Abstract
Objective
To estimate the risk of fatal and non-fatal myocardial infarction (MI) and stroke in patients with bipolar I disorder compared to people without bipolar I disorder.
Method
Utilizing a records-linkage system spanning 30 years (1966–1996), a population-based cohort of 334 subjects with bipolar I disorder and 334 age and sex-matched referents from Olmsted County, Minnesota, U.S. was identified. Longitudinal follow-up continued until incident MI or stroke (confirmed by board-certified cardiologist/neurologist), death, or study end date (December 31, 2013). Cox proportional hazards models assessed the hazard ratio (HR) for MI or stroke, adjusting for potential confounders.
Results
There was an increased risk of fatal or non-fatal MI or stroke (as a composite outcome) in patients with bipolar I disorder [HR 1.54, 95% confidence interval (CI) 1.02, 2.33; p=0.04]. However, after adjusting for baseline cardiovascular risk factors (alcoholism, hypertension, diabetes, and smoking), the risk was no longer significantly increased (HR 1.19, 95% CI 0.76, 1.86; p=0.46).
Limitations
Small sample size for the study design. Findings were not retained after adjustment for cardiovascular disease risk factors. Psychotropic medication use during the follow-up was not ascertained and was not included in the analyses.
Conclusion
This study in a geographically defined region in the U.S. demonstrated a significant increased risk of MI or stroke in bipolar I disorder, which was no longer significant after adjustment for cardiovascular risk factors.
Keywords: bipolar disorder, cardiovascular diseases, cohort studies, risk
Introduction
Bipolar disorder is a chronic, recurrent, and highly comorbid illness that negatively impacts the young U.S. population from the standpoint of disability, health care cost utilization, and mortality. Standardized mortality ratios from all causes are more than 2-fold higher in bipolar disorder patients compared to the general population (standardized mortality ratio: 2.5 for men and 2.7 for women), with elevated rates in neurological diseases (standardized mortality ratio: 1.6 for men and 2.3 for women) and cardiovascular diseases (standardized mortality ratio: 1.9 for men and 2.6 for women) (Angst et al., 2013; Osby et al., 2001; Westman et al., 2013).
Although cardiovascular disease (CVD)-associated mortality in bipolar disorder has been well recognized, the risk of non-fatal CVD or neurovascular events has not been firmly established (Callaghan and Khizar, 2010; Goldstein et al., 2015; Ramsey et al., 2010; Westman et al., 2013). For example, a recent meta-analysis performed by our group (Prieto et al., 2014), identified an increased pooled relative risk (RR) for stroke (RR: 1.74, 95% confidence interval [CI] 1.29, 2.35) but not for myocardial infarction (MI) (RR: 1.09, 95% CI 0.96, 1.24), highlighting potential differential risks contributing to neurovascular and cardiovascular events. Moreover, several important limitations were identified in the majority of prior studies including: 1) the use of administrative diagnostic codes or lay interviewers to ascertain both bipolar disorder and CVD diagnoses as well as potential confounders (Laursen et al., 2011; Lin et al., 2007, 2008; Ramsey et al., 2010; Westman et al., 2013); 2) the lack of assessment of and adjustment for critical CVD confounders, such as body mass index, diabetes, and smoking (Laursen et al., 2011; Lin et al., 2007, 2008; Westman et al., 2013); 3) the restriction of the sample to bipolar disorder inpatients (Lin et al., 2007, 2008; Westman et al., 2013); 4) the lack of adequate report of lost to follow-up; 5) the use of non-community based referent groups (i.e., inpatients who had undergone appendectomy) (Lin et al., 2007, 2008); and 6) the short-term follow-up (Lin et al., 2007, 2008).
Finally, the majority of these epidemiological studies assessing the risk of CVD/stroke in bipolar disorder have been performed in Scandinavian countries with rich data registries. This does, however, limit the generalizability of these findings to other demographically different countries, such as the United States. Due to the lack of a national health system in the US, there are few sources of epidemiological data to investigate the long-term outcomes of bipolar disorder such as CVD. One notable exception is the Rochester Epidemiology Project (REP) (St Sauver et al., 2012a; St Sauver et al., 2012b; St Sauver et al., 2011).
The REP is a unique population-based records-linkage system for the residents of Olmsted County, Minnesota (MN) that provides detailed medical data from health records and death certificates for all residents of the county since 1966. The availability of the complete text of historical medical records, including physical exam findings and laboratory tests, permits the confirmation of medical diagnoses using current diagnostic criteria. Administrative registries lack this capability. The long timeframe, nearly 50 years that this system encompasses, provides an exceptional opportunity to study long-term outcomes in diseases that arise in the young population, such as bipolar disorder.
The aim of this study was to investigate the subsequent risk of fatal and non-fatal MI and stroke in patients with bipolar I disorder compared with people without bipolar I disorder in Olmsted County, MN, USA.
Method
The Rochester Epidemiology Project (REP) maintains a records-linkage system for 90–96% of Olmsted County, Minnesota (USA) residents who have had their medical care in the county since 1966. This population is similar demographically to the State of Minnesota, the upper Midwest, and to a large segment of the entire U.S. population (St Sauver et al., 2012a). Validity and reliability of several variables within the REP dataset and medical records have been shown to be adequate in other studies that compared medical records’ information with data obtained by patient interview (St Sauver et al., 2012a). All medical contacts and procedures are coded using International Classification of Disease (ICD) diagnostic codes, and the full text of medical records is available for abstraction. Death certificate diagnostic codes are available through the State of Minnesota Death Registry and the National Death Index +.
This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Informed consent was not required per a State of Minnesota law that allows patients to authorize the use of their medical records for multiple studies (St Sauver et al., 2012b).
Cohort Identification
We identified a population-based cohort of all incident and prevalent cases of bipolar I disorder who were residents of Olmsted County, MN, USA, from January 1, 1966 through December 31, 1996. We defined bipolar I disorder as a manic episode confirmed by Diagnostic and Statistical Manual for Mental Disorders 4th Edition Text Revised (DSM-IV-TR) criteria, or a clinical diagnosis of a manic episode in the medical record. A board-certified psychiatrist with 8 years of experience in mood disorders (MLP), supervised by a board-certified psychiatrist with 20 years of similar background (MAF), reviewed medical records from 1,126 Olmsted county residents who: 1) provided research authorization, 2) had a bipolar I disorder-related diagnostic code, and 3) did not have any MI or stroke diagnostic codes before the first bipolar I disorder code. A total of 334 patients were confirmed as having bipolar I disorder while they resided in Olmsted County and as being free of MI or stroke before the index date (Figure 1). Psychiatric comorbidity was assessed by record review. We defined “history of psychosis” as ever having hallucinations or delusions within distinct mood episodes, assessed by medical record review. We randomly identified, from all the general population in Olmsted County who were part of the REP, one same-sex referent person for each bipolar I patient, who was born in the same year and was alive and free of MI, stroke, or bipolar I disorder codes before the index date in the matched pair. We utilized a stratified randomization by age and sex.
Figure 1.
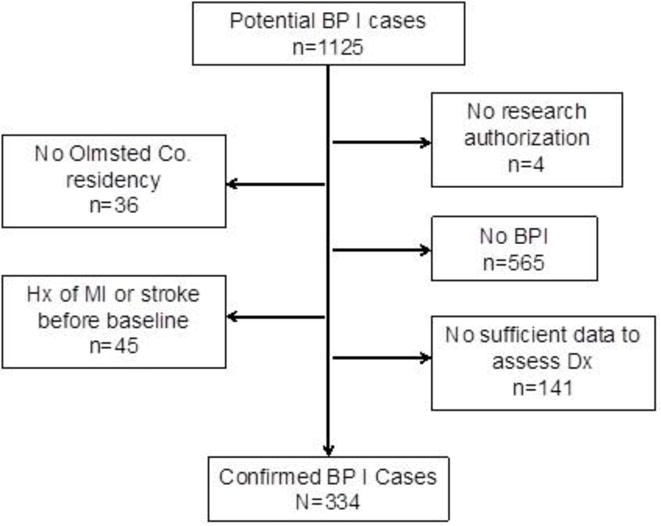
Hx: history. MI: myocardial infarction. BPI: bipolar I disorder. SCZ BP: schizoaffective disorder bipolar type. Co: county. Dx: diagnosis.
For patients with incident bipolar I disorder (i.e., patients who had their first manic episode in Olmsted County), the index date was the date of the first medical contact within the first manic episode. For prevalent cases, the index date was the first psychiatric outpatient visit or hospital admission when the patient was an Olmsted County resident. For referents, the index date was the index date of their matched pair.
Outcomes
Outcomes were ascertained from index through December 31, 2013. To ascertain MI, we used the Clinical Classification Software (CCS) for ICD-9-CM (Agency for Health Care Policy and Research, 1999), categories CCS 100 (acute MI) and selected codes from CCS 101 related to prior history of MI to screen for MI outcome events. The complete list of codes for all outcomes is available upon request. Then, to confirm whether each possible MI event identified by the prior procedure was a true MI, we used data from a population-based cohort of incident MI in Olmsted County, MN, USA (1979–2012) (Roger et al., 2002; Roger et al., 2010), which covered the same population during a similar timeframe. Patients who had an MI code before 1979 or after 2012 were manually reviewed. Details on the methodology of the MI cohort have been reported elsewhere (Roger et al., 2002; Roger et al., 2010). Briefly, patients with ICD-9-CM codes 410–411 were screened for an MI. Then, MI was validated by trained abstractors using epidemiological criteria that included cardiac pain, biomarkers and electrocardiogram changes. Using a similar procedure, possible stroke cases were identified using CCS category 109 (acute cerebrovascular disease) codes and selected codes from category 113 (late effects of cerebrovascular disease). Potential outcome events were further confirmed by record review by a neurologist (JPK) using standard clinical and imaging stroke criteria.
CCS category 100 (acute myocardial infarction) and 109 (acute cerebrovascular disease) codes listed anywhere on the death certificate were used to ascertain fatal MI or stroke, respectively. The date of death was obtained from the death certificate.
Outcomes analyses and cohort follow-up
We defined MI as a composite outcome including non-fatal MI and fatal MI. Stroke was grouped in a similar manner. We combined fatal and non-fatal events because outside of the hospital, MI is highly lethal (31%) (Norris, 1998). Our primary outcome was a combination of fatal and nonfatal MI and stroke (MI/stroke). The rationale for a composite outcome of MI or stroke was multifactorial including: a similar underlying biology of vascular endothelial disease for both myocardial and cerebrovascular infarction (Jashari et al., 2013), longitudinal contemporary clinical trials in cardiovascular disease typically utilize this composite outcome (Helft et al., 2015), and a large proportion of myocardial infarctions progress to cardiac arrest and death in the field (i.e. before getting medical treatment) (Norris, 1998). The date at start of follow-up was the index date for the patient with bipolar I disorder and for the matched referent person (index date of the pair). The end of follow-up was the first date in which the participant: 1) reached an outcome of interest (MI or stroke), 2) was lost to follow-up, 3) died, or 4) was alive and free of outcomes at the end of the study (December 31, 2013).
Assessment of covariates
Potential confounders of the association between bipolar disorder and CVD were assessed at index date. Diabetes was defined as a documented diagnosis, a fasting glucose level higher than 126 mg/dL, or as use of anti-diabetic drug therapy. Hypertension was defined as a documented diagnosis, as blood pressure measurement with average systolic ≥ 140 mm/Hg and diastolic ≥90 mm/Hg, or as use of antihypertensives. Hyperlipidemia was defined as a documented diagnosis, as fasting total cholesterol levels of ≥ 240 mg/dL, or as use of a lipid-lowering drug. Body mass index was calculated using: (weight in kg)/(height in cm)2. Smoking was determined by records’ review and classified as current, former, or never smoked.
Statistical analysis
We utilized Wilcoxon Rank Sum test or Kruskal-Wallis test for assessing the statistical significance of continuous variables, and the chi-squared test for categorical variables. Odds ratios (OR) and 95% CIs were estimated using logistic regression.
For time-to-event analyses, we developed cumulative incidence curves comparing patients with bipolar I disorder and referents, using the Kaplan-Meier method. To estimate the hazard ratio (HR) with its 95% CI, we used Cox Proportional Hazards models. We calculated unadjusted HRs and HRs adjusted for covariates at the index date that were potential confounders (i.e., were previously associated with bipolar I disorder and with any of the outcomes): hypertension (Goldstein et al., 2009), diabetes (Regenold et al., 2002), smoking (Waxmonsky et al., 2005), and alcohol use disorder (Greenland et al., 2003). The proportional hazards assumption was tested and confirmed using graphical methods. We used age as the time scale for all the analyses. Statistical significance was achieved at a two-tailed alpha level of 0.05. Statistical analysis was performed using SAS version 9.3 (SAS Institute, Inc.; Cary, NC) and JMP Pro version 10.0 (SAS Institute, Inc.; Cary, NC).
Results
Description of the cohort
A total of 334 patients with bipolar I disorder and 334 referent subjects were included in the study (Table 1). One person, who was selected as a referent, was diagnosed with bipolar I disorder later during follow-up, thus he/she was part of the bipolar disorder and referent cohorts. A total of 194 (57.7%) patients had their bipolar I disorder diagnosis confirmed by strict DSM-IV-TR criteria. The rest of the patients were confirmed by the clinician’s documented diagnosis in the medical records, and further confirmed by the reviewer’s assessment. The median age at first documented manic episode was 37 (interquartile range [IQR] 27, 52) years, and 56% of the patients had a history of psychosis. Among the referents, 4.6% of the subjects had history of major depressive disorder at the index date. The median follow-up time for the full cohort was 18.7 years (range 0–46.1 years): 17.3 years (range 0–46.0 years) for the bipolar cohort and 20.9 years (range 0–46.1 years) for the referent cohort (Wilcoxon p<0.0001).
Table 1.
Demographics and clinical characteristics of the patients with bipolar I disorder and the referent subjects at baseline.
| Bipolar I disorder | Referent subjects | OR (95% CI) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age at index date – median, IQR | 37 (28, 52) | 37 (28, 52) | 0.91 | |
| Sex (women) – n, % | 173 (52) | 173 (52) | 1.00 (0.74,1.36) | >0.99 |
| Education | 0.43 | |||
| High school graduate or less – | 128 (38) | 144 (43) | 1.00 (Ref) | |
| Some college – n, % | 60 (18) | 75 (22) | 0.89 (0.59, 1.36) | |
| Complete college or more – n, | 82 (25) | 73 (22) | 1.26 (0.85, 1.86) | |
| Marital status | <0.01 | |||
| Married – n, % | 145 (43) | 222 (66) | 1.00 (Ref) | |
| Single – n, % | 111 (33) | 71 (21) | 2.39 (1.66, 3.44) | |
| Divorced/separated – n, % | 63 (19) | 19 (6) | 5.08 (2.92, 8.83) | |
| Widowed – n, % | 14 (4) | 18 (5) | 1.19 (0.57, 2.47) | |
|
| ||||
| Clinical features | ||||
| Alcohol use disorder – n, % | 80 (24) | 15 (5) | 6.70 (3.77, 11.9) | <0.01 |
| Drug use disorder – n, % | 50 (15) | 4 (1.2) | 14.5 (5.2, 40.7) | <0.01 |
| Anxiety disorders – n, % | 7 (2.1) | 5 (1.5) | 1.4 (0.44, 4.5) | 0.56 |
|
| ||||
| CVD risk factors | ||||
| BMI – median (IQR) | 25.2 (22.3, 28.9) | 25.0 (22.3, 28.1) | 0.49 | |
| Obesity – n, % | 58 (19) | 59 (19) | 0.99 (0.66, 1.48) | 0.95 |
| Diabetes – n, % | 22 (6.6) | 11 (3.3) | 2.1 (0.98, 4.34) | 0.05 |
| Hypertension – n, % | 47 (14) | 26 (7.8) | 1.94 (1.17, 3.21) | 0.01 |
| Hyperlipidemia – n, % | 21 (6.3) | 16 (4.8) | 1.34 (0.68, 2.61) | 0.39 |
| Smoking | 0.01 | |||
| Never smoker – n, % | 114 (36) | 144 (45) | 1.00 (Ref) | |
| Former smoker – n, % | 63 (20) | 77 (24) | 1.03 (0.68, 1.56) | |
| Current smoker – n, % | 136 (44) | 100 (31) | 1.71 (1.20, 2.45) | |
IQR: interquartile range. DSM: Diagnostic and Statistical Manual for Mental Disorders. SD: standard deviation. BMI: body mass index.
Estimates of effect for myocardial infarction and stroke risk
There was no statistically significant increased risk of MI among patients with bipolar I disorder compared with the referent participants (HR 1.39, 95% CI 0.75, 2.60, p=0.30) (Table 2, Figure 2a). After adjusting for alcohol use disorder, hypertension, diabetes, and smoking, the estimate of effect decreased (HR 1.06, 95% CI 0.54, 2.08, p=0.86). There was a non-significant increased risk of stroke among patients with bipolar I disorder (HR 1.57, 95% CI 0.93, 2.64, p=0,09) (Table 2, Figure 2b). After adjusting for potential confounders, the HR decreased to 1.18 (95% CI 0.67, 2.09, p=0.68).
Table 2.
Survival analyses for fatal or non-fatal MI and/or stroke for patients with bipolar I disorder and for referent participants.
| Cohort or stratum | People at risk | Follow-up (person-years) | People with fatal or non-fatal MI and/or stroke | Unadjusted hazard ratio (95% CI) | p | Adjusted hazard ratio (95% CI)* | p |
|---|---|---|---|---|---|---|---|
| Myocardial infarction | |||||||
| Referents | 334 | 6778 | 21 | 1.00 (Ref) | 1.00 (Ref) | ||
| Patients with BPI | 334 | 5019 | 19 | 1.39 (0.75, 2.60) | 0.30 | 1.06 (0.55, 2.08) | 0.86 |
| With psychosis** | 185/185 | 2869/3853 | 8/13 | 0.95 (0.39, 2.30) | 0.91 | 0.80 (0.32, 2.01) | 0.63 |
| Without psychosis** | 149/149 | 2150/2925 | 11/8 | 1.94 (0.78, 4.83) | 0.16 | 1.67 (0.58, 4.79) | 0.34 |
|
| |||||||
| Stroke | |||||||
| Referents | 334 | 6738 | 29 | 1.00 (Ref) | 1.00 (Ref) | ||
| Patients with BPI | 334 | 4941 | 29 | 1.57 (0.93, 2.64) | 0.09 | 1.18 (0.67, 2.09) | 0.56 |
| With psychosis** | 185/185 | 2819/3851 | 18/16 | 1.96 (0.99, 3.88) | 0.05 | 1.70 (0.82, 3.51) | 0.15 |
| Without psychosis** | 149/149 | 2122/2887 | 11/13 | 1.22 (0.54, 2.75) | 0.63 | 0.83 (0.33, 2.12) | 0.70 |
|
| |||||||
| Myocardial infarction or stroke | |||||||
| Referents | 334 | 6647 | 46 | 1.00 (Ref) | 1.00 (Ref) | ||
| Patients with BPI | 334 | 4895 | 45 | 1.54 (1.02, 2.33) | 0.04 | 1.19 (0.76, 1.86) | 0.46 |
| With psychosis** | 185/185 | 2787/3795 | 24/25 | 1.72 (0.97, 3.03) | 0.06 | 1.52 (0.84, 2.78) | 0.17 |
| Without psychosis** | 149/149 | 2107/2852 | 21/21 | 1.38 (0.75, 2.55) | 0.30 | 0.97 (0.48, 1.96) | 0.93 |
Adjusted for alcohol use disorder, hypertension, diabetes and smoking.
Patients with bipolar I disorder in each stratum were compared with their corresponding non-affected pairs. For subgroup analyses, people at risk, follow-up person/years and participants with fatal or non-fatal MI and/or stroke are presented as number of cases in the subgroup/total number of referents in the subgroup. MI: myocardial infarction. BP: bipolar. CI: confidence interval. Ref: reference.
Figure 2a.
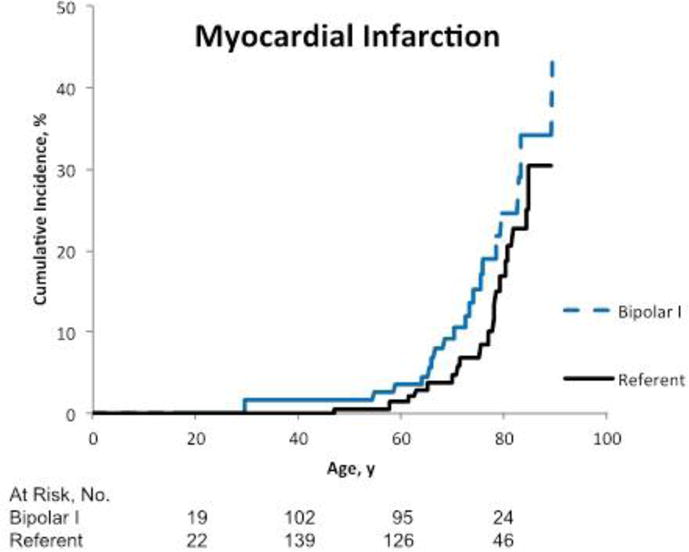
Cumulative incidence of fatal or non-fatal myocardial infarction among patients with bipolar I disorder compared to referents. The numbers of people at risk below the graphs reflect the occurrence of both left and right censoring (age is the time scale).
Figure 2b.
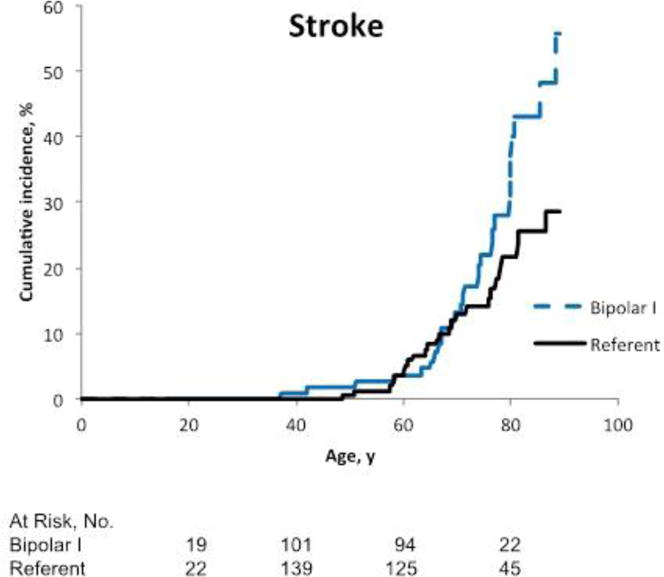
Cumulative incidence of fatal or non-fatal stroke among patients with bipolar I disorder compared to referents. The numbers of people at risk below the graphs reflect the occurrence of both left and right censoring (age is the time scale).
There was a significant increased risk of MI or stroke, when treated as a composite outcome, in bipolar I disorder patients compared with the referent group (HR 1.54, 95% CI 1.02, 2.33, p=0.04) (Table 2, Figure 2c). However, after adjusting for potential confounders, the HR decreased to 1.19 (95% CI 0.76, 1.86, p=0.46). In a secondary analysis, history of psychosis was associated with a marginally increased risk of MI or stroke (HR 1.71, 95% CI 0.97, 3.03, p=0.06). After adjusting for potential confounders, the HR in patients with history of psychosis reduced to 1.52 (95% CI 0.84, 2.78, p=0.17). The findings did not change in sensitivity analyses that excluded the referent subject who later developed bipolar disorder (HR 1.54, 95% CI 1.02, 2.33; p=0.04), or keeping the person in both cohorts but using robust sandwich covariance estimates (HR 1.54, 95% CI 1.01, 2.35; p=0.045).
Figure 2c.
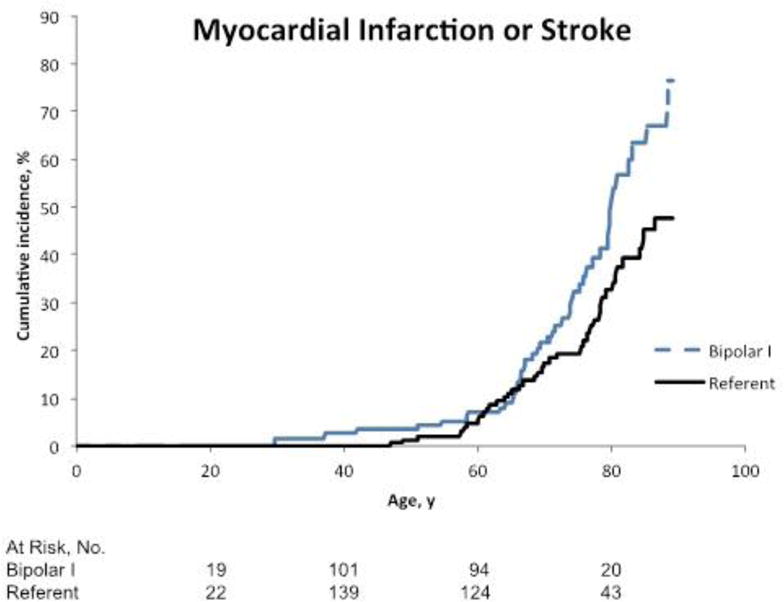
Cumulative incidence of fatal or non-fatal myocardial infarction or stroke among patients with bipolar I disorder compared to referents. The numbers of people at risk below the graphs reflect the occurrence of both left and right censoring (age is the time scale).
Discussion
We observed a significant increased risk for the composite outcome of fatal and non-fatal MI and stroke among patients with bipolar I disorder in Olmsted County, MN, USA, which was not retained after adjusting for CVD risk factors. The role of the potential confounders was particularly important for the risk of stroke as evidenced by a 0.39 reduction of the unadjusted HR to the adjusted HR. Based on a post-hoc analysis, there may be a differential risk of fatal or non-fatal MI/stroke in bipolar patients based on the presence or absence of psychosis; given the small sample size, this needs to be further investigated in subsequent studies. This study is strengthened by the quality of the assessment methods: bipolar I diagnosis confirmation by medical records review conducted by a board-certified experienced psychiatrist; CVD risk factors ascertainment by documented diagnosis, physiological measures, and laboratory results; and confirmation of MI and stroke by medical records review applying standard diagnostic criteria that included symptoms, signs, and laboratory measures.
Three register-based studies reported a significantly increased incidence rate ratio, admission rate ratio, or odds ratio of stroke in patients with bipolar I disorder, ranging from 1.39 (95% CI 1.26, 1.52) to 2.05 (95% CI 1.73, 3.54) (Laursen et al., 2009; Lin et al., 2007; Westman et al., 2013). Other studies have reported non-significant increased risk of MI in patients with bipolar disorder (Laursen et al., 2009; Lin et al., 2008; Ramsey et al., 2010). A large register-based cohort in Sweden showed no increased risk of MI (Westman et al., 2013). These differences in the risk of MI or stroke in other studies support the execution of more carefully designed studies that investigate these important clinical questions.
The possible increased risk of MI/stroke that we observed may be explained by several mechanisms. First, MI and stroke share several risk factors (Goldstein et al., 2006; Greenland et al., 2003) that are also associated with bipolar disorder [i.e., smoking (Waxmonsky et al., 2005), diabetes (Regenold et al., 2002), hypertension (Goldstein et al., 2009), obesity (McElroy et al., 2002), and low physical activity (Kilbourne et al., 2007b)]. Moreover, patients with bipolar disorder are exposed to pharmacotherapies (atypical antipsychotics, mood stabilizers, and lithium) that are associated with weight gain, obesity (McElroy et al., 2002), dyslipidemia, glucose abnormalities, and diabetes (Bobo et al., 2013; Guo et al., 2007; Olfson et al., 2006). Indeed, we observed an increased rate of diabetes, hypertension, and smoking in our bipolar I cohort, and when we incorporated these covariates in the multivariable models, the HR decreased substantially and became non-significant. However, physical activity and the use of psychotropic medication over time were not assessed, which may cause residual confounding. Thus, the increased risk for MI/stroke in patients with bipolar I disorder may be attributed in part to an increased exposure to CVD risk factors. It remains unclear whether bipolar disorder is associated with a subsequent development of CVD risk factors, or whether these patients are already highly exposed to these risk factors before the onset of the disorder.
Several lines of evidence may explain the higher CVD mortality in bipolar disorder. Patients with bipolar I disorder have less access to healthcare in general (Bradford et al., 2008), and cardiovascular interventional care in particular (Laursen et al., 2009). In addition, patients with bipolar I disorder may be more prone to post-MI complications and sudden cardiac death (Westman et al., 2013). One of the causes of sudden cardiac death is malignant arrhythmia, which may occur at higher rates in patients with bipolar disorder due to alterations of the autonomic nervous system (Cohen et al., 2003), or of the hypothalamus-pituitary-adrenal axis (Watson et al., 2004), or due to the use of atypical antipsychotics (Glassman and Bigger, 2001; Lin et al., 2014).
Although our study showed an important role of CVD risk factors on the risk of MI/stroke, this preliminary finding of a lower impact of CVD risk factors on the risk of MI/stroke in patients with history of psychosis suggests that bipolar I disorder with history of psychosis may increase CVD risk independently. In a cross-sectional study conducted by our group (Prieto et al., 2015), we identified an association between history of psychosis and cardiac disease in a sample of 988 patients with bipolar disorder, after adjusting for potential confounders. Psychosis in bipolar disorder has been associated with increased illness severity (Tohen et al., 2003), and may be associated with more severe neurobiological abnormalities with presumably more severe systemic manifestations.
A possible biological basis for the comorbidity of bipolar disorder and CVD is increased inflammation. Bipolar disorder has been associated with increased systemic inflammation, which may be stronger in patients with more severe symptoms (Dargel et al., 2015). Alterations in circulating inflammatory and anti-inflammatory cytokines have been identified in patients with bipolar disorder (Modabbernia et al., 2013). Atherosclerosis, the main pathophysiologic phenomenon in MI and ischemic stroke, is also mediated by inflammatory processes (Libby et al., 2009). In the context of increased levels of circulating inflammatory cytokines, endothelial cells express vascular cell adhesion molecules, which recruit monocytes and T lymphocytes, starting the atherosclerotic process [for details about atherosclerosis pathophysiology: (Libby et al., 2009)]. This early atherosclerotic plaque may start its development in young patients with bipolar disorder, who may not have developed CVD risk factors or these CVD risk factors may have not been present for sufficient time. Then, in the presence of CVD risk factors, the already damaged endothelium of these patients may be more prone to severe atherosclerosis in the context of heavy exposure to CVD risk factors. Therefore, systemic inflammation in bipolar disorder may trigger the beginning of atherosclerotic processes leading to increased risk for CVD early in life.
In addition, patients with bipolar I disorder and history of psychosis may share features of schizophrenia, such as increased inflammation (Potvin et al., 2008), increased exposure to CVD risk factors (Kilbourne et al., 2007a; Regenold et al., 2002), and increased CVD in longitudinal studies (Fan et al., 2013). We acknowledge that the increased risk of MI/stroke at the trend level among patients with bipolar I disorder and psychosis remains uncertain. Thus, this finding should be replicated in other cohorts.
Strengths and limitations
This study has a number of strengths. First, to our knowledge, this is the largest long-term U.S. population-based bipolar disorder cohort, which encompassed all patients with bipolar I disorder who were residents of Olmsted County during a 30-year timeframe, regardless of whether they were hospitalized or not at their index episode. There are no other bipolar disorder cohorts in the U.S. with this length of follow-up. Second, we were able to manually confirm each bipolar I case by psychiatrist chart review. Further, more than 50% of the cases were confirmed by strict DSM-IV-TR criteria. Only 30% of the potential cases identified with diagnostic codes were adjudicated as bipolar I disorder, which shows how strict our algorithm was. This rate is similar to rates of confirmed MI cases/potential MI cases identified by diagnostic codes using the REP (33%) (Roger et al., 2002). Third, the referent group consisted of persons without bipolar I disorder, but we did not exclude patients with other psychiatric disorders. Fourth, the ascertainment of MI and stroke was performed by records review by cardiologist-supervised trained abstractors (for MI) or a neurologist (for stroke). Fifth, the assessment of the covariates allowed adjusting for potential confounders of the association between bipolar I disorder, MI, and stroke. Sixth, the follow-up timeframe was up to 46 years, providing adequate time for the development of MI or stroke.
This study’s findings are limited by the small sample size and small number of events, which decrease our confidence in the estimates of effect. Further, stratified or subgroup analyses were limited by this lack of statistical power. We utilized a composite outcome, which has advantages from the statistical standpoint but limitations in its interpretation (Freemantle and Calvert, 2010). Even though we found a significantly increased risk of MI/stroke in this cohort, the CI was somewhat wide and thus the precision of the estimate of effect was limited. In addition, after adjusting for potential confounders at baseline, the HR decreased and became non-significant, which may have occurred due to small statistical power. We were not able to assess the development of CVD risk factors after baseline, nor the impact of CVD risk factors that arise during the course of the bipolar illness. Our recent systematic review (Prieto et al., 2014) has highlighted that CVD risk factors were not adjusted for in prior registries studies from Scandinavian countries (Laursen et al., 2011; Westman et al., 2013) and another two registry studies from Taiwan (Lin et al., 2007, 2008) conducted analyses that adjusted for hypertension, hyperlipidemia, renal disease and substance use, but did not adjust for diabetes, smoking (two well-established CVD risk factors) nor medication use. Finally, the two survey-based 2-wave studies (Ramsey et al., 2010; Goldstein et al., 2015) did adjust for CVD risk factors and medication use, however this study design is less robust because there could be missing events or missing CVD risk factors between each wave. Nevertheless, it is possible that the CVD risk factors are “mediators” or “intervening variables” in the chain of causality leading to MI or stroke. In this situation, the cardiovascular risk factors are not cofounders and its statistical adjustment may not be critical.
Because some patients in the disease and referent cohorts moved out of Olmsted County, MN, we may have missed MI or stroke events that occurred outside the county. However, the addition of mortality data, which is nationwide, allowed us to capture patients who died due to MI or stroke while out of the county. Our mortality data were based on death certificate diagnoses, and were not confirmed by autopsy; therefore, the validity of those diagnoses was not tested in this study. However, a prior study tested the validity of the death certificate diagnoses for coronary heart disease mortality with autopsy reports in Olmsted County (Goraya et al., 2000), and reported high sensitivity (91%) and high positive predictive value (96%) for out-of-hospital coronary heart disease death. This population-based cohort was based on treatment-seeking people who attended primary care clinics or hospitals in Olmsted County; therefore, we could not study patients who did not seek care. The little racial diversity of Olmsted County impeded any secondary analyses by race/ethnicity, and then these findings are only generalizable to Caucasian patients. Finally, we only collected data on covariates at baseline, and we did not include medication use over time because we were not able to ascertain medication use nor adherence using historical medical records. Therefore, we were not able to address the subsequent development of risk factors for CVD after the onset of bipolar disorder in incident cases, which may have underestimated the contribution of CVD risk factors to the risk of MI/stroke.
It is urgently needed to understand the mechanisms underlying this comorbidity of CVD and bipolar disorder. Few studies have investigated endothelial dysfunction in patients with bipolar disorder with contradictory findings (Fiedorowicz et al., 2012; Murray et al., 2012). Other areas of potential future research should include other biomarkers of early atherosclerosis, such as coronary calcium measured by computed tomography and oxidized low-density lipoprotein (oxLDL).
If the influence of CVD risk factors on the risk of MI/stroke in this population is high as showed in the adjusted analyses, the public health efforts should be focused on decreasing the prevalence of CVD risk factors among patients with bipolar disorder. A recent clinical trial investigated the efficacy of a multidisciplinary intervention specifically designed for patients with bipolar disorder (Kilbourne et al., 2013). It randomly assigned patients 58 patients to an intervention called Life Goals Collaborative Care and 60 patients to usual care. Its main outcome was blood pressure, cholesterol levels and quality of life. It showed a significant but small decrease in blood pressure (systolic: β=−2.94, p=0.05; diastolic: β=−2.18, p=0.03) and no changes in cholesterol levels. Therefore, more clinical trials should be designed to test interventions that may improve CVD health in these patients.
In conclusion, in a US population-based cohort of patients with bipolar I disorder observed for up to 46 years, we demonstrated a significant increase of MI/stroke as compared to people without bipolar I disorder. The association was no longer significant after adjustment for CVD risk factors that were present at baseline. If the risk of MI/stroke was confounded by these CVD risk factors, it will be fundamental to improve current preventive strategies to decrease the prevalence of smoking, alcohol use, hypertension and diabetes among patients with bipolar disorder. Moreover, we detected a possible higher risk of MI/stroke in the subgroup of patients with history of psychosis that certainly warrants replication. This study provided evidence of this association in a geographically-defined region in the U.S., despite the power limitations. Biomarkers of early atherosclerosis should be explored to identify patients with bipolar disorder at a particularly high risk of fatal or non-fatal CVD events.
Highlights.
Bipolar I disorder patients show an increased risk for myocardial infarction and stroke.
This association was not retained after adjusting for cardiovascular (CVD) risk factors.
Public health efforts may be directed towards the reduction of CVD risk factors in this population.
Acknowledgments
We thank Merle J. Belz, RN, Cynthia L. Nosek, RN, and Cynthia J. Stoppel, BS for their work abstracting the medical records, Jennifer St. Sauver, PhD for her advice on the Rochester Epidemiology Project, and Renato D. Alarcón, MD, MPH, Timothy W. Lineberry, MD, and Glenn E. Smith, PhD for their advice and support to this project.
Dr. Prieto has received honoraria for speaker activities and development of educational presentations from GlaxoSmithKline, has received travel support from GlaxoSmithKline, Lilly, Lundbeck, Pharmavita, and the National Institute of Mental Health, and has received scholarship support from the Government of Chile.
Dr. Bellivier has received honoraria for speaker activities from AstraZeneca, Bristol-Myers Squibb, Euthérapie, Lundbeck, Otsuka, European Space Agency, and for consulting from Bristol-Myers Squibb, Lundbeck, Otsuka, European Space Agency.
Dr. Frye has been a consultant (unpaid) for Allergan, Merck, Myriad, Sanofi-Aventis, Sunovion, Takeda Global Research, Teva Pharmaceuticals, United Biosource Corporation, has received grant support from Myriad, Pfizer, National Alliance for Schizophrenia and Depression (NARSAD), National Institute of Mental Health (NIMH), National Institute of Alcohol Abuse and Alcoholism (NIAAA), Mayo Foundation, and has received travel support from the Chilean Society of Neurology, Psychiatry and Neurosurgery (Sociedad de Neurologia, Psiquiatria y Neurocirugia), Advanced Health Media, GlaxoSmithKline, Colombian Society of Neuropsychopharmacology, AstraZeneca, Bristol-Myers-Squib, Otsuka, Sanofi-Aventis.
Grant support:
This project was made possible by the Rochester Epidemiology Project (grant number R01-AG034676; from the National Institute on Aging [NIA]). It was partially supported by Grant UL1-TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. It was partially supported by Mayo Foundation for Medical Education and Research, the Marriott Foundation and the Government of Chile.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 17th Annual Conference of the International Society for Bipolar Disorders.
Disclosures:
For the remaining authors, no further conflicts of interest were declared.
References
- Agency for Health Care Policy and Research. Clinical classifications for health policy research: tools for decision making and research. US Department of Health and Human Services; 1999. [Google Scholar]
- Angst J, Hengartner MP, Gamma A, von Zerssen D, Angst F. Mortality of 403 patients with mood disorders 48 to 52 years after their psychiatric hospitalisation. Eur Arch Psychiatry Clin Neurosci. 2013;263:425–434. doi: 10.1007/s00406-012-0380-1. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Cooper WO, Stein C, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70:1067–1075. doi: 10.1001/jamapsychiatry.2013.2053. [DOI] [PubMed] [Google Scholar]
- Bradford DW, Kim MM, Braxton LE, Marx CE, Butterfield M, Elbogen EB. Access to medical care among persons with psychotic and major affective disorders. Psychiatr Serv. 2008;59:847–852. doi: 10.1176/ps.2008.59.8.847. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Khizar A. The incidence of cardiovascular morbidity among patients with bipolar disorder: a population-based longitudinal study in Ontario, Canada. J Affect Disord. 2010;122:118–123. doi: 10.1016/j.jad.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kotler M, Mittelman I, Osher Y, Bersudsky Y. Impaired heart rate variability in euthymic bipolar patients. Bipolar Disord. 2003;5:138–143. doi: 10.1034/j.1399-5618.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- Dargel AA, Godin O, Kapczinski F, Kupfer DJ, Leboyer M. C-reactive protein alterations in bipolar disorder: a meta-analysis. J Clin Psychiatry. 2015;76:142–150. doi: 10.4088/JCP.14r09007. [DOI] [PubMed] [Google Scholar]
- Fan Z, Wu Y, Shen J, Ji T, Zhan R. Schizophrenia and the risk of cardiovascular diseases: a meta-analysis of thirteen cohort studies. J Psychiatr Res. 2013;47:1549–1556. doi: 10.1016/j.jpsychires.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG, Coryell WH, Rice JP, Warren LL, Haynes WG. Vasculopathy related to manic/hypomanic symptom burden and first-generation antipsychotics in a sub-sample from the collaborative depression study. Psychother Psychosom. 2012;81:235–243. doi: 10.1159/000334779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemantle N, Calvert MJ. Interpreting composite outcomes in trials. Bmj. 2010;341:c3529. doi: 10.1136/bmj.c3529. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT., Jr Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158:1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Fagiolini A, Houck P, Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11:657–662. doi: 10.1111/j.1399-5618.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Schaffer A, Wang S, Blanco C. Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J Clin Psychiatry. 2015;76:163–169. doi: 10.4088/JCP.14m09300. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, DeGraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- Goraya TY, Jacobsen SJ, Belau PG, Weston SA, Kottke TE, Roger VL. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted County, Minnesota. Mayo Clin Proc. 2000;75:681–687. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, Wilson PW. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- Guo JJ, Keck PE, Jr, Corey-Lisle PK, Li H, Jiang D, Jang R, L’Italien GJ. Risk of diabetes mellitus associated with atypical antipsychotic use among Medicaid patients with bipolar disorder: a nested case-control study. Pharmacotherapy. 2007;27:27–35. doi: 10.1592/phco.27.1.27. [DOI] [PubMed] [Google Scholar]
- Helft G, Steg PG, Le Feuvre C, Georges JL, Carrie D, Dreyfus X, Furber A, Leclercq F, Eltchaninoff H, Falquier JF, Henry P, Cattan S, Sebagh L, Michel PL, Tuambilangana A, Hammoudi N, Boccara F, Cayla G, Douard H, Diallo A, Berman E, Komajda M, Metzger JP, Vicaut E, Investigators OPDATT. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv481. [DOI] [PubMed] [Google Scholar]
- Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis. 2013;227:193–200. doi: 10.1016/j.atherosclerosis.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Kilbourne AM, Brar JS, Drayer RA, Xu X, Post EP. Cardiovascular disease and metabolic risk factors in male patients with schizophrenia, schizoaffective disorder, and bipolar disorder. Psychosomatics. 2007a;48:412–417. doi: 10.1176/appi.psy.48.5.412. [DOI] [PubMed] [Google Scholar]
- Kilbourne AM, Goodrich DE, Lai Z, Post EP, Schumacher K, Nord KM, Bramlet M, Chermack S, Bialy D, Bauer MS. Randomized controlled trial to assess reduction of cardiovascular disease risk in patients with bipolar disorder: the Self-Management Addressing Heart Risk Trial (SMAHRT) J Clin Psychiatry. 2013;74:e655–662. doi: 10.4088/JCP.12m08082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne AM, Rofey DL, McCarthy JF, Post EP, Welsh D, Blow FC. Nutrition and exercise behavior among patients with bipolar disorder. Bipolar Disord. 2007b;9:443–452. doi: 10.1111/j.1399-5618.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Munk-Olsen T, Agerbo E, Gasse C, Mortensen PB. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry. 2009;66:713–720. doi: 10.1001/archgenpsychiatry.2009.61. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Munk-Olsen T, Gasse C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS One. 2011;6:e24597. doi: 10.1371/journal.pone.0024597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Tsai SY, Lee HC. Increased risk of developing stroke among patients with bipolar disorder after an acute mood episode: a six-year follow-up study. J Affect Disord. 2007;100:49–54. doi: 10.1016/j.jad.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Lin HC, Tsai SY, Lee HC. No higher risk of myocardial infarction among bipolar patients in a 6-year follow-up of acute mood episodes. Psychosom Med. 2008;70:73–76. doi: 10.1097/PSY.0b013e31815c1e93. [DOI] [PubMed] [Google Scholar]
- Lin ST, Chen CC, Tsang HY, Lee CS, Yang P, Cheng KD, Li DJ, Wang CJ, Hsieh YC, Yang WC. Association between antipsychotic use and risk of acute myocardial infarction: a nationwide case-crossover study. Circulation. 2014;130:235–243. doi: 10.1161/CIRCULATIONAHA.114.008779. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Frye MA, Suppes T, Dhavale D, Keck PE, Jr, Leverich GS, Altshuler L, Denicoff KD, Nolen WA, Kupka R, Grunze H, Walden J, Post RM. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63:207–213. doi: 10.4088/jcp.v63n0306. [DOI] [PubMed] [Google Scholar]
- Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine Alterations in Bipolar Disorder: A Meta-Analysis of 30 Studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Murray DP, Metz NS, Haynes WG, Fiedorowicz JG. Vascular function is not impaired early in the course of bipolar disorder. J Psychosom Res. 2012;72:195–198. doi: 10.1016/j.jpsychores.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RM. Fatality outside hospital from acute coronary events in three British health districts, 1994–5. United Kingdom Heart Attack Study Collaborative Group. Bmj. 1998;316:1065–1070. [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Corey-Lisle P, Tuomari AV, Hines P, L’Italien GJ. Hyperlipidemia following treatment with antipsychotic medications. Am J Psychiatry. 2006;163:1821–1825. doi: 10.1176/ajp.2006.163.10.1821. [DOI] [PubMed] [Google Scholar]
- Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Prieto ML, Cuellar-Barboza AB, Bobo WV, Roger VL, Bellivier F, Leboyer M, West CP, Frye MA. Risk of myocardial infarction and stroke in bipolar disorder: a systematic review and exploratory meta-analysis. Acta Psychiatr Scand. 2014;130:342–353. doi: 10.1111/acps.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto ML, McElroy SL, Hayes SN, Sutor B, Kung S, Bobo WV, Fuentes ME, Cuellar-Barboza AB, Crow S, Osby U, Chauhan M, Westman J, Geske JR, Colby CL, Ryu E, Biernacka JM, Frye MA. Association between history of psychosis and cardiovascular disease in bipolar disorder. Bipolar Disord. 2015;17:518–527. doi: 10.1111/bdi.12302. [DOI] [PubMed] [Google Scholar]
- Ramsey CM, Leoutsakos JM, Mayer LS, Eaton WW, Lee HB. History of manic and hypomanic episodes and risk of incident cardiovascular disease: 11.5 year follow-up from the Baltimore Epidemiologic Catchment Area Study. J Affect Disord. 2010;125:35–41. doi: 10.1016/j.jad.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenold WT, Thapar RK, Marano C, Gavirneni S, Kondapavuluru PV. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J Affect Disord. 2002;70:19–26. doi: 10.1016/s0165-0327(01)00456-6. [DOI] [PubMed] [Google Scholar]
- Roger VL, Killian J, Henkel M, Weston SA, Goraya TY, Yawn BP, Kottke TE, Frye RL, Jacobsen SJ. Coronary disease surveillance in Olmsted County objectives and methodology. J Clin Epidemiol. 2002;55:593–601. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012a;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012b;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohen M, Zarate CA, Jr, Hennen J, Khalsa HM, Strakowski SM, Gebre-Medhin P, Salvatore P, Baldessarini RJ. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Ritchie JC, Ferrier IN, Young AH. Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. Br J Psychiatry. 2004;184:496–502. doi: 10.1192/bjp.184.6.496. [DOI] [PubMed] [Google Scholar]
- Waxmonsky JA, Thomas MR, Miklowitz DJ, Allen MH, Wisniewski SR, Zhang H, Ostacher MJ, Fossey MD. Prevalence and correlates of tobacco use in bipolar disorder: data from the first 2000 participants in the Systematic Treatment Enhancement Program. Gen Hosp Psychiatry. 2005;27:321–328. doi: 10.1016/j.genhosppsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Westman J, Hallgren J, Wahlbeck K, Erlinge D, Alfredsson L, Osby U. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ Open. 2013;3:e002373. doi: 10.1136/bmjopen-2012-002373. [DOI] [PMC free article] [PubMed] [Google Scholar]


