Abstract
Cannabis continues to rise in popularity as the perception of its harmfulness decreases and evidence of its deleterious developmental effect increases. While internalizing distress and suicide risk have been linked with cannabis use problems (DSM-5 cannabis use disorder (CUD); DSM-IV cannabis abuse and dependence) it remains unclear how this association varies over the course of development in treatment-seeking men and women. The current study utilized the National Drug Abuse Treatment Clinical Trials Network (NIDA CTN) to conduct a cross-sectional comparison of internalizing distress and suicide risk among men (n=437) and women (n=163) spanning ages 18–50 who met DSM-5 criteria for CUD. Interactions between gender and developmental stage (i.e., late adolescence, early adulthood, and middle adulthood) were observed for suicide risk and anxiety but not depression problems. Specifically, women seeking CUD treatment in late adolescence and middle adulthood exhibited significantly higher rates of anxiety and suicide risk compared to men seeking treatment during the same developmental stages. Internalizing distress and suicide risk did not differ between treatment-seeking men and women in the early adult stage. Overall, results suggest that the structure of risk for CUD may differ in men and women across the lifespan and that women presenting for CUD treatment during late adolescence and middle adulthood may uniquely benefit from intervention designed to address these elevations in anxiety and suicide risk.
Keywords: Cannabis, Marijuana, Internalizing symptoms, Suicide risk, Gender, Development
1. Introduction
Already the most commonly used illicit drug worldwide (United Nations Office on Drugs and Crime, 2010), cannabis continues to gain popularity (SAMHSA, 2012) as the perception of its harmfulness decreases (Compton, Grant, Colliver, Glantz, & Stinson, 2004; Johnston, O'Malley, Bachman, et al., 2013). Concurrently however, evidence of the deleterious developmental effect of cannabis use is prominent (Budney & Moore, 2002; Hall & Degenhardt, 2009; Volkow, Baler, Compton, & Weiss, 2014). Prolonged and compulsive cannabis use increases vulnerability to cannabis use disorder (DSM-5 cannabis use disorder (CUD); DSM-IV cannabis abuse and dependence) and its associated psychosocial impairments. In particular, internalizing symptoms like anxiety, depression, and suicide risk have been routinely linked with CUD yet the relationship between these factors and CUD severity remains unclear (Buckner et al., 2008; Degenhardt, Hall, & Lynskey, 2003; Grotenhermen, 2003; King, Iacono, & McGue, 2004; McQueeny et al., 2011; Van Dam, Bedi, & Earleywine, 2012). Prior research suggests that psychosocial problems – like internalizing symptoms – that co-occur with alcohol use disorders likely vary by gender and development (Foster, Hicks, Iacono, & McGue, 2014; Hicks, Iacono, & Mcgue, 2010) but these variations have yet to be characterized for CUD. Directly testing gender differences in the relative severity of anxiety, depression, and suicide risk among those with CUD from late adolescence through middle adulthood will be an important step in further clarifying their role in CUD severity and effectively tailoring clinical intervention.
As men constitute 75% of the population of cannabis users (SAMHSA, 2012), women have historically been underrepresented in investigations of cannabis use problems. Consequently, understanding of gender-specific risks and consequences that co-occur with CUD during the transition from adolescence through middle adulthood is limited. Prior research on use of both alcohol and cannabis in men and women has detected that women paradoxically exhibit more severe psychosocial risks and consequences for use compared to men (Foster, Hicks, Iacono, & McGue, 2015; Khan et al., 2013) even though they develop problems with both substances less frequently (e.g., 5.4% of adult women compared to 11.8% of adult men meet criteria for lifetime cannabis dependence; Stinson, Ruan, Pickering, & Grant, 2006). Specifically, women exhibit higher sensitivity to the acute effects of cannabis (e.g., Cooper & Haney, 2014), greater vulnerability to the deleterious neurodevelopmental effects of protracted cannabis use (e.g., McQueeny et al., 2011), and experience larger reductions in quality of life and greater social stigma surrounding cannabis use (e.g., Lev-Ran et al., 2012). With these significant deterrents averting cannabis use in women, higher levels of premorbid risk exposure are likely more prevalent among the small proportion of women who develop CUD. Subsequently, higher rates of both psychosocial risks and consequences are likely to coincide with CUD in women compared to men.
Though internalizing distress is more prevalent among women, this distress appears to have a gender specific relationship with alcohol and cannabis use problems (Foster et al., 2014; Foster et al., 2015; Khan et al., 2013). For example, women with CUD exhibit higher rates of major depression than men with CUD (Khan et al., 2013). Additionally, anxiety has been shown to temporally predict the emergence of cannabis use problems later in development (Buckner et al., 2008). While research comparing these effects in men and women is limited, internalizing symptoms are a stronger predictor for alcohol and other illicit drug use problems in women compared to men (Foster et al., 2015), suggesting that the same pattern is likely present for CUD. Furthermore, both depression and anxiety have also been identified as important predictors of CUD relapse for women compared to men (Flórez-Salamanca et al., 2013). While these studies establish the importance of expanding knowledge regarding gender differences in cannabis use, gender differences in the relative importance of anxiety, depression, and suicide risk in CUD severity has not been thoroughly explored.
The time when CUD is present during development may also be a key determinant of the severity of psychosocial problems in men and women. Cannabis problems generally emerge in adolescence (Swift, Coffey, Carlin, Degenhardt, & Patton, 2008; Volkow et al., 2014; Wagner & Anthony, 2002), escalate through early adulthood (Jager, Schulenberg, O’Malley, & Bachman, 2013; Tucker, Ellickson, Orlando, Martino, & Klein, 2005), and stabilize through middle and later adulthood (Chen & Kandel, 1995; Coffey, Lynskey, Wolfe, & Patton, 2000; Kandel & Davies, 1992). Cannabis problems emergent during atypical periods of risk (i.e., adolescence or middle adulthood) may be associated with more severe psychosocial problems compared to those that emerge during a more common period (i.e., early adulthood) (Chen & Kandel, 1995; Hicks et al., 2010; Schuster, O’Malley, Bachman, Johnston, & Schulenberg, 2001; Tucker, Ellickson, Orlando, Martino, & Klein, 2005a).
While developmental typologies are understudied in CUD samples, those for other substance use problems are well documented. For instance, men with alcohol use problems emergent during early adulthood (i.e., when risk for substance use problems escalates and role transition begins) often exhibit a developmentally-limited course wherein few preceding risks and long-term consequences are evident (Babor et al., 1992; Hicks et al., 2010; Leggio, Kenna, Fenton, Bonenfant, & Swift, 2009). Similar developmental patterns are not evident in women as risks and consequences associated with alcohol use problems appear uniformly severe (Foster, Hicks, Iacono, & Mcgue, 2014). To determine if similar typologies of risk are present for CUD, studies are needed to estimate the severity of co-occurring psychosocial problems like internalizing symptoms across these key periods of transition in the lifespan (i.e., adolescent, early adulthood, and middle adulthood) when social roles and CUD prevalence typically shift.
When taken together, previous literature suggests that internalizing distress is likely elevated for CUD in women compared to men and that this relationship likely varies by development. While multiple studies have estimated the base rates of internalizing disorders co-occurring with CUD, few studies have directly compared the degree of severity of these symptoms in a clinical sample of men and women with CUD. Prior study has typically focused on a single developmental period (i.e., either adolescence or adulthood), precluding estimation of how shifting role responsibilities across developmental periods (e.g., increasing independence from primary caregivers, career initiation, marital relationships, parenthood) moderate the psychosocial problems linked with CUD. Defining these risk factors and their prominence across key demographic variables of developmental stage will provide additional insight into treatment barriers that require additional attention.
To address these limitations, the current study estimated anxiety, depression, and suicide risk among men and women who meet DSM-5 criteria for CUD during the transition from late adolescence to middle adulthood (i.e., age 18–50). A cross-sectional developmental framework was used to organize our analyses around direct gender comparisons at periods of development that coincide with shifts in CUD prevalence (i.e., late adolescent onset, young adult escalation, and middle adult persistence) and key social role transitions (i.e., increasing personal independence in late adolescence, increasing responsibility in young adulthood, and stabilization through middle adulthood). Severity of internalizing distress among those with CUD is hypothesized to vary by both gender and developmental stage such that women with CUD would have higher levels of internalizing distress relative to men with CUD and that these differences would increase in parallel with developmental shifts in CUD risk.
2. Material and methods
2.1 Clinical sample and setting
Participants (n = 600) were treatment-seeking men and women between 18 and 50 years of age screened for their eligibility for a 12-week clinical efficacy trial of N-acetylcysteine (NAC) for cannabis cessation (see McClure et al., 2014 for details of the larger trial). The current sample was comprised of all participants who met DSM-5 criteria for CUD in the previous 30 days, irrespective of their eligibility for the larger trial. Participating clinical sites were identified through the National Drug Abuse Treatment Clinical Trials Network (NIDA CTN) that spans clinical settings across the United States. To increase the representativeness of the CUD sample, efforts were made to recruit the same proportions of minorities present in the communities of each site. Using their age, participants were further divided into developmental groups that reflect distinct periods of CUD prevalence rates and social role responsibilities: late adolescence (i.e., age 18–22; when personal independence increases and CUD onset occurs in both genders), early adulthood (i.e., age 23–40; when CUD prevalence escalates and significant role transitions involving career, marital relationship(s), and parenthood occur), and middle adulthood (i.e., age 41 and over; when CUD prevalence and social responsibilities typically stabilize) (Chen & Jacobson, 2012; Englund et al., 2013; Perkonigg et al., 2008).
2.2. Assessment
Following brief pre-screening to ascertain the probability of CUD via phone or in-person, participants provided IRB-approved written informed consent prior to entering a baseline assessment phase. Over one-week, trained study personnel conducted a battery of diagnostic, medical and psychosocial assessments to attain initial information about each participant before being randomized into the clinical trial.
2.2.1 CUD Diagnosis and Substance Use Variables
During the baseline assessment, trained staff administered the DSM-IV checklist to diagnose lifetime cannabis abuse and dependence (DSM-IV) along with a separate query for craving status in the previous 30-day period. These data were subsequently combined during analysis to classify CUD based on the current DSM-5 nosology. In addition to reporting CUD symptoms in the diagnostic interview, participants also reported the age of onset for CUD symptoms in order to control for developmental timing of symptoms. Participants also completed a battery of measures to characterize multiple aspects of cannabis and other substance use across groups. Cannabis use frequency was quantified using a 30-day timeline followback calendar (Sobell, Brown, Leo, & Sobell, 1996; Sobell & Sobell, 1973). CUD use was also confirmed via urine drug screen (UDS) test of cannabinoid levels obtained as a part of the intake assessment. Craving was rated using the Marijuana Craving Questionnaire (Heishman, Singleton, & Liguori, 2001; Heishman & Singleton, 2006), in which participants rate their level of agreement with statements like “I could not easily limit how much marijuana I smoked right now” and “It would be great to smoke marijuana right now” resulting in a sum score ranging from 12–84. Participants also rated the degree to which they experienced 19 different types of problems as a result of smoking cannabis in the past month (i.e., Marijuana Problems Scale - MPS, range 0–38) (Stephens, Roffman, & Curtin, 2000). The MPS has demonstrated internal consistency (Buckner & Zvolensky, 2014; Stephens et al., 2000) for the measurement of problems ranging from interpersonal conflict to financial and legal difficulties. A sum of the number of other substances that participants reported previously using estimated polysubstance use. Nicotine dependence was assessed via self-report of nicotine use (i.e., Fagerström Test for Nicotine Dependence; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
2.2.2 Internalizing distress and suicide risk
Internalizing symptoms were indexed using the Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, 1983), a 14-item self-report measure of general symptoms associated with anxiety and depression. Responses are provided on a 4-point scale (score range, 0–42) that reflects the degree to which each symptom is endorsed. Factor analysis of the HADS has confirmed its two-factor structure, compromised of separate, 7-item subscales for Anxiety and Depression (subscale score ranges, 0–21). While pathogenic and phenotypic overlap for these risk factors produces collinearity in these subscale measurements (i.e., r = 0.70; (Bjelland, Dahl, Haug, & Neckelmann, 2002), prior literature distinguishes the relationships between these factors and heavy cannabis use (Buckner et al., 2008). To assess concurrent suicide risk, participants completed the patient-report version of the Concise Health Risk Tracking Scale (CHRT; Trivedi et al., 2011) at each assessment visit. This 12-item measure (score range, 12–60) is typically used to assess level of suicide risk in clinical settings. Factor analysis has confirmed its three-factor structure in the assessment of current suicidal thoughts and plans, perceived lack of social support and hopelessness (Trivedi et al., 2011).
2.2.3 Indices of overall physical and psychiatric health
Assessments of overall physical and psychiatric health were also included for two reasons: 1) to confirm expected similarities between gender and developmental groups and 2) to determine if abnormalities in medical or psychiatric health coincide with potential group differences for primary internalizing measures. This is particularly important for cannabis, as it is commonly used to manage medical symptoms (e.g., for alleviation of chronic pain, reduction of nausea associated with chemotherapy, and treatment of glaucoma; Ogborne, Smart, & Adlaf, 2000) that may independently contribute to internalizing symptoms. Objective measures of general health included Body Mass Index (BMI), blood pressure, and resting heart rate. Each participant also underwent a general medical history evaluation in which a medical clinician assessed multifarious domains of physical health (e.g., respiratory, cardiovascular, musculoskeletal, endocrine, nervous system, and others). All prior and current diagnoses reported were summed to further index the physical health of each individual across the developmental stages measured. Non-substance related DSM-IV psychiatric diagnoses were also obtained. Diagnoses meeting full criteria were summed to compute a single index of overall mental health, separate from substance use diagnoses.
2.3 Analytic Plan
A 2 × 3 factorial analysis of covariance was conducted using the following model to estimate the effects of the categorical (i.e., gender and developmental stage) and continuous (i.e., CUD symptom count and age of CUD onset) factors on levels of each internalizing problem (i.e., depression, anxiety, and suicide risk):
Yijk = μ + αi + βj + (αβ)ij + γ1x1ijk + γ2x2ijk + εijk
Overall, the level of internalizing distress (i.e., separate models were fit for each dependent variable; Yijk) was predicted using an additive model which adjusted the grand mean (i.e., μ) for the effects of Gender (i.e., αi), Developmental Stage (i.e., βj), and the interaction between Gender and Developmental Stage, (i.e., (αβ)ij). Marginal means were additionally adjusted using continuous covariates for CUD severity (i.e., CUD symptom count; γ1x1ijk) and age of CUD onset (i.e., γ2x2ijk) in order to account for the independent influences of these variables on the internalizing problem. Bonferroni corrections were applied to account for the use of multiple comparisons and Cohen's d effect sizes quantified the relative magnitude of each effect between men and women across developmental periods. Patterns across marginal means for each developmental group were used to infer temporal changes in the common rates of internalizing factors for men and women with CUD during the transition from late adolescence to middle adulthood.
3. Results
General medical, psychiatric and substance use characteristics of the sample are demarcated by gender and developmental stage in Table 1. Effect sizes (i.e., Cohen’s d values) are included to establish expected equivalence between groups and additionally highlight the magnitude of key differences between men and women at each developmental stage. Overall, men and women exhibited similarity in average age and pulse rate, with expected differences in control variables of body mass index (d = 0.36, p < 0.01) and diastolic blood pressure (d = −0.37, p < 0.01). With regard to cannabis related variables, the frequency of cannabis use over the previous 30 days was significantly higher in men compared to women (d = −0.99, p < 0.05) and generally increased with age, though these differences were not significant. While gender differences in cannabis use frequency across developmental groups were present and often large (i.e., late adolescent group, d = −0.99; early adulthood group, d = −1.00; middle adulthood group, d = −0.91), these gender differences were not significant due to large standard deviations in frequency of use for all groups. CUD symptom count, urine cannabinoid tests, ratings of cannabis craving, and the severity of cannabis related problems were similar between men and women. Importantly, the number of psychiatric diagnoses (including internalizing disorders but not substance use disorders) was also equivalent across groups of men and women at each developmental stage. Notable gender differences were present for the average age of onset for CUD, with men reporting a significantly younger age of onset (M = 18.8) than women (M = 20.7; d = 0.31, p < 0.01). Additionally, women reported significantly more medical problems than men during both late adolescence (d = 0.52, p < 0.05) and middle adulthood (d = 0.61, p < 0.01). While no gender differences were present for tobacco use, polysubstance use (i.e., a count of additional substances used) was higher in men compared to women during the early adulthood stage (d = −0.29, p < 0.05).
Table 1.
Means, Standard Deviations, and Cohen’s d Effect Sizes for Gender and Developmental Group Differences in the Cannabis Use Disorder Sample
| Overall | Late Adolescence (age 18–22) |
Early Adulthood (age 23–40) |
Middle Adulthood (age 41+) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n=437) |
Female (n=163) |
d | Male (n=93) |
Female (n=29) |
d | Male (n=241) |
Female (n=99) |
d | Male (n=103) |
Female (n=35) |
d | ||
| General | |||||||||||||
| Age | 31.6 (9.4) | 31.7 (9.1) | 0.01 | 20.2 (1.3) | 20.2 (1.4) | 0.01 | 30.3 (4.9) | 30.2 (5.1) | −0.00 | 45.2 (3.0) | 45.5 (2.8) | 0.09 | |
| Body Mass (BMI) | 26.8 (6.6) | 29.23 (7.1) | 0.36** | 24.0 (4.4) | 27.7 (6.0) | 0.70** | 27.0 (6.3) | 28.6 (7.2) | 0.23 | 29.6 (8.4) | 33.2(6.7) | 0.46 | |
| Systolic Blood Pressure | 125.9 (14.3) | 120.1 (16.4) | −0.37** | 120.9(10.7) | 112.8 (12.5) | −0.69* | 127.1 (14.1) | 119.4 (12.9) | −0.57** | 128.9 (17) | 130.5 (24.0) | 0.08 | |
| Diastolic Blood Pressure | 76.6 (10.9) | 75.5 (11.1) | −0.10 | 70.0 (9.4) | 69.0 (10.1) | −0.09 | 77.9 (10.2) | 75.9 (9.6) | −0.20 | 81.4(11.1) | 81.2 (13.5) | −0.01 | |
| Pulse Rate | 69.5 (11.7) | 71.0 (10.6) | 0.14 | 69.9 (12.4) | 70.6 (10.2) | 0.06 | 68.7 (11.0) | 71.4 (10.2) | 0.26 | 71.2 (12.6) | 70.0 (12.7) | −0.09 | |
| Medical Problems | 5.4 (5.8) | 8.0 (7.8) | 0.39** | 4.3 (5.1) | 7.7 (7.9) | 0.52* | 5.7 (5.6) | 7.4 (7.9) | 0.25 | 5.9 (7.0) | 10.3 (7.6) | 0.61** | |
| Psychiatric Problems | 1.8 (3.0) | 2.1 (2.9) | 0.09 | 1.5 (2.5) | 2.0 (2.1) | 0.20 | 1.9 (3.1) | 1.9 (2.7) | −0.01 | 2.1 (3.4) | 2.9 (3.8) | 0.24 | |
| Cannabis | |||||||||||||
| Frequency (30-day TLFB)a | 89.7 (78.4) | 72.1 (53.3) | −0.99* | 69.9 (49.0) | 55.8 (39.8) | −0.99 | 92.1 (81.8) | 73.0 (53.8) | −1.00 | 108.0 (93.1) | 85.0 (61.0) | −0.91 | |
| CUD Symptoms | 6.2 (2.5) | 6.1 (2.3) | −0.04 | 6.4 (2.4) | 5.9 (1.9) | −0.25 | 6.3 (2.4) | 6.1 (2.3) | −0.08 | 5.8 (2.9) | 6.3 (2.7) | 0.17 | |
| Age of CUD Onset | 18.8 (5.4) | 20.7 (6.9) | 0.31** | 16.5 (2.2) | 17.1 (2.2) | 0.27 | 18.6 (4.6) | 20.1 (5.2) | 0.30* | 21.6 (8.0) | 25.1 (10.7) | 0.37 | |
| UDS Cannabinoidsb | 0.9 (0.3) | 0.9 (0.3) | 0.03 | 0.9 (0.3) | 0.9 (0.4) | −0.11 | 0.9 (0.3) | 0.9 (0.3) | 0.06 | 0.8 (0.4) | 0.8 (0.4) | 0.05 | |
| Marijuana Craving | 46.7 (16.0) | 47.2 (16.3) | 0.03 | 44.6 (15.1) | 49.4 (16.5) | 0.30 | 47.4 (16.5) | 47.1 (15.8) | −0.01 | 47.3 (15.8) | 45.1 (18.5) | −0.12 | |
| Marijuana Problems | 8.5 (6.1) | 8.8 (6.2) | 0.05 | 7.2 (4.9) | 7.4 (4.8) | 0.024 | 8.9 (6.4) | 8.3 (5.6) | −0.10 | 8.6 (6.3) | 12(8.2) | 0.47 | |
| Other Substance Use | |||||||||||||
| Tobacco Use (Faegerström) | 1.4 (2.2) | 1.2 (2.1) | −0.12 | 1.5 (2.4) | 0.8 (1.3) | −0.35 | 1.29 (1.924) | 1.2 (2.1) | −0.05 | 1.8 (2.6) | 1.6 (2.7) | −0.07 | |
| Other Drug Use | 2.4 (1.6) | 2.0 (1.7) | −0.26* | 2.3(1.6) | 1.8(1.8) | −0.31 | 2.6 (1.7) | 2.1(1.8) | 0.29* | 2.1(1.5) | 1.9(1.1) | −0.13 | |
| Internalizing Symptoms | |||||||||||||
| HADS Anxiety | 6.3 (4.0) | 7.0 (3.9) | 0.19 | 5.6 (3.5) | 8.3 (3.0) | 0.63** | 6.8 (4.1) | 6.8 (4.0) | −0.07 | 6.0 (4.0) | 8.3 (4.4) | 0.64** | |
| HADS Depression | 4.0 (3.3) | 3.7 (3.3) | −0.09 | 3.5 (3.0) | 3.6 (3.1) | 0.05 | 4.1 (3.2) | 3.4 (2.8) | −0.26 | 4.5 (3.6) | 5.2 (4.5) | 0.17 | |
| 12-item CHRI Suicide Risk | 16.3 (7.0) | 16.1(6.8) | −0.03 | 14.9 (5.7) | 19.3 (6.6) | 0.56* | 16.9 (7.2) | 15.5 (6.3) | −0.21 | 17.8 (7.4) | 18.4 (8.3) | 0.08 | |
p <.05,
p <.01; Cohen’s d values reflect comparison of women to men (i.e., positive d values reflect higher levels of variable in women compared to men;
TLFB frequency reflects number of times used in prior 30 days, accounting for multiple uses in one day;
Urine drug screen values reflect proportion of the group with a positive cannabinoid test at the intake assessment.
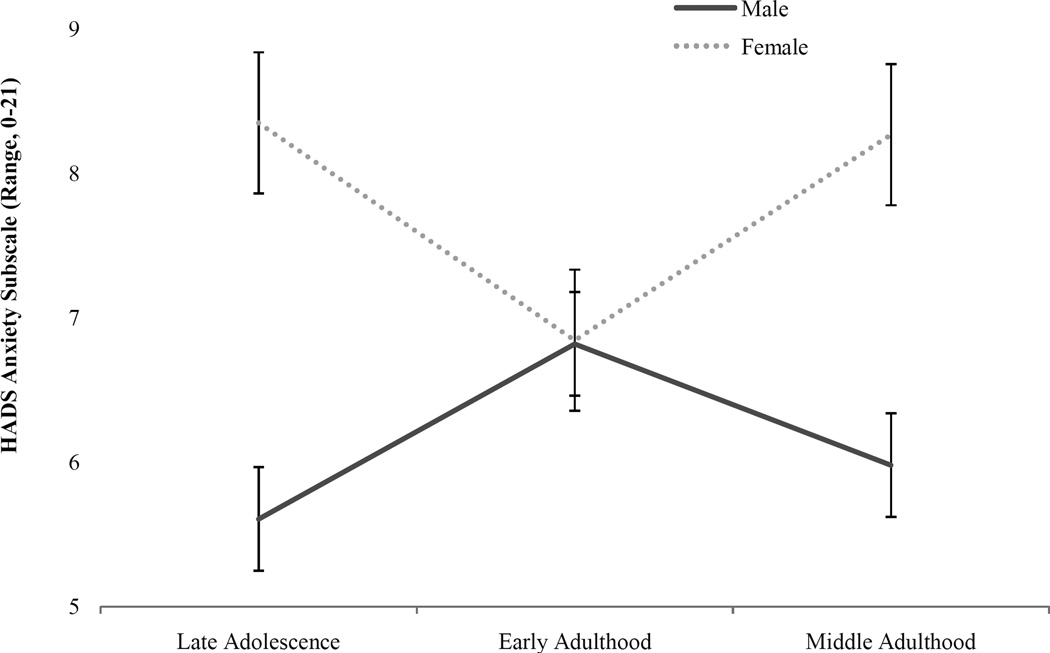
3.1. Anxiety symptoms
Marginal means for anxiety level (i.e., HADS anxiety subscale ratings; presented in Figure 1) significantly varied by CUD severity (F(1, 378) = 20.30, p = .00), gender (F(1, 378) = 10.41, p = .00) and the interaction between gender and developmental stage (F(1, 378) = 3.65, p = .02). Compared to men of the same developmental period, women exhibited significantly higher levels of anxiety problems in late adolescence (d = 0.63, p < 0.01) and middle adulthood (d = 0.63, p < 0.01). On average, women in the late adolescent and middle adulthood groups exhibited anxiety ratings exceeding the normative range for this measure (i.e., 0–7; (Zigmond & Snaith, 1983), suggesting that this level of anxiety causes clinical concern and may warrant additional treatment. In juxtaposition, rates of anxiety among men and women were nearly identical (d = −0.07, p > 0.05) during early adulthood. No significant relationship was observed between anxiety problems and CUD onset age (F(1, 378) = 1.59, p = .20) or developmental stage (F(1, 378) = 0.126, p = .88).
Figure 1.
Anxiety level associated with CUD by gender and developmental stage.
3.2 Depression symptoms
Marginal means for depression level (i.e., HADS depression subscale ratings; provided in Table 1) significantly varied by CUD severity (F(1, 378) = 21.09, p = .00) only. Depression symptoms were generally higher among women compared to men during late adolescence and middle adulthood, though these differences were not significant (p > 0.05). In early adulthood, men exhibited slightly higher levels of depression symptoms, though this difference was also not significant. Furthermore, depression problems did not vary notably due to CUD onset age (F(1, 378) = 0.44, p = .50), gender (F(1, 378) = 0.16, p = .68), developmental stage (F(1, 378) = 1.86, p = .16) or the interaction between gender and developmental stage (F(1, 378) = 1.53, p = .21).
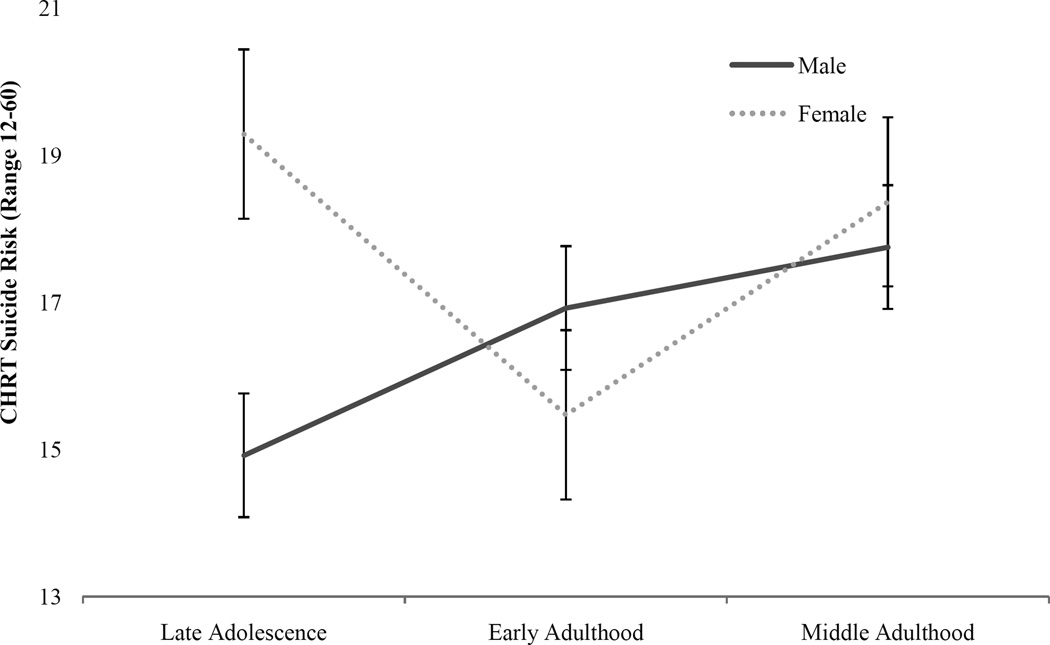
3.3 Suicide risk
Marginal means for suicide risk (i.e., CHRT scale ratings; presented in Figure 2) significantly varied by CUD severity (F(1, 378) = 11.02, p = .00) and the interaction between gender and developmental stage (F(1, 378) = 4.08, p = .02). Compared to men of the same developmental period, women exhibited significantly higher levels of suicide risk during late adolescence (d = 0.56, p < 0.05), while rates of suicide risk were similar at following developmental stages (i.e., early adulthood, d = −0.21, p > 0.05; middle adulthood, d = 0.08, p > 0.05). No significant relationship was observed between suicide risk and CUD onset age (F(1, 378) = 0.03, p = .86), gender (F(1, 378) = 1.7, p = .20), or developmental stage (F(1, 378) = 1.70, p = .18).
Figure 2.
Suicide risk associated with CUD in men and women at each developmental stage.
4. Discussion
The current study uniquely estimated the effects of developmental stage and gender on levels of internalizing distress and suicide risk among a large, treatment-seeking sample of men and women with CUD presenting for treatment. Both gender and developmental stage moderated large effects in concurrent suicide risk and anxiety but not depression after controlling for differences in the onset and severity of CUD symptoms. Effects observed were similar in magnitude to biological gender differences in body mass index and blood pressure (Table 1). Regardless of the onset or duration of their CUD problems, women in late adolescence (i.e., age 18–22) and middle adulthood (i.e., age 41 and over) in our sample were at especially high risk for concurrent suicide risk and anxiety relative to men of the same developmental periods. In juxtaposition, men and women exhibited similar levels of internalizing distress during the early adulthood stage, when CUD prevalence is highest in the broader population. As CUD has a lower prevalence in late adolescence and middle adulthood, a higher proportion of women who exhibit CUD during those atypical periods likely have a more severe form of the disorder relative to those who develop it in the early adulthood stage when it is more common. In juxtaposition, gender differences may diminish during the early adulthood stage of transition when CUD prevalence is higher as cases that are less severe may occur in higher proportion during this period of increasing personal and financial independence and rapid transition of social roles.
The gender and developmental differences detected for anxiety and suicide risk in our CUD sample extend previous work on gender differences in substance use disorders to highlight that substance use problems in women compared to men may be accompanied by more severe psychosocial risks and impairment, particularly internalizing symptoms. While women appear more vulnerable to the deleterious effects of substance use, these results suggest that anxiety and suicide risk are not simply gender-specific consequences proximal to heavy cannabis use. That is, the severity of anxiety and suicide risk varied across developmental groups with equivalent levels of CUD severity, indicating that heavy cannabis exposure alone could not account for gender differences in these problems. Instead, noted elevations in anxiety and suicide risk may reflect a phenotype for CUD that begins early in the development of women. Prior longitudinal investigations of CUD have supported a developmental cascade of risk in which childhood factors like maltreatment and internalizing and externalizing problems contribute to subsequent onset of CUD (Oshri, Rogosch, Burnette, & Cicchetti, 2011; Rogosch, Oshri, & Cicchetti, 2010). The lack of parallel findings for depression is not surprising given prior findings that anxiety – particularly social anxiety - has a more specific and robust association with CUD than other internalizing disorders (Buckner & Zvolensky, 2014; Buckner et al., 2008). Overall, our results provide additional insight into how previously identified childhood determinants of CUD may differ by gender and vary in association with CUD across stages of development. From a treatment perspective, these results signal that specification of treatment practices for CUD may be necessary to account for gender and developmental differences in its severity.
Despite evident innovations presented in the current study, important limitations must be noted. While cross-sectional designs are routinely utilized to make developmental inferences, these analyses do not allow for direct estimation of effects of CUD within an individual over time. We are thus unable to determine if these results reflect an internalizing pathway for CUD problems in women but not men, are dictated by the chronicity of CUD problems throughout development, or represent a consequence of gender differences in a third variable like a history of physical or sexual trauma or a chronic medical condition. Importantly, the higher prevalence of sexual and physical trauma in women compared to men may provide an alternative account for the development of both CUD and internalizing distress over the developmental period measured in this study. Additionally, higher rates of medical problems among women relative to men in a developmental pattern that coincided with those effects observed for anxiety and suicide risk. Additional longitudinal research is needed to unpack the temporal role these medical conditions play in the rates of internalizing distress among treatment-seeking women with CUD. While we utilized a clinical sample of CUD patients, lack of a control comparison precluded direct comparison of in internalizing severity between men and women with and without CUD. However, in representative samples, gender differences in both internalizing disorders and negative emotional experiences are uniformly higher in women across the lifespan (Angold, 1993; Grossman & Wood, 1993; Nolen-Hoeksema & Girgus, 1994; Stapley & Haviland, 1989), which does not coincide with the pattern of risks observed in our sample. Consequently, normative gender differences in anxiety and suicide risk cannot fully account for these effects. Finally, the gender difference in suicide risk measured by the Concise Health Risk Tracking (CHRT) scale was statistically significant but moderate in size (i.e., d = 0.56, 14.9 for men vs. 19.3 for women in late adolescence). As the CHRT is a relatively new measure, a paucity of research evidence is available to help determine the precise clinical relevance of this statistically significant difference. Future research should advance this work by using a more comprehensive measure of suicide risk with specific anchors for indicating clinical relevance.
5. Conclusions
Overall, the current work is the first to utilize a gender differences framework to estimate how concurrent psychosocial problems associated with CUD may be moderated by developmental stage in those presenting for care. The elevated internalizing symptoms co-occurring among women with CUD in late adolescence and middle adulthood pose additional, distinct risks for poor outcomes and suggest that gender and age-specific intervention may be paramount for ensuring treatment efficacy in these groups. Future work in this area should build on these findings through use of a longitudinal framework beginning at earlier stages in development before cannabis use typically onsets (i.e., late childhood) to establish if anxiety and suicide risk precede CUD symptoms, emerge after CUD onset, or can be linked with chronicity of CUD problems through adulthood.
Highlights.
Cannabis has gained popularity as evidence of its deleterious effects has increased.
Co-occurrence of internalizing and cannabis problems may differ by development and gender.
Internalizing and suicide risks were compared in 437 men and 163 women seeking cannabis use disorder (CUD) treatment from late adolescence through middle adulthood.
Co-occurring risks were higher in women than men during late adolescence and middle age but not early adulthood, suggesting that CUD intervention addressing these risks may improve outcomes in women during these developmental stages.
Acknowledgments
This work was supported by National Institute on Drug Abuse (NIDA) grants U10DA013727, R25DA020537, and K01DA03679; National Institute on Alcoholism and Alcohol Abuse grant F31AA023121; and the American Academy of Child and Adolscent Psychiatry/NIDA Jeanne Spurlock Research Fellowship in Substance Abuse and Addiction for Minority Medical Students.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angold A. Puberty onset of gender differences in rates of depression: A developmental, epidemiologic and neuroendocrine perspective. Journal of Affective Disorders. 1993;29(2–3):145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Babor T, Hofmann M, Delboca F, Hesselbrock V, Meyer R, Dolinksky Z, Rounsaville B. Types of alcoholoics: Evidence for an empirically derived typology based on indicators of vulnerability and severity. Archives of General Psychiatry. 1992;49(8):599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. Journal of Psychosomatic Research. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. Journal of Psychiatric Research. 2008;42(3):230–239. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ. Cannabis and related impairment: The unique roles of cannabis use to cope with social anxiety and social avoidance. American Journal of Addiction. 2014;23(6):598–603. doi: 10.1111/j.1521-0391.2014.12150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney A, Moore B. Development and consequences of cannabis dependence. Journal of Clinical Pharmacology. 2002;42(11):28S–33S. doi: 10.1002/j.1552-4604.2002.tb06000.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. American Journal of Public Health. 1995;85(1):41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Jacobson KC. Developmental trajectories of substance use from early adolescence to young adulthood: Gender and racial/ethnic differences. Journal of Adolescent Health. 2012;50(2):154–163. doi: 10.1016/j.jadohealth.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey C, Lynskey M, Wolfe R, Patton GC. Initiation and progression of cannabis use in a population-based Australian adolescent longitudinal study. Addiction. 2000;95(11):1679–1690. doi: 10.1046/j.1360-0443.2000.951116798.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. Journal of the American Medical Association. 2004;291(17):2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug and Alcohol Dependence. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98(11):1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Englund MM, Siebenbruner J, Oliva EM, Egeland B, Chung CT, Long JD. The developmental significance of late adolescent substance use for early adult functioning. Developmental Psychology. 2013;49(8):1554–1564. doi: 10.1037/a0030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flórez-Salamanca L, Secades-Villa R, Budney AJ, García-Rodríguez O, Wang S, Blanco C. Probability and predictors of cannabis use disorders relapse: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2013;132(1–2):127–133. doi: 10.1016/j.drugalcdep.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KT, Hicks BM, Iacono WG, McGue M. Alcohol use disorder in women: Risks and consequences of an adolescent onset and persistent course. Psychology of Addictive Behaviors. 2014;28(2):322–335. doi: 10.1037/a0035488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KT, Hicks BM, Iacono WG, McGue M. Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychological Medicine. 2015;45(14):3047–3058. doi: 10.1017/S0033291715001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Wood W. Sex differences in intensity of emotional experience: A social role interpretation. Journal of Personality and Social Psychology. 1993;65(5):1010–1022. doi: 10.1037//0022-3514.65.5.1010. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical Pharmacokinetics. 2003;42(4):327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG. Assessment of cannabis craving using the Marijuana Craving Questionnaire. Methods in Molecular Medicine. 2006;123(2):209–216. doi: 10.1385/1-59259-999-0:209. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: Development and initial validation of a self-report instrument. Addiction. 2001;96(7):1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Iacono WG, McGue M. Consequences of an adolescent onset and persistent course of alcohol dependence in men: Adolescent risk factors and adult outcomes. Alcoholism: Clinical and Experimental Research. 2010;34(5):819–833. doi: 10.1111/j.1530-0277.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager J, Schulenberg JE, O’Malley PM, Bachman JG. Historical variation in drug use trajectories across the transition to adulthood: the trend toward lower intercepts and steeper, ascending slopes. Development and Psychopathology. 2013;25(2):527–543. doi: 10.1017/S0954579412001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Davies M. In: Vulnerability to drug abuse. Glantz MD, Pickens RW, editors. Washington, DC, US: American Psychological Association; 1992. [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: Results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug and Alcohol Dependence. 2013;130(1–3):101–108. doi: 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99(12):1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM. Typologies of alcohol dependence: From Jellinek to genetics and beyond. Neuropsychology Review. 2009;19(1):115–129. doi: 10.1007/s11065-008-9080-z. [DOI] [PubMed] [Google Scholar]
- Lev-Ran S, Imtiaz S, Taylor BJ, Shield KD, Rehm J, Le Foll B. Gender differences in health-related quality of life among cannabis users: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2012;123(1–3):190–200. doi: 10.1016/j.drugalcdep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Mcclure EA, Sonne SC, Winhusen T, Carroll KM, Ghitza UE, McRae-Clark AL, Gray KM. Achieving cannabis cessation — Evaluating N-acetylcysteine treatment (ACCENT): Design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemporary Clinical Trials. 2014;39(2):211–223. doi: 10.1016/j.cct.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behavioural Brain Research. 2011;224(1):128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Ogborne AC, Smart RG, Adlaf EM. Self-reported medical use of marijuana: A survey of the general population. Journal of the Canadian Medical Association. 2000;162(12):1685–1686. [PMC free article] [PubMed] [Google Scholar]
- Oshri A, Rogosch FA, Burnette ML, Cicchetti D. Developmental pathways to adolescent cannabis abuse and dependence: Child maltreatment, emerging personality, and internalizing versus externalizing psychopathology. Psychology of Addictive Behaviors. 2011;25(4):634–644. doi: 10.1037/a0023151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkonigg A, Goodwin RD, Fiedler A, Behrendt S, Beesdo K, Lieb R, Wittchen HU. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103(3):439–451. doi: 10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Oshri A, Cicchetti D. From child maltreatment to adolescent cannabis abuse and dependence: A developmental cascade model. Development and Psychopathology. 2010;22(4):883–897. doi: 10.1017/S0954579410000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C, O’Malley PM, Bachman JG, Johnston LD, Schulenberg J. Adolescent marijuana use and adult occupational attainment: A longitudinal study from age 18 to 28. Substance Use & Misuse. 2001;36(8):997–1014. doi: 10.1081/ja-100104486. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug and Alcohol Dependence. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. A self-feedback technique to monitor drinking behavior in alcoholics. Behaviour Research and Therapy. 1973;11(2):237–238. doi: 10.1016/s0005-7967(73)80014-2. [DOI] [PubMed] [Google Scholar]
- Stapley JC, Haviland JM. Beyond depression: Gender differences in normal adolescents’ emotional experiences. Sex Roles. 1989;20(5–6):295–308. [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68(5):898–908. [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: Prevalence, correlates and co-morbidity. Psychological Medicine. 2006;36(10):1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Swift W, Coffey C, Carlin JB, Degenhardt L, Patton GC. Adolescent cannabis users at 24 years: Trajectories to regular weekly use and dependence in young adulthood. Addiction. 2008;103(8):1361–1370. doi: 10.1111/j.1360-0443.2008.02246.x. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Wisniewski SR, Morris DW, Fava M, Gollan JK, Warden D, Rush AJ. Concise Health Risk Tracking scale: A brief self-report and clinician rating of suicidal risk. The Journal of Clinical Psychiatry. 2011;72(6):757–764. doi: 10.4088/JCP.11m06837. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Ellickson PL, Orlando M, Martino SC, Klein DJ. Substance use trajectories from early adolescence to emerging adulthood: A comparison of smoking, binge drinking and marijuana use. Journal of Drug Issues. 2005;35(2):307–331. [Google Scholar]
- Van Dam NT, Bedi G, Earleywine M. Characteristics of clinically anxious versus non-anxious regular, heavy marijuana users. Addictive Behaviors. 2012;37(11):1217–1223. doi: 10.1016/j.addbeh.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. The New England Journal of Medicine. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence: Developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26(4):479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]