hypointensity volume and estimated total intracranial volume. Bolded values represent p<0.05 and italicized values represent trend-level effects (p<0.10).
2.3 Statistical analysis
Data were analyzed using the IBM SPSS Statistic 19 (SPSS Inc., Chicago, IL) and the Glmnet package version 2.0-2 within R version 3.2.1. The first step was to identify brain regions (based on white and/or gray matter volume) that distinguished fallers from non-fallers at baseline. To do so, we used an elastic net regularized logistic regression model via Glmnet. Elastic net regression is a mixture of lasso and ridge regression models and introduces penalties to the estimated coefficients to address the problem of having numerous correlated predictor variables (Friedman et al. 2010), as is the case when using many brain regions as predictor variables. The elastic net penalizes the size of the coefficients for correlated predictors, with some coefficients being shrunk exactly to zero. Glmnet requires the user to set two parameters related to the penalty: alpha was set at 0.5 to represent an exact mixture of lasso and ridge regression and lambda was determined using k-fold cross-validation to minimize misclassification error. Fifty-seven brain regions were included as predictors and 3 additional variables (age, total intracranial volume, and log-transformed white matter hypointensity) were included as covariates, based on biological relevance (Whitman et al. 2001; Debette and Markus 2010). These covariates were not subjected to the elastic net penalty. The brain regions that were selected in the elastic net regression model (i.e., whose coefficients were not shrunk to zero) were utilized for the subsequent analysis of covariance (ANCOVA) to statistically test for significant differences in regional brain volume between older fallers and non-fallers.
Change scores were calculated by subtracting the baseline score from the 12-month score and this was done for each of the four executive function measures. Thus, a negative change score reflects improved performance in selective attention and conflict resolution, set shifting, and working memory. Conversely, a positive change score reflects improved psychomotor speed.
Finally, to investigate whether fall-related structural alterations were independently associated with subsequent changes in executive functions, we constructed multiple linear regression models using regional brain volume as independent variables, and changes in executive functions as dependent variables. To maximize statistical power, these analyses included all 66 participants and included falls status, age and total white matter hypointensity volume as covariates in the first step. Under the premise that fallers and non-fallers may have different trajectories in cognitive aging across time, and that the start of these different trajectories might precede the baseline assessment, baseline executive functioning was not included as a covariate. The brain regions of interest were entered in the second step to determine their unique contribution to change in executive functions. The overall alpha level was set at p ≤ 0.05; for trend-level effects, the alpha level was set at p ≤ 0.10. To check the assumptions of the linear regression models, we examined histograms of the residual values and scatterplots of the predictor variables versus the residuals. These showed that the residual values were normally distributed, free of outliers, and homoscedastic.
3. RESULTS
3.1 Participants
Table 1 provides descriptive characteristics of the study sample categorized by their falls status. All participants were right-hand dominant as indicated by the Edinburgh Handedness Inventory (Oldfield 1971). At baseline, compared with non-fallers, fallers showed statistically significantly slower gait speed (p=0.02; Table 1) and evidence of a trend for higher GDS score (p=0.08; Table 1) as well as higher white matter hypointensity volume (p=0.06; Table 1). The trend level significance for GDS persisted across the 12-month period with fallers showing a reduced score over time (p=0.07; Table 2); however, there were no statistically significant between-group differences in change for all other measures over the 12-month study period (Table 2).
Table 1.
Study Sample Characteristics at Baseline
| Baseline | |||||
|---|---|---|---|---|---|
| Non-fallers (N=36) | SD | Fallers (N=30) | SD | p-value* | |
| Age (yr) | 74.25 | 2.86 | 73.83 | 3.06 | 0.57 |
| Height (cm) | 165.74 | 8.44 | 163.12 | 6.66 | 0.18 |
| Weight (kg) | 72.33 | 17.03 | 72.06 | 12.53 | 0.94 |
| Sex (M/F) | 25/11 | - | 24/6 | - | 0.22 |
| BMI (kg/m2) | 26.10 | 4.87 | 26.83 | 4.59 | 0.41 |
| MMSE (30 pts max) | 28.31 | 1.51 | 28.48 | 1.53 | 0.64 |
| MOCA (30 pts max) | 24.72 | 3.24 | 24.59 | 3.33 | 0.87 |
| MCI, n (%) | 20 (56%) | - | 16 (53%) | - | 0.86 |
| GDS | 0.25 | 0.65 | 0.80 | 1.69 | 0.08 |
| FCI | 2.67 | 2.16 | 3.28 | 1.71 | 0.22 |
| PPA | 0.22 | 0.83 | 0.50 | 0.87 | 0.19 |
| SPPB (12 pts max) | 10.64 | 1.57 | 10.10 | 1.74 | 0.20 |
| Gait Speed (m/s) | 1.30 | 0.23 | 1.17 | 0.22 | 0.02 |
| TUG (s) | 7.46 | 1.63 | 8.44 | 3.64 | 0.15 |
| Stroop CW (s) | 108.22 | 28.58 | 111.78 | 35.23 | 0.65 |
| Stroop C (s) | 56.66 | 12.71 | 56.28 | 13.31 | 0.91 |
| Stroop Cw - Stroop C (s) | 51.56 | 23.52 | 55.50 | 26.84 | 0.53 |
| Trail B (s) | 107.29 | 43.93 | 128.28 | 41.52 | 0.43 |
| Trail A (s) | 59.69 | 18.69 | 56.86 | 13.60 | 0.56 |
| Trail B - A (s) | 47.60 | 40.67 | 71.42 | 147.30 | 0.35 |
| (Trail B – A)/A | 0.84 | 0.67 | 1.08 | 1.62 | 0.43 |
| Digit Forward | 8.36 | 2.39 | 7.63 | 2.74 | 0.21 |
| Digit Backward | 4.36 | 2.07 | 4.33 | 2.37 | 0.92 |
| Digit Forward - Backward | 4.00 | 2.29 | 3.14 | 2.10 | 0.12 |
| DSST | 29.36 | 5.51 | 28.66 | 6.43 | 0.64 |
| White matter hypointensity (mm^3) | 3713.72 | 1848.94 | 6643.76 | 7315.72 | 0.06 |
Statistical comparisons were conducted by performing ANCOVA on SPSS.
Note: BMI = Body Mass Index; MCI = Mild Cognitive Impairment (i.e. MOCA < 26); MMSE = Mini-Mental Status Examination; MoCA = Montreal Cognitive Assessment; GDS = Geriatric Depression Scale; FCI = Functional Comorbidity Index; ABC = Activities-specific Balance Confidence scale; PPA = Physiological Profile Assessment; SPPB = Short Physical Performance Battery; TUG = Time-Up-and-Go test; Stroop CW: Stroop Colour-Words condition; Stroop C: Stroop Coloured-X’s condition; DSST = Digit Symbol Substitution Test.
For Stroop CW - Stroop C, Trail B - A, and Digit Forward - Backward, smaller difference reflect better performance.
Bolded values represent p<0.05 and italicized values represent trend-level effects (p<0.10).
Table 2.
Study Sample Characteristics – Change in Physical and Cognitive Performance.
| Δ over 12-Months╫ | Non-fallers (N=35) | SD | Fallers (N=29) | SD | p-value* |
|---|---|---|---|---|---|
| MMSE (30 pts max) | −0.31 | 1.53 | −0.14 | 1.73 | 0.75 |
| MOCA (30 pts max) | −0.49 | 3.14 | −0.38 | 2.14 | 0.98 |
| GDS | 0.53 | 2.19 | −0.28 | 1.00 | 0.07 |
| FCI | 0.32 | 1.53 | 0.52 | 1.55 | 0.62 |
| PPA | −0.03 | 0.94 | −0.06 | 0.93 | 0.83 |
| SPPB (12 pts max) | 0.40 | 1.12 | 0.34 | 1.61 | 0.77 |
| Gait Speed (m/s) | −0.07 | 0.23 | −0.002 | 0.18 | 0.38 |
| TUG (s) | 0.57 | 4.20 | −0.45 | 3.17 | 0.29 |
| Stroop CW (s) | −5.79 | 18.38 | −2.01 | 28.19 | 0.58 |
| Stroop C (s) | −1.65 | 8.02 | 0.02 | 6.56 | 0.43 |
| Stroop CW - Stroop C (s) | −4.23 | 16.22 | −2.02 | 25.28 | 0.76 |
| Trail B (s) | −5.83 | 27.77 | −0.93 | 36.68 | 0.69 |
| Trail A (s) | −1.97 | 17.70 | −2.06 | 14.31 | 0.47 |
| Trail B - A (s) | −3.72 | 28.26 | 1.13 | 40.16 | 0.48 |
| (Trail B – A)/A | −0.03 | 0.55 | 0.13 | 0.78 | 0.33 |
| Digit Forward | −0.34 | 2.15 | 0.24 | 2.36 | 0.12 |
| Digit Backward | −0.17 | 1.90 | 0.14 | 1.51 | 0.33 |
| Digit Forward - Backward | −0.17 | 3.17 | 0.10 | 2.97 | 0.55 |
| DSST | 1.60 | 4.83 | −0.24 | 3.53 | 0.18 |
Statistical comparisons were conducted by performing ANCOVA on SPSS.
Note: BMI = Body Mass Index; MMSE = Mini-Mental Status Examination; MoCA = Montreal Cognitive Assessment; GDS = Geriatric Depression Scale; FCI = Functional Comorbidity Index; ABC = Activities-specific Balance Confidence scale; PPA = Physiological Profile Assessment; SPPB = Short Physical Performance Battery; TUG = Time-Up-and-Go test; Stroop CW: Stroop Colour-Words condition; Stroop C: Stroop Coloured-X’s condition; DSST = Digit Symbol Substitution Test.
Two participants (one non-faller and one faller) did not participate in the 12-month assessments.
For Stroop CW - Stroop C, Trail B - A, and Digit Forward - Backward, negative scores reflect better performance over 12-months.
All comparisons were adjusted for baseline age, and total white matter hypointensity volume.
Δ over 12-Months is calculated as 12-month performance minus baseline performance.
3.2. Structural MRI
The elastic net logistic regression analysis reduced the total number of regional brain volumes from 56 (see Supplementary Tables 1 and 2) to 14 cortical and subcortical gray matter regions (Table 3) and 5 cerebral white matter regions (Table 4). Together with the covariates, these regions significantly—though modestly—distinguished fallers from non-fallers, based on an area under the receiver operating curve of 0.70, 95% CI [0.59, 0.80]. Analysis of covariance results showed that compared with non-fallers, fallers had lower volume in gray matter and subcortical structures (Table 3), as well as lower volume in regional cerebral white matter at baseline (Table 4). Specifically, after adjusting for baseline age, total white matter hypointensity volume, and estimated total intracranial volume, fallers had significantly lower gray matter volume in left lateral orbitofrontal cortex (p=0.03) and right insula (p=0.01). For subcortical structures, fallers had significantly lower total volume in total pallidum (p=0.01) and total hippocampus (p=0.02). Left insula gray matter volume (p=0.05) and right rostral anterior cingulate cortex volume (p=0.06) showed trend level differences between the two groups, with fallers exhibiting lower left insula and higher right rostral anterior cingulate cortex volumes. For cerebral white matter volume, fallers had significantly lower volume in left lateral orbitofrontal cortex (p=0.01), left pars triangularis (p=0.03), and right pars triangularis (p=0.02).
Table 4.
Regional White Matter Volumetric Differences between Fallers and Non-Fallers
| Regions | Non-fallers (N=36) | Fallers (N=30) | ||||
|---|---|---|---|---|---|---|
| Structural Volume (mm^3) | SD | Structural Volume (mm^3) | SD | p-value* | ||
| Frontal Areas | Laterality | |||||
| Caudal anterior cingulate | RH | 2868.55 | 512.26 | 2858.64 | 577.98 | 0.59 |
| Lateral orbitofrontal | LH | 6551.07 | 863.94 | 6042.02 | 704.27 | 0.01 |
| Pars triangularis | LH | 2928.88 | 474.45 | 2625.87 | 358.52 | 0.03 |
| Pars triangularis | RH | 3139.51 | 454.87 | 2817.54 | 412.86 | 0.02 |
| Parietal Areas | ||||||
| Inferior parietal | LH | 10238.39 | 1688.24 | 9412.48 | 1482.45 | 0.07 |
Statistical comparisons were conducted by performing ANCOVA on SPSS.
Note: LH = Left-hemisphere; RH= Right-hemisphere.
All comparisons were adjusted for baseline age, total white matter hypointensity volume, and estimated total intracranial volume. Bolded values represent p<0.05 and italicized values represent trend-level effects (p<0.10).
3.3. Linear Regression Models
We constructed two multiple linear regression models across all 66 participants: one to test the unique contribution of left lateral orbitofrontal cortex white matter volume to changes in set shifting performance (i.e., Trail-Making Test performance; (B-A)/A) and a second to test the unique predictive association between hippocampal volume and changes in information processing, working memory, and psychomotor speed (i.e., DSST performance).
Change in Set Shifting Performance
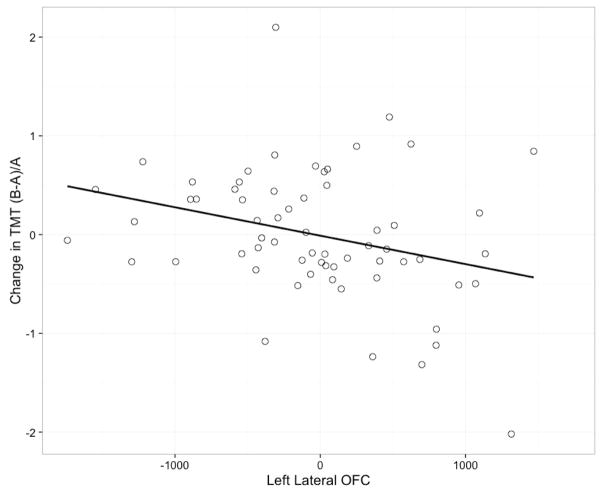
In the final model, left lateral orbitofrontal cortex white matter volume was significantly associated with change in set shifting performance. Falls status, age and white matter hypointensity volume together accounted for 2.00% of the variance. Adding left lateral orbitofronal white matter volume to the model resulted in a R2 change of 9.00% (F Change 5.84, p=0.02) and a total R2 of 11.00% (Table 5).
Table 5.
Linear Regression Model for Change in Set Shifting Performance
| Δ Set Shifting╫ | |||||
|---|---|---|---|---|---|
| Independent Variable | R2 | R2 Change | Unstandardized B (SE) | Standardized B | p-value* |
| Model 1 | 0.02 | ||||
| Falls status | 0.18 (0.17) | 0.14 | 0.31 | ||
| Age (yr) | 0.01 (0.03) | 0.04 | 0.79 | ||
| White matter hypointensity (mm^3) | −0.08 (0.31) | −0.04 | 0.78 | ||
| Model 2 | 0.11 | 0.09 | 0.02 | ||
| Falls status | 0.01 (0.18) | 0.01 | 0.95 | ||
| Age (yr) | −0.02 (0.03) | −0.08 | 0.58 | ||
| White matter hypointensity (mm^3) | 0.20 (0.32) | 0.09 | 0.54 | ||
| LH-lateral orbitofrontal white matter volume(mm^3) | −0.01 (0.01) | −0.35 | 0.02 |
Note: LH = Left-hemisphere.
Bolded values represent p<0.05
Calculated as 12-month (TMTB-TMTA)/TMTA – baseline (TMTB-TMTA)/TMTA
Change in Information Processing, Working Memory, and Psychomotor Speed Performance
In the final model, there was a non-significant association between total hippocampal volume and change in information processing and psychomotor speed performance (p=0.13). Falls status, age and white matter hypointensity volume together accounted for 8.00% of the variance. Adding hippocampal volume to the model resulted in a R2 change of 4.00% (F Change 2.41, p=0.13) and a total R2 of 12.00% (Table 6)
Table 6.
Linear Regression Model Summary for Change in Information Processing, Working Memory, and Psychomotor Speed Performance
| Δ DSST╫ | |||||
|---|---|---|---|---|---|
| Independent Variable | R2 | R2 Change | Unstandardized B (SE) | Standardized B | p-value* |
| Model 1 | 0.08 | ||||
| Falls status | −1.53 (1.10) | −0.18 | 0.17 | ||
| Age (yr) | 0.02 (0.19) | 0.02 | 0.91 | ||
| White matter hypointensity (mm^3) | −2.89 (1.94) | −0.19 | 0.14 | ||
| Model 2 | 0.12 | 0.04 | 0.13 | ||
| Falls status | −1.06 (1.13) | −0.12 | 0.35 | ||
| Age (yr) | 0.11 (0.19) | 0.07 | 0.58 | ||
| White matter hypointensity (mm^3) | −2.68 (1.93) | −0.18 | 0.17 | ||
| Hippocampus (mm^3) | <0.01 (<0.01) | 0.21 | 0.13 |
Bolded values represent p<0.05
Calculated as 12-month DSST – baseline DSST
4. DISCUSSION
Compared with non-fallers, we found that community-dwelling older fallers without dementia demonstrated significantly lower gray matter volume, subcortical volume, and cerebral white matter volume. Specifically, fallers demonstrated lower gray matter in left lateral orbitofrontal cortex, right insula, pallidum, and hippocampus. Fallers also showed lower cerebral white matter volume in left lateral orbitofrontal cortex and bilateral pars triangularis. Moreover, there was a trend for fallers to demonstrate lower gray matter volume in the left insula and right rostral anterior cingulate cortex. These areas significantly contribute to executive functions (i.e. left lateral orbitofrontal cortex, bilateral pars triangularis, and left superior frontal gyrus) (Bench et al. 1993; Elliott et al. 2000; Aron et al. 2003; Cools et al. 2004), motor control (i.e. pallidum and hippocampus) (Wichmann and DeLong 1996; Rosso et al. 2014; Beauchet et al. 2015), and memory (i.e., left pars triangularis and hippocampus) (Squire 1992; Badre and Wagner 2007). Notably, lower left lateral orbitofrontal cortex white matter volume at baseline was independently associated with greater decline in set shifting performance over the 12-month period. This significant association was observed even after adjusting for baseline age and total white matter hypointensity volume. Thus, a recent history of multiple falls among older adults without a diagnosis of dementia may indicate the presence of subclinical alterations in regional brain volume that are associated with subsequent decline in executive functions.
Our cross-sectional neuroimaging findings concur with previous findings of associations between impaired mobility and lower gray matter and subcortical volume among community-dwelling older adults without a diagnosis of dementia. Among 326 community-dwelling older adults, Rosano and colleagues (Rosano et al. 2007) demonstrated that lower volume in the basal ganglia and superior parietal cortex were significantly associated with impaired balance control. Recently, Rosso and colleagues (Rosso et al. 2014) extended these previous findings by demonstrating that lower integrity (or high mean diffusivity as measured via diffusion tensor imaging) of normal-appearing gray matter, especially of the hippocampus and anterior cingulate gyrus, was associated with higher step length variability (i.e., poor gait control). A number of cross-sectional studies have found that lower hippocampal volume is associated with poor gait control (Zimmerman et al. 2009; Shimada et al. 2013; Rosso et al. 2014; Beauchet et al. 2015). Specifically, Beauchet and colleagues (Beauchet et al. 2015) recently showed that lower hippocampal volume was associated with higher gait variability among older adults with MCI. However, among other cognitively healthy older adults, higher hippocampal volume was associated with higher gait variability. Thus, more research is needed to better understand the role of the hippocampus in mobility. With some exceptions, the majority of the evidence to date suggests that gray matter is more strongly associated with impaired mobility among community-dwelling older adults without a diagnosis of dementia, compared to white matter, (Rosano et al. 2007; Zimmerman et al. 2009; Shimada et al. 2013; Rosso et al. 2014; Beauchet et al. 2015). Furthermore, we extend previous studies by demonstrating differences in regional cerebral white matter volume between fallers and non-fallers. To our knowledge, this has not been previously demonstrated. Nevertheless, it does concur with observations that show covert white matter lesions are associated with impaired mobility (Guttmann et al. 2000; Benson et al. 2002).
Our finding of regional volumetric differences between fallers and non-fallers may have potential implication in the association between impaired executive functions and mobility (Anstey et al. 2009). Specifically, compared with non-fallers, fallers demonstrated lower volume in the left lateral orbitofrontal cortex, right insula, bilateral pars triangularis, and left superior frontal gyrus. These regions significantly contribute to executive functions. Executive functions are higher order cognitive processes that control, integrate, organize, and maintain other cognitive abilities (Stuss and Alexander 2000). Current evidence suggest that reduced executive functions are associated with falls (Rapport et al. 1993; Lundin-Olsson et al. 1997; Rapport et al. 1998; Lord and Fitzpatrick 2001; Anstey et al. 2006) and with increased risk of a major fall-related injury (Nevitt et al. 1991). Moreover, Anstey and colleagues (Anstey et al. 2009) demonstrated that compared with non-fallers, fallers performed significantly worse in processing speed and set-shifting tasks. Intriguingly, however, we did not observe statistical differences in overt cognitive performance—at baseline or over time—between fallers and non-fallers in our sample, although fallers consistently had lower scores numerically. We postulate that this observation may be attributed to: 1) a 12-month follow-up may not provide sufficient time for fallers to show significant decline in cognitive function; 2) our study population consisted of older adults with significant falls history but were without a formal diagnosis of cognitive impairment or dementia, which could constrict the variance in executive function performance with the sample. Also, this observation supports those findings of Buracchio and colleagues (Buracchio et al. 2010), who demonstrated that impaired mobility precedes clinical evidence of cognitive decline.
The right insula is implicated in mediating the switching between the executive and default-mode networks (Sridharan et al. 2008), the latter of which is associated with mind wandering epochs (Mason et al. 2007; Christoff et al. 2009). We previously demonstrated that falls are associated with an increased propensity to mind wander, or having one’s thoughts and attention transiently drift away from the on-going task at hand (Nagamatsu et al. 2013). Thus, our current finding of lower grey matter volume in the right insula in fallers, relative to non-fallers, suggests that fallers may be susceptible to falls because they have reduced ability to re-engage executive functions once in a mind wandering state, such that they are slower in applying executive resources in response to dynamic, external task demands. While this remains speculative at this point, this possibility is an intriguing line of future inquiry.
In addition, we found that fallers had significantly lower hippocampal volume compared with non-fallers. There is also growing recognition of an association between impaired memory and mobility (van Schoor et al. 2002; Montero-Odasso et al. 2009; Beauchet et al. 2014). Specifically, higher stride time variability was significantly associated with lower episodic memory performance among community-dwelling older adults (Beauchet et al. 2014). We note that while the hippocampus is a key brain structure for memory, there is evidence to suggest that the left pars triangularis is involved in the cognitive control of memory (Petrides 2002; Badre et al. 2005; Badre and Wagner 2007). Moreover, of relevance to our study, the right pars triangularis is involved in the executive process of motor inhibition control (Levy and Wagner 2011). Emerging evidence in the literature also suggests that the hippocampus may play a larger role in falls-relevant cognitive processing than being solely involved in memory function (Shohamy and Turk-Browne 2013), including spatial-processing (Johnson and Redish 2007), navigation (Pfeiffer and Foster 2013), and attention (Reas and Brewer 2013).
Notably, our study also provides novel data that demonstrate the prospective impact of regional cerebral white matter volumetric differences associated with impaired mobility, indexed as a recent history of multiple falls in this study, on cognitive performance among community-dwelling older adults without a diagnosis of dementia. Specifically, lower left lateral orbitofrontal cortex white matter volume at baseline was significantly associated with greater decline in set shifting performance. Previous studies have shown that the lateral orbitofrontal region is involved in set shifting (Cools et al. 2004; Remijnse et al. 2005; Hampshire et al. 2012) and inhibitory control (Bench et al. 1993; Elliott et al. 2000; Aron et al. 2003; Cools et al. 2004).
In line with this, impaired set shifting has been identified as a significant cognitive risk factor for falls (Nevitt et al. 1991; Lord and Fitzpatrick 2001). Specifically, in a prospective study with 325 community-dwelling older adults, Nevitt and colleagues (Nevitt et al. 1991) demonstrated that slower Trail Making B test time was independently associated with greater risk for injurious falls. Among older adults between the age of 62 to 95 years, Lord and colleagues (Lord and Fitzpatrick 2001) also found reduced set-shifting ability was associated with reduced performance in choice-stepping task. Moreover, those with a history of falls displayed poorer performance in choice-stepping reaction time compared with non-fallers. Our current prospective findings extends the current understanding of the neural correlates that underpin the observed relationship between reduced inhibitory control, reduced set shifting, and impaired mobility. Specifically, a recent history of falls was associated with less left lateral orbitofrontal cortex white matter volume, which was independently associated with subsequent decline in set shifting performance over a 12-month period. Together with previous research, our current study highlights the concomitant, bi-directional relationship between falls risk and cognitive decline in community-dwelling older adults.
We recognize the limitations of our study. The validity of our findings depends on accurate identification of recurrent fallers and non-fallers and previous research has demonstrated that falls recall in older adults is subject to retrospective recall bias (Cummings et al. 1988). However, we corroborated falls history with immediate family members or close friends. Our study sample consisted exclusively of independent community-dwelling older adults specifically between the age-range of 70–80 who were without significant physical or cognitive impairment. Thus, our results may not generalize beyond this population of older adults and we may have underestimated the association between falls and cortical and subcortical volume. With respect to study inclusion/exclusion, we have only excluded older adults taking psychotropic medication and thus our study sample may include older adults taking medication of other pharmacological classes or under the influence of polypharmacy. In addition, atlas registration procedure performed during the MRI analysis may have reduced inter-subject variability and impacted the accuracy of gray matter volume estimation. Furthermore, we did not perform a comprehensive assessment of memory function within the scope of the study; thus, we cannot eliminate the possibility that our study sample may be consisted of a mix of older adults with and without amnestic MCI. Also, our study may have been underpowered, as indicated by the several trend-level associations we found. Also due to the small sample size and concern over power, we did not adjust the significance level to account for multiple testing, which might lead to false positive findings. Although we observed a prospective relationship between baseline brain volume and changes in cognition, future longitudinal studies—in which both brain volume and cognition are assessed repeatedly over time—would provide more direct evidence that changes in the brain regions identified herein among fallers precede changes in executive function. Lastly, fMRI studies could shed light on the functional importance of the brain differences observed between fallers and non-fallers.
In summary, community-dwelling older adults with a recent history of multiple falls demonstrated lower gray matter volume, subcortical volume, and cerebral white matter volume, compared with non-fallers. Importantly, lower left lateral orbitofrontal cortex white matter volume at baseline was independently associated with greater decline in executive functions over the 12-month period. A better understanding of the neural correlates that underlie the association between impaired mobility and cognitive decline improves our capacity to refine and develop intervention to maintain mobility among older adults. Moreover, in consideration of our findings as well as those of previous studies, health care professionals working with older adults should consider falls history in their assessment to potentially identify those who may be at greater risk for subsequent cognitive decline.
Figure 1.
Change in Cognitive Performance vs. Left Lateral Orbitofrontal Cortex
Note: Values on the graph reflect residuals after adjusting for falls status, age, and white matter hypointensity.
Acknowledgments
CLH is an Alzheimer Society Research Program Doctoral trainee. JRB is a Canadian Institutes of Health Research and Michael Smith Foundation of Health Research Post-Doctoral Fellow. TLA is a Canada Research Chair (Tier II) in Physical Activity, Mobility, and Cognitive Neuroscience. This work was supported by the Canadian Institute of Health Research (MOB-93373) and Alzheimer’s Society of Canada Research Program to TLA.
References
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26(9):1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279–1282. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, von Sanden C, Luszcz MA. An 8-year prospective study of the relationship between cognitive performance and falling in very old adults. J Am Geriatr Soc. 2006;54(8):1169–1176. doi: 10.1111/j.1532-5415.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Wood J, Kerr G, Caldwell H, Lord SR. Different cognitive profiles for single compared with recurrent fallers without dementia. Neuropsychology. 2009;23(4):500–508. doi: 10.1037/a0015389. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60(3):393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Allali G, Montero-Odasso M, Sejdic E, Fantino B, Annweiler C. Motor phenotype of decline in cognitive performance among community-dwellers without dementia: population-based study and meta-analysis. PLoS One. 2014;9(6):e99318. doi: 10.1371/journal.pone.0099318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Launay CP, Annweiler C, Allali G. Hippocampal volume, early cognitive decline and gait variability: which association? Exp Gerontol. 2015;61:98–104. doi: 10.1016/j.exger.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, Dolan RJ. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31(9):907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Benson RR, Guttmann CR, Wei X, Warfield SK, Hall C, Schmidt JA, Kikinis R, Wolfson LI. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58(1):48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, Srikanth VK. Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc. 2013;61(7):1074–1079. doi: 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacol Bull. 1988;24(4):689–692. [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24(5):1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings S, Nevitt M, Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. Journal of the American Geriatrics Society. 1988;36:613–616. doi: 10.1111/j.1532-5415.1988.tb06155.x. [DOI] [PubMed] [Google Scholar]
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbaere K, Close JC, Heim J, Sachdev PS, Brodaty H, Slavin MJ, Kochan NA, Lord SR. A multifactorial approach to understanding fall risk in older people. J Am Geriatr Soc. 2010;58(9):1679–1685. doi: 10.1111/j.1532-5415.2010.03017.x. [DOI] [PubMed] [Google Scholar]
- Delbaere K, Close JCT, Brodaty H, Sachdev P, Lord SR. Determinants of disparities between perceived and physiological risk o falling among elderly people: cohort study. British Medical Journal. 2010 doi: 10.1136/bmj.c4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Ezzati A, Katz MJ, Lipton ML, Lipton RB, Verghese J. The association of brain structure with gait velocity in older adults: a quantitative volumetric analysis of brain MRI. Neuroradiology. 2015 doi: 10.1007/s00234-015-1536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Graf P, Uttl B, Tuokko H. Color- and picture-word Stroop tests: performance changes in old age. J Clin Exp Neuropsychol. 1995;17(3):390–415. doi: 10.1080/01688639508405132. [DOI] [PubMed] [Google Scholar]
- Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N Engl J Med. 1995;332(9):556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann CR, Benson R, Warfield SK, Wei X, Anderson MC, Hall CB, Abu-Hasaballah K, Mugler JP, 3rd, Wolfson L. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54(6):1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chaudhry AM, Owen AM, Roberts AC. Dissociable roles for lateral orbitofrontal cortex and lateral prefrontal cortex during preference driven reversal learning. Neuroimage. 2012;59(4):4102–4112. doi: 10.1016/j.neuroimage.2011.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer K, Lamb SE, Jorstad EC, Todd C, Becker C. Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age Ageing. 2006;35(1):5–10. doi: 10.1093/ageing/afi218. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Voss MW, Handy TC, Davis JC, Nagamatsu LS, Chan A, Bolandzadeh N, Liu-Ambrose T. Disruptions in brain networks of older fallers are associated with subsequent cognitive decline: a 12-month prospective exploratory study. PLoS One. 2014;9(4):e93673. doi: 10.1371/journal.pone.0093673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, Harris TB, Rosano C. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29(3–4):156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol. 2006;59(4):673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27(45):12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Lord S, Clark R, Webster I. Physiological factors associated with falls in an elderly population. Journal of American Geriatrics Society. 1991;39:1194–1200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Lord S, Fitzpatrick R. Choice stepping reaction time: A composite measure of fall risk in older people. Journal of Gerontology. 2001;10:M627–632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- Lord S, Ward J, Williams P, Anstey K. Physiological factors associated with falls in older community-dwelling women. Journal of American Geriatrics Society. 1994;42:1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- Lord SR, Castell S. Physical activity program for older persons: effect on balance, strength, neuromuscular control, and reaction time. Arch Phys Med Rehabil. 1994;75(6):648–652. doi: 10.1016/0003-9993(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. J Am Geriatr Soc. 1991;39(12):1194–1200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Lord SR, Fitzpatrick RC. Choice stepping reaction time: a composite measure of falls risk in older people. J Gerontol A Biol Sci Med Sci. 2001;56(10):M627–632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc. 1994;42(10):1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- Lundin-Olsson L, Nyberg L, Gustafson Y. Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349(9052):617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009;9:41. doi: 10.1186/1471-2318-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60(11):2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65(4):565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu LS, Kam JW, Liu-Ambrose T, Chan A, Handy TC. Mind-wandering and falls risk in older adults. Psychol Aging. 2013;28(3):685–691. doi: 10.1037/a0034197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46(5):M164–170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261(18):2663–2668. [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16(7):907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Petrides M. The mid-ventrolateral prefrontal cortex and active mnemonic retrieval. Neurobiol Learn Mem. 2002;78(3):528–538. doi: 10.1006/nlme.2002.4107. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497(7447):74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport LJ, Hanks RA, Millis SR, Deshpande SA. Executive functioning and predictors of falls in the rehabilitation setting. Archives of Physical Medicine and Rehabilitation. 1998;79(6):629–633. doi: 10.1016/s0003-9993(98)90035-1. [DOI] [PubMed] [Google Scholar]
- Rapport LJ, Webster JS, Flemming KL, Lindberg JW, Godlewski MC, Brees JE, Abadee PS. Predictors of falls among right-hemisphere stroke patients in the rehabilitation setting. Arch Phys Med Rehabil. 1993;74(6):621–626. doi: 10.1016/0003-9993(93)90160-c. [DOI] [PubMed] [Google Scholar]
- Reas ET, Brewer JB. Imbalance of incidental encoding across tasks: an explanation for non-memory-related hippocampal activations? J Exp Psychol Gen. 2013;142(4):1171–1179. doi: 10.1037/a0033461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26(2):609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62(9):1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- Rosso AL, Olson Hunt MJ, Yang M, Brach JS, Harris TB, Newman AB, Satterfield S, Studenski SA, Yaffe K, Aizenstein HJ, Rosano C. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture. 2014;40(1):225–230. doi: 10.1016/j.gaitpost.2014.03.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Olson Hunt MJ, Yang M, Brach JS, Harris TB, Newman AB, Satterfield S, Studenski SA, Yaffe K, Aizenstein HJ, Rosano C A. B. C. s. Health. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture. 2014;40(1):225–230. doi: 10.1016/j.gaitpost.2014.03.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009;44(4):1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott V, Wagar B, Sum A, Metcalfe S, Wagar L. A public health approach to fall prevention among older persons in Canada. Clin Geriatr Med. 2010;26(4):705–718. doi: 10.1016/j.cger.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shimada H, Ishii K, Ishiwata K, Oda K, Suzukawa M, Makizako H, Doi T, Suzuki T. Gait adaptability and brain activity during unaccustomed treadmill walking in healthy elderly females. Gait Posture. 2013;38(2):203–208. doi: 10.1016/j.gaitpost.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Turk-Browne NB. Mechanisms for widespread hippocampal involvement in cognition. J Exp Psychol Gen. 2013;142(4):1159–1170. doi: 10.1037/a0034461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- Spreen OSE. A Compendium of Neurological Tests. 2. New York: Oxford University Press, Inc; 1998. [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SL, Roe CM, Grant EA, Hollingsworth H, Benzinger TL, Fagan AM, Buckles VD, Morris JC. Preclinical Alzheimer disease and risk of falls. Neurology. 2013;81(5):437–443. doi: 10.1212/WNL.0b013e31829d8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63(3–4):289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- United Nations, D. o. E. a. S. A., Population Division. World Population Ageing 2013. 2013 ST/ESA/SER.A/348. [Google Scholar]
- van Marwijk HW, Wallace P, de Bock GH, Hermans J, Kaptein AA, Mulder JD. Evaluation of the feasibility, reliability and diagnostic value of shortened versions of the geriatric depression scale. Br J Gen Pract. 1995;45(393):195–199. [PMC free article] [PubMed] [Google Scholar]
- van Schoor NM, Smit JH, Pluijm SM, Jonker C, Lips P. Different cognitive functions in relation to falls among older persons. Immediate memory as an independent risk factor for falls. J Clin Epidemiol. 2002;55(9):855–862. doi: 10.1016/s0895-4356(02)00438-9. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347(22):1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): Evidence for validity. J Clin Epidemiol. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised. The Psychological Corporation; Harcourt Brace Jovanovich: 1981. [Google Scholar]
- Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57(6):990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6(6):751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24(4):709–711. [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]