Abstract
Tumors of the urethra, whether primary or metastatic, are very rare. The true nature of urethral neoplasm is not always obvious clinically nor in routine histological sections. Immunostains should be performed on such lesions because of management implications. We present a case of multiple metastases to the urethra from a prostatic carcinoma, masquerading as multiple urothelial carcinomas. Pathologists and urologists should be aware of the possibility of metastasis from the prostate.
Keywords: Prostate cancer, Urethral cancer, Metastatic prostatic cancer, Mimmic of urothelial cancer
Tumors of the urethra, whether primary or metastatic, are very rare.1 We present a case of multiple metastases to the urethra from a prostatic carcinoma, masquerading as multiple urothelial carcinomas.
An 84 year old man presented in January 2014 with urinary retention and symptoms of urinary tract infection. Cystoscopy (Fig. 1, panels A, B and C) revealed 3 polypoid urethral lesions. One of the lesions was in the membranous urethra and the other 2 in the spongy (bulbar and penile) urethra. The bladder was normal. Cold cup biopsies of the urethral lesions and transurethral resection of the prostate (TURP) were performed.
Figure 1.
Cystoscopic images of the urethra. Panel A shows an exophytic growth in the penile part of the spongy urethra. Panel B is the tumor in the bulbar part of the spongy urethra. Panel C displays the lesion in the membranous urethra near the verumontanum. Panel D depicts the open prostatic urethra after the TURP.
All the urethral biopsies showed high grade malignant papillary neoplasms with exophytic growth patterns (Fig. 2, panels A to D). The tumors in the spongy/distal parts of the urethra were non-invasive but the tumor in the membranous urethra had invaded muscle (Fig. 2, panel A). The prostatic tissue was benign except for loose fragments of papillary neoplasm, similar to the urethral tumors. The urethral cancers were initially regarded as high grade urothelial carcinomas but in view of the unusual presentation of multiple urethral tumor in the absence of bladder involvement at cystoscopy, immunostains were performed. These showed strong PSA positivity (Fig. 2, panel E). Alpha-Methylacyl-CoA racemase (AMACR) was also positive (Fig. 2, panel F). Special stains for mucin were negative. Retrospective evaluation of the routine Hematoxylin and Eosin stained sections showed the tumor to be made up of tall pseudostratified cells with some glandular arrangements (Fig. 2, panel D).
Figure 2.
The main histological and immunehistochemical features of the tumors in the penile urethra are illustrated. Panel A shows an invasive neoplasm with a papillary surface growth pattern and extension into the underlying urethral wall muscle. A clear papillary architecture is seen Panels A to D. Pseudostratified columnar cells and gland like spaces are seen in Panel D. PSA and AMACR positivity are seen in the tumor cells in Panels E and F respectively. The urothelium overlying the invasive tumor is high molecular weight cytokeratin 34βE12 positive (Panel F). Occasional PSA positive (brown) cells, indicative of focal surface involvement by prostatic carcinoma are noted on the surface (Panel E). AMACR (red) is positive in the invasive tumor cells and also within some of the surface urothelium (Panel F). Panel F is a dual stain for AMACR detected with red chromogen (Fast Red) and 34βE12 detected with brown chromogen (Diaminobenzidine).
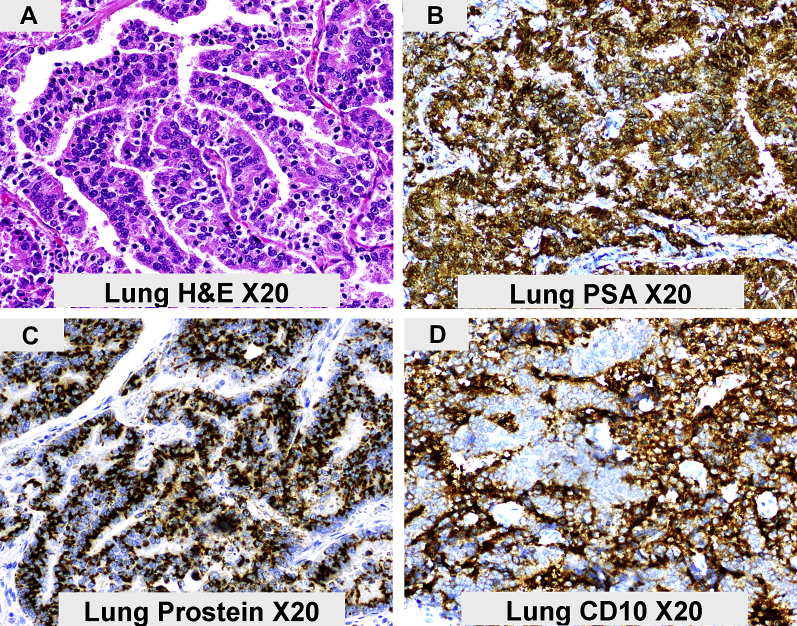
Review of the past medical history indicated an indolent prostate cancer diagnosed in 2004 on digital rectal examination and by raised serum prostate specific antigen (PSA) level of 10.3 ug/L (age adjusted reference interval is <6.5 ug/L). He had been managed with active surveillance and his PSA levels between 2004 and 2014 had fluctuated between 9 ug/L and 11 ug/L. TURP, performed in 2011 for urinary retention, yielded benign prostate tissue only. He presented in September 2014, with metastatic cancer in his right lung. The tumor, a poorly differentiated prostatic adenocarcinoma, strongly expressed PSA (Fig. 3, panel B), prostein/p501s (Fig. 3, panel C) and CD10 (Fig. 3, panel D). He is currently clinically stable on androgen deprivation therapy with a PSA level of 15 ug/L.
Figure 3.
The main morphological and immunohistochemical characteristics of the lung metastasis are shown. The tumor (Panel A) is a poorly differentiated adenocarcinoma with tall columnar cells displaying some pseudostratification. The tumor cells expresse PSA (Panel B), Prostein/p501s (Panel C) and CD10 (Panel D). Immunoreactivity is detected by the chromogen Diaminobenzidine (brown).
Primary penile urethral carcinoma is a very rare male genital tract cancer. Metastatic tumors are even less frequent. These usually originate in the bladder, prostate or the gastrointestinal system.1 In a recent review of 29 cases of metastatic prostate cancers to the penis, Ellis and Epstein found 16 (55.2%) of them had originated from prostatic ductal adenocarcinomas and most of the remainder had their origin in usual acinar adenocarcinomas.2 The tumor in our case has features of a high grade prostatic acinar carcinoma.
Metastases from prostate carcinoma to the urethra show a number of architectural patterns, including papillary, cribriform, single glands, features reminiscent of high grade prostatic intraepithelial neoplasia (PIN) and Pagetoid morphology.2, 3 Because the tumors, in our case, had a prominent papillary growth pattern, they were initially considered as primary urethral cancers of urothelial origin. Primary urothelial cancers of the urethra often accompany urothelial cancers in the bladder or other parts of urinary system. Finding multiple cancers in the urethra without bladder involvement made us consider other possibilities and perform immunostains. The true nature of the tumor only became apparent after immunostains. This is a recognized pitfall, acknowledged by Ellis and Epstein and Tu and colleagues. In the series by Ellis and Epstein, 3 of the 29 cases were initially diagnosed as urothelial carcinomas. This experience was also shared by Tu and colleagues who misinterpreted one of their cases of penile metastatic tumor from the prostate as a primary urethral cancer.4
Urothelial and some prostatic carcinomas appear to overlap morphologically and some of the poorly differentiated prostatic carcinomas can be negative for PSA by immunohistochemistry. Even serum PSA may not be elevated.5 Other immunostains to prove prostatic origin and exclude urothelial cancer should be employed as no unequivocal defining morphological to distinguish prostatic ductal carcinoma from high grade urothelial carcinoma exist.
The route of spread to the urethra is unclear and no single mechanism can explain it adequately because the urethra is a complex structure and its 4 parts lie in different anatomical compartments. The spongy anterior urethra with its bulbar and penile parts is situated in the navicular fossa which lies below the pelvic floor. The membranous (rhabdosphincter) part of the urethra lies within the pelvic floor in the perineal region. The posterior (prostatic/bladder neck) part of the urethra which is located above the pelvic floor descends through the anterior part of the prostate. The pre-prostatic urethra which also lies above the pelvic floor extends vertically from bladder neck to the posterior aspect of the prostate and is associated with the internal urethral sphincter. Direct involvement, seeding and retrograde venous or lymphatic spread have been suggested as possibilities.2 In our patient, the biopsies from the anterior (distal) urethra showed surface involvement only, whereas the sample from the membranous (middle) urethra also showed invasive carcinoma. The proximal urethra was uninvolved clinically. The TURP were histologically uninvolved.
In general, patients with metastatic prostate cancer to the urethra have a poor prognosis because they are usually seen in advanced stages of prostate cancer. Approximately 50% of patients with follow-up information are dead within 2 years following the penile metastasis.6 A case report and review of the literature by Kotake and colleagues of 25 cases from 3 publications of prostate carcinoma, appears to confirm the above observations.7
The lung is an uncommon site for distant spread from the prostate in the absence of bony metastases. Hematogenous spread to the spine, believed to take place via a backward metastatic pathway, is the most common method of distant metastasis in prostate cancer. Bony metastases usually precede spread via the vena cava and there is an inverse relationship between spine and lung metastases. Furthermore spine involvement is more frequent with smaller prostatic tumors when compared to those that metastasize to lung or liver.8 Our patient does not have bony metastases and his primary tumor is relatively small. However, his lung metastases also express CD10, which has been shown to represent a marker for a more aggressive phenotype with a higher malignant and possibly metastatic potential.9
The true nature of urethral neoplasm is not always obvious in routine sections. Immunostains should be performed on such lesions because of management implications. Pathologists should be aware of the possibility of metastasis from the prostate.
Conflict of interest and source of funding
No conflicts of interest to disclose.
Acknowledge
We thank Professor Warick Delprado (Douglas Hanly Moir Pathology) for his assistance with the case.
References
- 1.Hofstadter F., Amin M.B., Delahunt B., Hartmann A. Tumours of the urethra. In: Eble J.N., Sauter G., Epstein J.I., Sesterhenn I.A., editors. WHO Tumours of the Urinary System and Male Genital Organs. IARC Press; Lyon: 2004. [Google Scholar]
- 2.Ellis C.L., Epstein J.I. Metastatic prostate adenocarcinoma to the penis: a series of 29 cases with predilection for ductal adenocarcinoma. Am J Surg Pathol. 2015;39:67–74. doi: 10.1097/PAS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 3.Roma A.A., Magi-Galluzzi C., Wood H. Metastatic prostate adenocarcinoma to the penis presenting as pagetoid carcinoma. A phenomenon not previously reported. Am J Surg Pathol. 2015;39:724–726. doi: 10.1097/PAS.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 4.Tu S.M., Reyes A., Maa A. Prostate carcinoma with testicular or penile metastasis. Clinical, pathologic and immunohistochemical features. Cancer. 2002;94:2610–2617. doi: 10.1002/cncr.10546. [DOI] [PubMed] [Google Scholar]
- 5.Pierro A., Cilla S., Digesu C., Morganti A.G. Penile metastases of recurrent prostatic adenocarcinoma without PSA level increase: a case report. J Clin Imaging Sci. 2012;2:44. doi: 10.4103/2156-7514.99178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung C.F., Lee C.H., Hung S.W. Invasive adenocarcinoma of the prostate with urethral tumour. J Chin Med Assoc. 2010;73:101–103. doi: 10.1016/S1726-4901(10)70010-1. [DOI] [PubMed] [Google Scholar]
- 7.Kotake Y., Gohji K., Suzuki T. Metastases to the penis from carcinoma of the prostate. Int J Urol. 2001;8:83–86. doi: 10.1046/j.1442-2042.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 8.Bubendorf L., Schöpfer A., Wagner U. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 9.Dall'Era M.A., True L.D., Siegel A.F. Differential expression of CD10 in prostate cancer and its clinical implication. BMC Urol. 2007;7:3. doi: 10.1186/1471-2490-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]