Abstract
Epidemiological studies demonstrate robust correlations between green tea consumption and reduced risk of type 2 diabetes and its cardiovascular complications. However, underlying molecular, cellular, and physiological mechanisms remain incompletely understood. Health promoting actions of green tea are often attributed to epigallocatechin gallate (EGCG), the most abundant polyphenol in green tea. Insulin resistance and endothelial dysfunction play key roles in the pathogenesis of type 2 diabetes and its cardiovascular complications. Metabolic insulin resistance results from impaired insulin-mediated glucose disposal in skeletal muscle and adipose tissue, and blunted insulin-mediated suppression of hepatic glucose output that is often associated with endothelial/vascular dysfunction. This endothelial dysfunction is itself caused, in part, by impaired insulin signaling in vascular endothelium resulting in reduced insulin-stimulated production of NO in arteries, and arterioles that regulate nutritive capillaries. In this review, we discuss the considerable body of literature supporting insulin-mimetic actions of EGCG that oppose endothelial dysfunction and ameliorate metabolic insulin resistance in skeletal muscle and liver. We conclude that EGCG is a promising therapeutic to combat cardiovascular complications associated with the metabolic diseases characterized by reciprocal relationships between insulin resistance and endothelial dysfunction that include obesity, metabolic syndrome and type 2 diabetes. There is a strong rationale for well-powered randomized placebo controlled intervention trials to be carried out in insulin resistant and diabetic populations.
Keywords: EGCG, endothelial function, green tea, insulin action, insulin sensitivity, metabolism, muscle blood flow, type 2 diabetes
1. INTRODUCTION
Accumulating laboratory and clinical studies suggest that polyphenol-rich plants have health-promoting effects with respect to cardiovascular and metabolic health [1–9], cancer prevention [10–13], and neurodegenerative diseases [14, 15].Within polyphenol-rich plants, green tea and its flavan-3-ols are among the most extensively studied for their putative health benefits.
Tea comes from the Camellia sinensis plant. Tea leaves are categorized into different classes based on the degree of fermentation (or leaf oxidation) during processing. During fermentation, flavan-3-ols, the bioactive polyphenols in tea leaves, undergo polyphenol oxidase-dependent oxidative polymerisation, resulting in the formation of theaflavins and thearubigins [16]. Green tea is unfermented and contains the highest concentration of flavan-3-ols. Oolong tea is a partially fermented product and therefore contains a mixture of flavan-3-ols, theaflavins, and thearubigins. Black tea is the most fermented tea, and as a result, contains abundant theaflavins and thearubigins, and limited or no flavan-3-ols. There are five major types of flavan-3-ols in green tea including catechin, epicatechin, epicatechin gallate, epigallocatechin, and epigallocatechin gallate (EGCG).
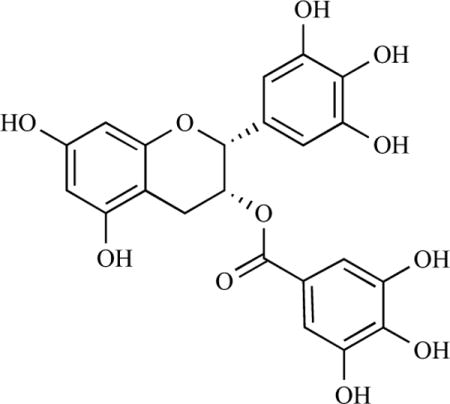
EGCG is a polyphenol belonging to the catechin family, a group of polyphenolic compounds (see chemical structure). Catechins are found in a variety of foods including fruits, vegetables, chocolate, wine, and tea. However, EGCG is predominantly found in tea and is the most abundant polyphenol accounting for as much as 50% of green tea polyphenols [16, 17]. In recent years, extensive research has investigated potential health-promoting effects as well as underlying molecular mechanisms of green tea or purified EGCG relevant to cardiovascular and metabolic diseases [2, 4, 7, 9, 18–21] including type 2 diabetes.
Insulin resistance plays a key role in the pathogenesis of type 2 diabetes and precedes the onset of diabetes. Insulin resistance is characterized by impaired insulin-mediated glucose disposal in skeletal muscle and is often associated with endothelial dysfunction. The conventional treatments available for type 2 diabetes are not sufficient to adequately control the disease which is increasing in prevalence and incidence as a result of the obesity epidemic in the developed world. Therefore, novel complementary therapeutic approaches including green tea and purified EGCG that oppose endothelial dysfunction and ameliorate metabolic insulin resistance in skeletal muscle and liver may augment current conventional treatments. There is growing public interest in the use of complementary and alternative approaches (such as green tea and its flavan-3-ols), for treating insulin resistance and type 2 diabetes because of unmet medical needs and the inherent safety benefits with functional foods including green tea.
2. EPIDEMIOLOGICAL STUDIES
Epidemiological studies show a positive relationship between tea consumption and reduced risk for type 2 diabetes [5–9, 22]. In 2009, two meta-analyses by Huxley et al. [23] and Jing et al. [24] report that, compared to non-tea drinkers, tea (green and black tea) consumption of >3 cups per day is associated with a 17 – 35 % lower risk of type 2 diabetes. Furthermore, in a British cohort [6] tea (green and black tea) consumption of >3 cups per day was associated with a 34% lower risk of diabetes. Whether theaflavins and thearubigins have similar bioactivities with EGCG, relevant to cardiovascular and metabolic diseases, is not known. Among Japanese adults, Iso et al. [9] report an inverse and dose-dependent association between green tea consumption and risk for type 2 diabetes. This study suggested that high green tea consumption (≥6 cups.d−1) lowers risk for developing type 2 diabetes by 33% compared to those who drink < 1 cup per week. More recently, a meta-analysis by Zheng et al. [25] demonstrates green tea catechin treatment for ≥ 12 weeks, but not shorter term (< 12 weeks), is associated with lower fasting blood glucose levels. Large epidemiologic studies have also shown green tea consumption is associated with decreased cardiovascular and all-cause mortality [26, 27]. In this same study there was no effect on cancer risk demonstrating the specificity of green tea for metabolic and cardiovascular disease [26]. However, not all studies report these positive associations. Studies in Japanese [28] and Singapore Chinese [29] populations failed to uncover an association between green tea consumption and reduced risk of type 2 diabetes.
3. CELLULAR ACTIONS OF EGCG
The classical metabolic actions of insulin on glucose homeostasis include glucose uptake by skeletal muscle and adipose tissue, and suppression of hepatic glucose production. There is evidence from in vitro studies that EGCG has insulin-mimetic metabolic actions on myocytes [30–32], adipocytes [33–36] and hepatocytes [37, 38] (see Fig. 1)
Fig. 1. Schematic of proposed metabolic actions of EGCG in myocytes, adipocytes and hepatocyes.
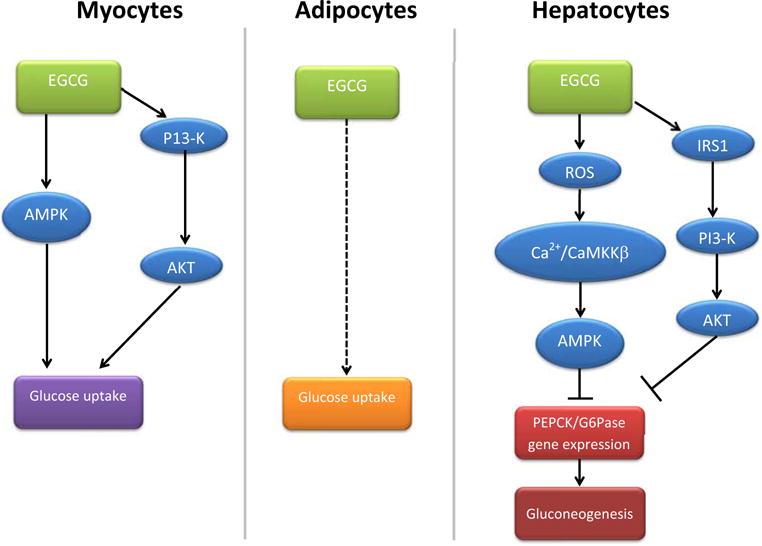
Arrows indicate activation ⊥ while indicate inhibition. Dotted line represents an unknown pathway.
Skeletal muscle is the major site for insulin-mediated glucose utilization and thereby contributes to postprandial blood glucose levels. In isolated myocytes, green tea or EGCG stimulates GLUT4 translocation and results in increased glucose uptake [30, 31, 39]. Similar to insulin, EGCG has been reported to stimulate muscle glucose uptake via the PI3-K/Akt signaling pathway in cultured myotubes [31, 32, 40]. Although it has been proposed that EGCG has insulin-mimetic actions on glucose uptake in myocytes [30–32, 39, 40], there is no evidence of EGCG directly activating the insulin receptor tyrosine kinase [31]. In addition to the insulin-mimetic pathway, EGCG can also stimulate muscle glucose uptake by alternative pathways such as the adenosine monophosphate-activated protein kinase (AMPK, with doses > 20 μM) [40]. Therefore, it is likely that EGCG-mediated muscle glucose uptake in vitro involves multiple signaling pathways some of which are insulin-mimetic.
Chronic green tea supplementation (4–12 weeks) increases glucose uptake [33–35], and promotes GLUT4 translocation [34, 35] in isolated adipocytes, however the molecular pathway leading to glucose uptake is unknown. Interestingly, green tea catechins (especially EGCG) augment insulin-stimulated glucose metabolism in adipose cells using an in vitro assay that assesses insulin-dependent breakdown of glucose to CO2 [36].
EGCG suppresses gluconeogenesis in cultured hepatocytes. At high doses (> 25 μM), EGCG suppresses hepatic gluconeogenesis through the same pathway as insulin, wherein EGCG promotes signaling through IRS-1/PI3-K/Akt, resulting in inhibition of PEPCK and G6Pase gluconeogenic enzyme activity [38]. However, we have demonstrated in isolated hepatocytes that EGCG, at relatively low concentrations (1 μM), inhibits glucose production via inhibition of gluconeogenesis and expression of key gluconeogenic genes [37]. This involves activation of AMPK through reactive oxygen species and the Ca2+/calmodulin-dependent protein kinase kinase β (CaMKK β) pathway [37].
The microsomal enzyme 11β-hydroxysteroiddeydrogenase type 1 (11β-HSD1) catalyzes the interconversion of cortisone to cortisol. Strong evidence exists for an important etiological role of 11β-HSD1 in various metabolic disorders including insulin resistance, type 2 diabetes, hypertension, dyslipidemia and obesity. EGCG strongly inhibits 11β-HSD1 activity [41] thus implicating another potential mechanism for EGCG to ameliorate metabolic diseases.
In summary, the glucose lowering effects of EGCG may involve pathways that directly supress hepatic glucose production while simultaneously stimulating glucose uptake in skeletal muscle and adipose tissue. However, the direct metabolic or cellular actions of EGCG on glucose metabolism have only been assessed in vitro and the effect of EGCG in vivo or in a vascularly intact model needs to be confirmed. In addition to these classical metabolic actions, EGCG also has vascular actions that may directly contribute to muscle glucose uptake (detailed below).
4. VASCULAR ACTIONS OF EGCG
4.1. Endothelial Cell Culture
EGCG stimulates nitric oxide (NO) production from vascular endothelial cells similar to insulin (see Figs. 2 and 3). Both insulin- and EGCG-mediated NO production is dependent on the activation of PI3-K, since wortmannin blocks NO production by both insulin and EGCG [20]. Furthermore, like insulin, EGCG requires the activation of Akt and endothelial nitric oxide synthase (eNOS) for NO production. Fig. (2) shows the time-course for EGCG on activation of Akt and eNOS. Thus, low micromolar concentrations of EGCG (achievable with consumption of 5 cups of green tea) can stimulate Akt and eNOS within 15 minutes in bovine aortic endothelial cells. Interestingly, these results also highlight that EGCG-mediated signaling pathways share features in common with the insulin signaling pathway that lead to activation of eNOS and NO production in endothelial cells [42–44]. However, EGCG does not activate the insulin receptor or VEGF receptor tyrosine kinase, suggesting that the insulin and EGCG pathways converge at a point downstream from the insulin receptor.
Fig. 2. Stimulation of NO from endothelial cells via a PI3-K/Akt/eNOS pathway.
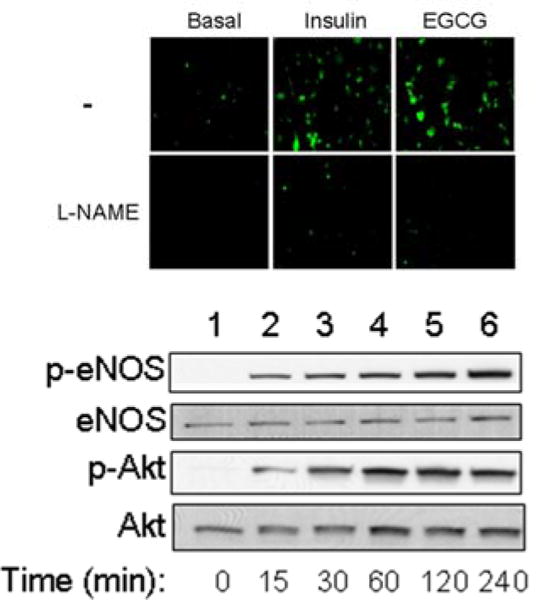
Bovine aortic endothelial cells were loaded with 4,5-diaminofluoresceine diacetate (DAF2-DA). In the presence of NO, DAF2-DA emits green fluorescence. Both insulin (100 nM, 5 min) and EGCG (50 μM, 5 min) stimulates NO production in endothelial cells. This effect was inhibited by the presence of the NOS inhibitor L-NAME [20].
Fig. 3. Schematic of proposed vasoactive pathways of EGCG in the vasculature.
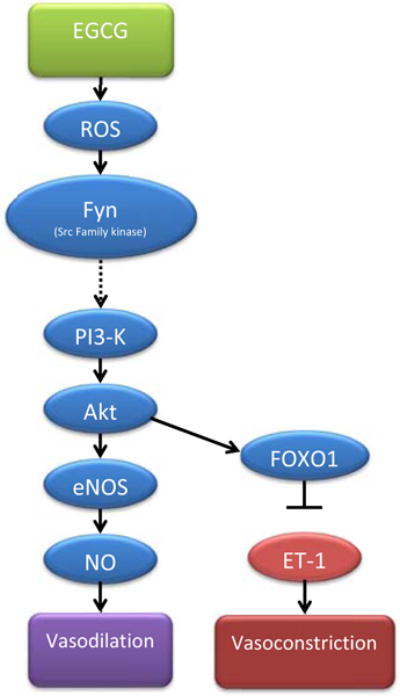
Arrows indicate activation while ⊥ indicate inhibition.
It has been proposed that the laminin receptor is a specific cell surface receptor for EGCG that mediates some of its biological actions [45]. However, we have shown that EGCG-mediated activation of the laminin receptor does not play a significant role in the vascular endothelium relating to NO production [20]. Instead, EGCG-mediated signaling requires low level production of reactive oxygen species such as H2O2 [20]. EGCG-mediated production of intracellular H2O2 can be abrogated by N-acetylcysteine, a scavenger of reactive oxygen species [20]. Reactive oxygen species activate Fyn, a member of the Src family tyrosine kinases that is required for activation of PI3-K, eNOS and NO production in vascular endothelial cells. Again, pre-treatment with N-acetylcysteine blocks EGCG-mediated activation of Fyn [20]. The importance of these overlapping pathways highlight the possibility that EGCG may have insulin-mimetic and/or insulin-potentiating effects in the vascular system.
Insulin stimulates production of both NO and endothelin-1 (ET-1) from the endothelium. Insulin stimulates NO production (vasodilator) via a PI3-K dependent pathway and ET-1 production (vasoconstrictor) via a MAP kinase dependent pathway [46]. These agents have opposing vasoactive actions that are in balance under normal healthy conditions. In the presence of insulin, NO production dominates favoring vasodilation. Disruption of the balance between NO and ET-1 production is believed to contribute to the development of hypertension, type 2 diabetes and atherosclerosis [47]. Importantly, EGCG-stimulated phosphorylation of FoxO1, downstream from PI3-K/Akt inhibits both insulin and EGCG-stimulated synthesis and secretion of ET-1 [48, 49]. These data provide a second mechanism that involves PI3-K-dependent pathways to impair ET-1 secretion and favor vasodilator conditions. During insulin resistance, the PI3-K pathway is impaired [50] and the MAP kinase pathway is upregulated [51]. Ultimately this decreases NO production, while increasing ET-1 production. Thus, EGCG, which promotes NO while simultaneously inhibiting ET-1 production, may have a significant advantage as a therapeutic agent in the treatment of insulin resistance. The NO-favoring actions of EGCG have a primary benefit in improving cardiovascular homeostasis, while also improving the metabolic actions of insulin by allowing greater hormone and substrate access to metabolic targets including skeletal muscle. Fig. (3) details the proposed molecular pathways EGCG uses to promote vasodilation.
4.2. Isolated Vessels
Studies have shown that EGCG is a potent vasodilator in isolated aortic rings [52, 53], bovine ophthalmic arteries [54], coronary artery rings [55], and mesenteric vascular beds [4]. Green tea catechins and EGCG have been reported to improve endothelial function in the spontaneous hypertensive rat [4, 56], pre-diabetic OLETF rat [57, 58] and the high fat-fed mouse [59]. These actions are PI3-K- and Fyn-dependent.
4.3. Skeletal Muscle
Our research implicates vascular dysfunction in skeletal muscle as one major cause of muscle insulin resistance. Insulin stimulates both total blood flow to skeletal muscle [60, 61] and increases microvascular perfusion of myocytes [62–67]. However, insulin’s macrovascular and microvascular actions are temporally distinct events, and insulin-mediated glucose uptake is significantly altered by changes in microvascular rather than macrovascular responses [63, 68].
Systemic and local hindleg infusion of the NOS inhibitor L-NAME blocks most, if not all, of insulin-mediated microvascular perfusion in muscle and inhibits 30–40% of insulin-mediated muscle glucose uptake [62, 63, 69]. Therefore, insulin-mediated microvascular recruitment in muscle is, at least in part, NOS-dependent and plays an integral role in regulating muscle glucose uptake. We have demonstrated that insulin, whether infused intravenously or secreted from the pancreas following a mixed meal, stimulates microvascular blood flow in skeletal muscle in both experimental animals [62–65, 67–73] and human subjects [74–76]. This action of insulin to enhance microvascular recruitment in skeletal muscle facilitates delivery of glucose and insulin to the myocytes and enhances glucose disposal. Previously, we have demonstrated that insulin resistant rats [68, 70, 73] and humans [74, 75] have impaired insulin-mediated microvascular and metabolic responses in muscle, suggesting that the loss of microvascular insulin action may contribute to insulin resistance. We recently reported that high fat-induced insulin resistance can originate from impaired microvascular insulin responses and that this microvascular defect precedes the development of myocyte insulin resistance [68]. As mentioned previously, an imbalance between the vasodilator NO, and the vasoconstrictor ET-1, contributes to endothelial dysfunction. We have shown that acute ET-1 infusion inhibits insulin-mediated microvascular recruitment and muscle glucose uptake in vivo [77]. This further highlights that disruption of the balance between NO and ET-1 results in reduced insulin-stimulated muscle glucose uptake, indicative of muscle insulin resistance.
The above findings demonstrate a strong link between insulin resistance and endothelial dysfunction, positioning insulin’s microvascular action as a critical factor in the development of insulin resistance and type 2 diabetes. Finding novel treatments that have insulin-mimetic and/or insulin-potentiating actions on these vascular targets is a novel approach for treating insulin resistance, endothelial dysfunction as well as type 2 diabetes and its cardiovascular complications.
Serotonin infusion induces muscle insulin resistance in both the perfused rat hindlimb preparation [78] and in vivo [67] via vascular actions. We have preliminary data (unpublished) demonstrating that EGCG vasodilates in the presence of serotonin in the constant flow perfused rat hindlimb. Thus, EGCG can oppose vasoconstriction associated with insulin resistance and impaired muscle nutrient exchange. Importantly, we have unpublished data demonstrating EGCG-mediated vasodilation in this preparation is NOS-dependent at low doses (≤ 10 μM), and at least in part NOS-independent at high doses (100 μM).
The dependency of EGCG-induced vasodilation on NOS varies with the dose/concentration of EGCG. EGCG-mediated vasodilation in rat thoracic aorta is NOS dependent at low concentrations (0.01 – 10 μM), but not at higher doses of EGCG [52]. Some [52, 53], but not all [20, 54] studies have reported that high concentration of EGCG (100 μM) can induce vasodilation through different pathways by acting directly on vascular smooth muscle cells [52] or by activating the cAMP-dependent protein kinase pathway [53]. EGCG has also been shown to increase PGI2 production in bovine aortic endothelial cells [79]. Together, these data are supportive of our unpublished studies that high dose EGCG-induced vasodilation involves NO-independent mechanisms.
As described above, insulin has important microvascular actions in skeletal muscle. Acute infusion of EGCG in rats in vivo (to raise the plasma EGCG level to 10 μM) does not alter femoral artery blood flow, but stimulates microvascular recruitment in muscle (unpublished observations). This suggests that these differential vascular actions of EGCG (macro- vs. micro-vascular) may be concentration-dependent. The magnitude of the increase in microvascular recruitment was similar in effect to raising plasma insulin concentrations to 1.5 nM. However, the microvascular actions of EGCG and insulin did not appear to be additive, i.e. EGCG has insulin-mimetic but not synergistic actions in muscle microvasculature. If similar effects are observed in human studies, this would suggest that daily chronic green tea consumption may promote enhanced microvascular function and oppose endothelial dysfunction.
5. ANIMAL STUDIES
Most of the compelling evidence for the anti-diabetic actions of green tea have come from animal studies including normal healthy [30, 33, 39], insulin resistant [2, 34, 39, 57–59, 80–82], type 2 diabetic [83–85] and hypertensive [4] rodent models (detailed in Table 1).
Table 1.
Summary of acute and chronic effects of green tea and EGCG in humans and animals.
| Treatment | Subjects | Effects | References | |
|---|---|---|---|---|
| Human | Acute | Healthy |
↑ GTT ↑ Insulin sensitivity ↔ GTT ↔ Fasting blood/plasma glucose ↔ Fasting seruminsulin ↑ Post-prandial plasma glucose ↔ Post-prandial serum insulin |
[83] [105] [105] [83, 105] [105] [98] [98] |
| Chronic (a3wks) | Healthy | ↔ Fasting plasma glucose | [92] | |
| Overweight/Obese/Insulin Resistant |
↓ Fasting plasma/serum glucose ↓ Fasting serum insulin ↓ HOMA-IR ↔ HOMA-IR ↔ GTT ↔ Fasting blood/plasma glucose ↔ Fasting plasma insulin |
[93, 107] [107] [107] [88] [88] [87–89, 94, 99, 100, 102, 106] [88, 89, 94, 99, 102] |
||
| Type 2 diabetes |
↓ Fasting insulin ↓ HOMA-IR ↓ HbA1c ↓ Fasting plasmaglucose ↔ Fasting blood/plasma glucose ↔ Fasting plasma insulin ↔ HOMA-IR ↔ HbA1c |
[97] [97] [97, 108]* [109]* [95, 101, 104] [95, 104] [95, 104] [95] |
||
| Animal | Acute (≤2hrs) | Healthy |
↔ Blood glucose ↓ GTT ↓ Insulin sensitivity |
[83] [113] [113] |
| Insulin resistant |
↑ GTT ↑ Insulin sensitivity ↓ Plasma glucose ↓ Plasma insulin |
[113] [113] [113] [113] |
||
| Type 2 diabetes | ↓ Blood glucose | [83] | ||
| Chronic (≥2wks) | Healthy |
↓ Fasting blood/plasma glucose ↓ Fasting plasma insulin ↔ GTT ↑ insulin sensitivity |
[33, 39] [33] [33, 39] [33] |
|
| Insulin resistant |
↓ Fasting blood/plasma glucose ↓ Fasting plasma/serum insulin ↑ GTT ↔ GTT ↑ QUICKI ↓ HOMA-IR ↑ Endothelial function |
[39, 57, 58, 81, 82] [57–59, 80–82] [2, 34, 39, 80] [82] [4, 59] [81, 82] [4, 57–59] |
||
| Type 2 diabetes |
↓ Fasting blood glucose ↑ GTT |
[84, 85] [84, 85] |
GTT: glucose tolerance test;
: improve;
: reduce;
: no effects;
:oolong tea.
In healthy rats, green tea treatment for 3 weeks (raising plasma EGCG levels to ~40 nM) significantly reduces adiposity and circulating lipids; while increasing (~25%) muscle glucose uptake and GLUT-4 translocation in vitro [30]. In rats, Wu and colleagues [33] showed that 12 weeks of green tea treatment (containing mixed catechins 56 mg.d−1; EGCG 38 mg.d−1) significantly lowered fasting plasma glucose, insulin, and circulating lipids while concurrently improving glucose tolerance and insulin sensitivity. In mice, green tea (EGCG 610 mg.L−1 in drinking water) supplementation for 14 weeks lowers fasting blood glucose levels. However, no concomitant changes in serum lipid levels were observed [39]. Interestingly, glucose tolerance and muscle glucose uptake in these mice were not altered by green tea therapy while adipose tissue glucose uptake was significantly impaired [39]. Thus, the effects of green tea or EGCG in healthy animals are inconclusive due to a limited number of studies. It should be noted, however, that it is extremely difficult to demonstrate improvement in these parameters, even with conventional drugs, if the animal start out with values in the normal range. The more compelling work indicating positive benefits of ECGC has been conducted in insulin resistant or diabetic animals.
Green tea, green tea extract or EGCG treatment have been reported to ameliorate diet-induced insulin resistance in a number of rodents studies [2, 34, 39, 59, 80–82] and also studies involving genetic rodent models of insulin resistance and type 2 diabetes [4, 57, 58, 83–85]. Green tea (EGCG 1 g.L−1 in drinking water) treatment for 12 weeks improves glucose tolerance, GLUT4 content and glucose uptake in adipocytes in vitro from fructose-fed rats [34]. Green tea treatment (EGCG 150 or 300 mg.kg−1.d−1) of fructose-fed hamsters dose-dependently improves glucose tolerance, increases serum adiponectin levels and reduces fasting serum insulin levels [80]. Similarly, Bose et al. [81] reported that 4 weeks of EGCG (3.2 g.kg−1 diet) treatment to high fat-fed mice, significantly reduced fasting blood glucose and plasma insulin levels. However, the food intake of the mice was not reported, and therefore it is unclear how much food (or EGCG) was consumed by the mice each day. Chronic green tea treatment in high fat-fed mice for 14 weeks (EGCG 610 mg.L−1) [39] significantly improves glucose tolerance, GLUT4 translocation in muscle, and in vitro muscle glucose uptake, and reduces fasting plasma glucose. Similarly, EGCG treatment for 16 weeks (3.2 g.kg−1 diet) [81] or 22 weeks (EGCG 2 mg.kg−1.d−1) [82] in high fat-fed mice reduces fasting plasma glucose, insulin, and the homeostasis model assessment of insulin resistance (HOMA-IR; a surrogate index of insulin resistance). In addition EGCG (50 mg.kg−1.d−1) treatment for 10 weeks in high fat-fed mice [59] lowers fasting serum glucose and insulin, thus improving quantitative insulin sensitivity check index (QUICKI, a surrogate index of insulin sensitivity) [86].
The db/db mouse is a commonly used genetic model of type 2 diabetes. One study showed that db/db mice treated with EGCG (at 100 mg.kg−1.d−1 but not at 30 mg.kg−1.d−1) for 2 weeks improves glucose tolerance [84]. Wolfram et al. [84] also showed that EGCG treatment for 7 weeks (EGCG 0.25 – 1 g.kg−1 diet) improves glucose tolerance and reduces blood glucose in this diabetic mouse model in a dose-dependent manner. Another study [85] reports that db/db mice treated with EGCG (1 g.kg−1 diet) for 10 weeks significantly improves glucose tolerance and reduces fasting blood glucose with an effect size comparable to the anti-diabetic insulin sensitizer rosiglitazone. Interestingly, this study also shows EGCG intake reduces the number of pathologically altered islets of Langerhans, while increasing the number and the size of islets, thereby increasing beta cell mass and presumably insulin secretory capacity [85]. These effects correlated with a reduction in islet endoplasmic reticulum stress [85]. Acute green tea treatment (mixed catechins 22 mg.kg−1, EGCG 17 mg.kg−1) lowers fasting blood glucose levels in db/db mice, but not wild type mice [83]. In pre-diabetic OLETF rats, treatment with green tea catechins (25 – 30 mg.kg−1.d−1) for 12 weeks lowers blood pressure and fasting plasma glucose and insulin levels [57, 58]. EGCG treatment (200 mg.kg−1.d−1) for 3 weeks prevents development of insulin resistance and reduces systolic blood pressure significantly in spontaneously hypertensive rats [4].
The similar metabolic effects of green tea and EGCG in the above studies suggest that improvements in insulin sensitivity by green tea treatment might be attributable to the actions of EGCG, given that EGCG makes up such a large percentage of green tea polyphenols. However, there are multiple components in green tea that may have synergistic actions.
6. CLINICAL INVESTIGATIONS
Numerous clinical studies have been undertaken to investigate the effects of green tea or green tea extracts on glucose tolerance, insulin sensitivity, glucose homeostasis and other cardiometabolic outcomes in people ranging from normal and healthy to insulin resistant individuals and those with type 2 diabetes [18, 83, 87–107] (detailed in Table 1). The effect of green tea/EGCG consumption in healthy subjects appears to be heterogeneous with some reporting improved insulin sensitivity [105] and glucose tolerance [83], while others show no relationship [92, 98]. Again, as with the animal studies, determining differences in these metabolic parameters in healthy people is challenging even with conventional therapeutic agents.
In postmenopausal women with impaired glucose tolerance, EGCG treatment (300 mg.d−1) for 12 weeks reduces fasting plasma glucose by 5% [93]. Green tea extract treatment (EGCG 208 mg.d−1) for 12 weeks significantly reduces fasting blood glucose and insulin [107] levels in obese insulin resistant subjects. In contrast to these aforementioned studies, EGCG treatment (800 mg.d−1) for 8 weeks had no effects on glucose tolerance, fasting glucose and insulin levels in overweight and obese men [88].
In patients with type 2 diabetes, green tea extract (EGCG 860 mg.d−1 for 16 weeks) significantly reduces HOMA-IR, HbA1c, and fasting insulin levels [97]. Conversely, Ryu et al. [104] showed that metabolic markers including blood lipids, glucose, insulin, and adiponectin levels were not altered following 4 weeks of green tea treatment (polyphenol content not reported). Similarly, another study [95] shows green tea treatment (540 mg.d−1 polyphenols, EGCG content unknown) for 2 months has no apparent effect on metabolic markers such as fasting serum glucose and insulin, HbA1c, and HOMA-IR.
Some studies have assessed the metabolic effects of oolong tea in patients with type 2 diabetes. As previously mentioned, oolong tea is partially fermented and contains moderate amounts of EGCG. Shimada et al. [108] reported that oolong tea treatment for 4 weeks (45 mg.day−1 of EGCG) significantly increases plasma adiponectin levels by 9.9% and lowers HbA1c levels by 3.3% in patients with various coronary risk factors. Additionally, there was a slight, but not significant, decrease in the fasting plasma glucose levels. Hosoda et al. [109] used a higher dose of oolong tea treatment (EGCG 390 mg.d−1) for 4 weeks and reported lower fasting plasma glucose levels in people with type 2 diabetes. The mechanism of the anti-hyperglycemic effects of the oolong tea is unclear. However, oolong tea appears to have a concentration-dependent effect on glycemia and this tea contains moderate amounts of EGCG.
Although these clinical studies are not conclusive, they suggest a relationship between green tea or EGCG consumption and reduced risk of type 2 diabetes. The potential of complementary alternative medicines as adjuncts to conventional therapy deserves further investigation by direct interventional studies that include biomarker and pharmacokinetic analyses as well as study outcomes that are clinically meaningful.
CONCLUSION
Animal and cellular studies provide strong evidence that EGCG has beneficial actions to improve and/or augment insulin sensitivity. The molecular and cellular mechanisms, although not fully resolved, are under active investigation and likely involve signaling pathways that are shared with insulin as well as insulin-independent pathways (Figs. 1 and 3). In the vascular system, ECGC stimulates NO production with resulting vasodilation and microvascular recruitment, and inhibits vasoconstriction by opposing ET-1 release and inhibiting serotonin mediated vasoconstriction. Ultimately, these favourable actions of EGCG in skeletal muscle may contribute to ameliorating insulin resistance by improving microvascular delivery of hormones and nutrients to relevant target tissues regulating glucose homeostasis.
Along with robust epidemiological studies related to tea consumption and cardiometabolic health, molecular, cellular, and physiological studies in animals and humans provide a strong rationale for well-powered randomized placebo controlled intervention trials to be carried out in insulin resistant and diabetic populations. These studies should evaluate the efficacy of EGCG as an insulin sensitizer and adjunct functional food treatment for diabetes and its cardiovascular complications. It is important that these future clinical studies of EGCG intervention include formal pharmacokinetic and pharmacodynamic aspects that are often overlooked in human studies of functional foods and nutritional supplements [21]. As for example, in recent clinical intervention studies of glucosamine [110], cocoa [111], and vitamin C [112].
Randomized controlled trials of EGCG in combination with current anti-diabetic drugs are also worthwhile as the vascular actions of EGCG may provide additional benefits in terms of overcoming cardiovascular pathophysiology associated with insulin resistance and type 2 diabetes. These combination studies are also important because EGCG or green tea may be most effective as an adjunctive rather than a primary therapy.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (APP1009962). This work was also supported by the American Diabetes Association (1-09-JF-33; 1-12-BS-99 to J.K), American Heart Association (13GRNT17220057 to J.K). We gratefully acknowledge Dr John C Keske for drawing the chemical structure of EGCG.
LIST OF ABBREVIATIONS
- AKT
Protein kinase B
- AMPK
Adenosine monophosphate-activated protein kinase
- CaMKK β
Ca2+/calmodulin-dependent protein kinase kinase β
- DAF2-DA
4,5-diaminofluoresceine diacetate
- EGCG
Epigallocatechin gallate
- ET-1
Endothelin-1
- GLUT-4
Glucose transporter 4
- HOMA-IR
Homeostasis model assessment of insulin resistance
- PGI2
Prostaglandin I2
- QUICKI
Quantitative insulin sensitivity check index
- HbA1c
Hemoglobin A1c
- L-NAME
L-NG-Nitroarginine Methyl Ester
- NOS
Nitric oxide synthase
- NO
Nitric oxide
- OLETF
Otsuka Long Evans Tokushima Fatty
- PI3-K
Phosphoinositide 3-kinase
- 11β-HSD1
11β-hydroxysteroiddeydrogenase type 1
- VEGF
Vascular endothelial growth factor
Biography
Michelle A. Keske

Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Tresserra-Rimbau A, Rimm EB, Medina-Remon A, Martinez-Gonzalez MA, de la Torre R, Corella D, Salas-Salvado J, Gomez-Gracia E, Lapetra J, Aros F, Fiol M, Ros E, Serra-Majem L, Pinto X, Saez GT, Basora J, Sorli JV, Martinez JA, Vinyoles E, Ruiz-Gutierrez V, Estruch R, Lamuela-Raventos RM. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2014 doi: 10.1016/j.numecd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Chen N, Bezzina R, Hinch E, Lewandowski PA, Cameron-Smith D, Mathai ML, Jois M, Sinclair AJ, Begg DP, Wark JD, Weisinger HS, Weisinger RS. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr Res. 2009;29(11):784–793. doi: 10.1016/j.nutres.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Stoclet JC, Kleschyov A, Andriambeloson E, Diebolt M, Andriantsitohaina R. Endothelial no release caused by red wine polyphenols. J Physiol Pharmacol. 1999;50(4):535–540. [PubMed] [Google Scholar]
- 4.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab. 2007;292(5):E1378–1387. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg JA, Axen KV, Schnoll R, Boozer CN. Coffee, tea and diabetes: the role of weight loss and caffeine. Int J Obes (Lond) 2005;29(9):1121–1129. doi: 10.1038/sj.ijo.0802999. [DOI] [PubMed] [Google Scholar]
- 6.Hamer M, Witte DR, Mosdol A, Marmot MG, Brunner EJ. Prospective study of coffee and tea consumption in relation to risk of type 2 diabetes mellitus among men and women: the Whitehall II study. Br J Nutr. 2008;100(5):1046–1053. doi: 10.1017/S0007114508944135. [DOI] [PubMed] [Google Scholar]
- 7.Polychronopoulos E, Zeimbekis A, Kastorini CM, Papairakleous N, Vlachou I, Bountziouka V, Panagiotakos DB. Effects of black and green tea consumption on blood glucose levels in non-obese elderly men and women from Mediterranean Islands (MEDIS epidemiological study) Eur J Nutr. 2008;47(1):10–16. doi: 10.1007/s00394-007-0690-7. [DOI] [PubMed] [Google Scholar]
- 8.Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr. 2005;24(5):376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 9.Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144(8):554–562. doi: 10.7326/0003-4819-144-8-200604180-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson PJ, Kurowska E, Freeman DJ, Chambers AF, Koropatnick DJ. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J Nutr. 2004;134(6):1529–1535. doi: 10.1093/jn/134.6.1529. [DOI] [PubMed] [Google Scholar]
- 11.Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86(11):855–858. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- 12.Komori A, Yatsunami J, Okabe S, Abe S, Hara K, Suganuma M, Kim SJ, Fujiki H. Anticarcinogenic activity of green tea polyphenols. Jpn J Clin Oncol. 1993;23(3):186–190. [PubMed] [Google Scholar]
- 13.Wang ZY, Agarwal R, Bickers DR, Mukhtar H. Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis. 1991;12(8):1527–1530. doi: 10.1093/carcin/12.8.1527. [DOI] [PubMed] [Google Scholar]
- 14.Darvesh AS, Carroll RT, Bishayee A, Geldenhuys WJ, Van der Schyf CJ. Oxidative stress and Alzheimer’s disease: dietary polyphenols as potential therapeutic agents. Expert Rev Neurother. 2010;10(5):729–745. doi: 10.1586/ern.10.42. [DOI] [PubMed] [Google Scholar]
- 15.Silva AR, Pinheiro AM, Souza CS, Freitas SR, Vasconcellos V, Freire SM, Velozo ES, Tardy M, El-Bacha RS, Costa MF, Costa SL. The flavonoid rutin induces astrocyte and microglia activation and regulates TNF-alpha and NO release in primary glial cell cultures. Cell Biol Toxicol. 2008;24(1):75–86. doi: 10.1007/s10565-007-9017-y. [DOI] [PubMed] [Google Scholar]
- 16.Lin YS, Tsai YJ, Tsay JS, Lin JK. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J Agric Food Chem. 2003;51(7):1864–1873. doi: 10.1021/jf021066b. [DOI] [PubMed] [Google Scholar]
- 17.Vuong QV, Golding JB, Nguyen M, Roach PD. Extraction and isolation of catechins from tea. J Sep Sci. 2010;33(21):3415–3428. doi: 10.1002/jssc.201000438. [DOI] [PubMed] [Google Scholar]
- 18.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010;29(1):31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 19.Hininger-Favier I, Benaraba R, Coves S, Anderson RA, Roussel AM. Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J Am Coll Nutr. 2009;28(4):355–361. doi: 10.1080/07315724.2009.10718097. [DOI] [PubMed] [Google Scholar]
- 20.Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem. 2007;282(18):13736–13745. doi: 10.1074/jbc.M609725200. [DOI] [PubMed] [Google Scholar]
- 21.Munir KM, Chandrasekaran S, Gao F, Quon MJ. Mechanisms for food polyphenols to ameliorate insulin resistance and endothelial dysfunction: therapeutic implications for diabetes and its cardiovascular complications. Am J Physiol Endocrinol Metab. 2013;305(6):E679–686. doi: 10.1152/ajpendo.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Woudenbergh GJ, Kuijsten A, Drogan D, Van Der A D, Romaguera D, Ardanaz E, Amiano P, Barricarte A, Beulens JWJ, Boeing H. Tea consumption and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. PLoS One. 2012;7(5):e36910. doi: 10.1371/journal.pone.0036910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169(22):2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 24.Jing Y, Han G, Hu Y, Bi Y, Li L, Zhu D. Tea consumption and risk of type 2 diabetes: a meta-analysis of cohort studies. J Gen Intern Med. 2009;24(5):557–562. doi: 10.1007/s11606-009-0929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng XX, Xu YL, Li SH, Hui R, Wu YJ, Huang XH. Effects of green tea catechins with or without caffeine on glycemic control in adults: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97(4):750–762. doi: 10.3945/ajcn.111.032573. [DOI] [PubMed] [Google Scholar]
- 26.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296(10):1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 27.Peters U, Poole C, Arab L. Does tea affect cardiovascular disease? A meta-analysis. Am J Epidemiol. 2001;154(6):495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 28.Oba S, Nagata C, Nakamura K, Fujii K, Kawachi T, Takatsuka N, Shimizu H. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br J Nutr. 2010;103(3):453–459. doi: 10.1017/S0007114509991966. [DOI] [PubMed] [Google Scholar]
- 29.Odegaard AO, Pereira MA, Koh WP, Arakawa K, Lee HP, Yu MC. Coffee, tea, and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr. 2008;88(4):979–985. doi: 10.1093/ajcn/88.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashida H, Furuyashiki T, Nagayasu H, Bessho H, Sakakibara H, Hashimoto T, Kanazawa K. Anti-obesity actions of green tea: possible involvements in modulation of the glucose uptake system and suppression of the adipogenesis-related transcription factors. Biofactors. 2004;22(1–4):135–140. doi: 10.1002/biof.5520220126. [DOI] [PubMed] [Google Scholar]
- 31.Ueda M, Nishiumi S, Nagayasu H, Fukuda I, Yoshida K, Ashida H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem Biophys Res Commun. 2008;377(1):286–290. doi: 10.1016/j.bbrc.2008.09.128. [DOI] [PubMed] [Google Scholar]
- 32.Jung KH, Choi HS, Kim DH, Han MY, Chang UJ, Yim SV, Song BC, Kim CH, Kang SA. Epigallocatechin gallate stimulates glucose uptake through the phosphatidylinositol 3-kinase-mediated pathway in L6 rat skeletal muscle cells. J Med Food. 2008;11(3):429–434. doi: 10.1089/jmf.2007.0107. [DOI] [PubMed] [Google Scholar]
- 33.Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004;52(3):643–648. doi: 10.1021/jf030365d. [DOI] [PubMed] [Google Scholar]
- 34.Wu LY, Juan CC, Hwang LS, Hsu YP, Ho PH, Ho LT. Green tea supplementation ameliorates insulin resistance and increases glucose transporter IV content in a fructose-fed rat model. Eur J Nutr. 2004;43(2):116–124. doi: 10.1007/s00394-004-0450-x. [DOI] [PubMed] [Google Scholar]
- 35.Yan J, Zhao Y, Suo S, Liu Y, Zhao B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic Biol Med. 2012;52(9):1648–1657. doi: 10.1016/j.freeradbiomed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Anderson RA, Polansky MM. Tea enhances insulin activity. J Agric Food Chem. 2002;50(24):7182–7186. doi: 10.1021/jf020514c. [DOI] [PubMed] [Google Scholar]
- 37.Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem. 2007;282(41):30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002;277(38):34933–34940. doi: 10.1074/jbc.M204672200. [DOI] [PubMed] [Google Scholar]
- 39.Nishiumi S, Bessyo H, Kubo M, Aoki Y, Tanaka A, Yoshida K, Ashida H. Green and black tea suppress hyperglycemia and insulin resistance by retaining the expression of glucose transporter 4 in muscle of high-fat diet-fed C57BL/6J mice. J Agric Food Chem. 2010;58(24):12916–12923. doi: 10.1021/jf102840w. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZF, Li Q, Liang J, Dai XQ, Ding Y, Wang JB, Li Y. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine. 2010;17(1):14–18. doi: 10.1016/j.phymed.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Hintzpeter J, Stapelfeld C, Loerz C, Martin HJ, Maser E. Green tea and one of its constituents, Epigallocatechine-3-gallate, are potent inhibitors of human 11beta-hydroxysteroid dehydrogenase type 1. PLoS One. 2014;9(1):e84468. doi: 10.1371/journal.pone.0084468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) J Biol Chem. 2001;276(32):30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 43.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem. 2002;277(3):1794–1799. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 44.Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol. 2002;16(8):1931–1942. doi: 10.1210/me.2002-0074. [DOI] [PubMed] [Google Scholar]
- 45.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11(4):380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 46.Eringa EC, Stehouwer CD, van Nieuw Amerongen GP, Ouwehand L, Westerhof N, Sipkema P. Vasoconstrictor effects of insulin in skeletal muscle arterioles are mediated by ERK1/2 activation in endothelium. Am J Physiol Heart Circ Physiol. 2004;287(5):H2043–2048. doi: 10.1152/ajpheart.00067.2004. [DOI] [PubMed] [Google Scholar]
- 47.Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51(12):3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 48.Chen H, Lin AS, Li Y, Reiter CE, Ver MR, Quon MJ. Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3-kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion. J Biol Chem. 2008;283(43):29228–29238. doi: 10.1074/jbc.M802906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiter CE, Kim JA, Quon MJ. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology. 2010;151(1):103–114. doi: 10.1210/en.2009-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104(4):447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289(2):H813–822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 52.Aggio A, Grassi D, Onori E, D’Alessandro A, Masedu F, Valenti M, Ferri C. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur J Nutr. 2013;52(1):263–272. doi: 10.1007/s00394-012-0320-x. [DOI] [PubMed] [Google Scholar]
- 53.Lorenz M, Wessler S, Follmann E, Michaelis W, Dusterhoft T, Baumann G, Stangl K, Stangl V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279(7):6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 54.Romano MR, Lograno MD. Epigallocatechin-3-gallate relaxes the isolated bovine ophthalmic artery: involvement of phosphoinositide 3-kinase-Akt-nitric oxide/cGMP signalling pathway. Eur J Pharmacol. 2009;608(1–3):48–53. doi: 10.1016/j.ejphar.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 55.Auger C, Kim JH, Chabert P, Chaabi M, Anselm E, Lanciaux X, Lobstein A, Schini-Kerth VB. The EGCg-induced redox-sensitive activation of endothelial nitric oxide synthase and relaxation are critically dependent on hydroxyl moieties. Biochem Biophys Res Commun. 2010;393(1):162–167. doi: 10.1016/j.bbrc.2010.01.112. [DOI] [PubMed] [Google Scholar]
- 56.Galleano M, Bernatova I, Puzserova A, Balis P, Sestakova N, Pechanova O, Fraga CG. (−)-Epicatechin reduces blood pressure and improves vasorelaxation in spontaneously hypertensive rats by NO-mediated mechanism. IUBMB Life. 2013;65(8):710–715. doi: 10.1002/iub.1185. [DOI] [PubMed] [Google Scholar]
- 57.Ihm SH, Jang SW, Kim OR, Chang K, Oak MH, Lee JO, Lim DY, Kim JH. Decaffeinated green tea extract improves hypertension and insulin resistance in a rat model of metabolic syndrome. Atherosclerosis. 2012;224(2):377–383. doi: 10.1016/j.atherosclerosis.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Ihm SH, Lee JO, Kim SJ, Seung KB, Schini-Kerth VB, Chang K, Oak MH. Catechin prevents endothelial dysfunction in the prediabetic stage of OLETF rats by reducing vascular NADPH oxidase activity and expression. Atherosclerosis. 2009;206(1):47–53. doi: 10.1016/j.atherosclerosis.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 59.Jang HJ, Ridgeway SD, Kim JA. Effects of the green tea polyphenol epigallocatechin-3-gallate on high-fat diet-induced insulin resistance and endothelial dysfunction. Am J Physiol Endocrinol Metab. 2013;305(12):E1444–1451. doi: 10.1152/ajpendo.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267(2 Pt 1):E187–202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 61.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94(3):1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285(1):E123–129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 63.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53(6):1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 64.Youd JM, Rattigan S, Clark MG. Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-alpha. Diabetes. 2000;49(11):1904–1909. doi: 10.2337/diabetes.49.11.1904. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes. 2004;53(2):447–453. doi: 10.2337/diabetes.53.2.447. [DOI] [PubMed] [Google Scholar]
- 66.Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 2002;51(1):42–48. doi: 10.2337/diabetes.51.1.42. [DOI] [PubMed] [Google Scholar]
- 67.Rattigan S, Clark MG, Barrett EJ. Acute vasoconstriction-induced insulin resistance in rat muscle in vivo. Diabetes. 1999;48(3):564–569. doi: 10.2337/diabetes.48.3.564. [DOI] [PubMed] [Google Scholar]
- 68.Premilovac D, Bradley EA, Ng HL, Richards SM, Rattigan S, Keske MA. Muscle insulin resistance resulting from impaired microvascular insulin sensitivity in Sprague Dawley rats. Cardiovasc Res. 2013;98(1):28–36. doi: 10.1093/cvr/cvt015. [DOI] [PubMed] [Google Scholar]
- 69.Bradley EA, Richards SM, Keske MA, Rattigan S. Local NOS inhibition impairs vascular and metabolic actions of insulin in rat hindleg muscle in vivo. Am J Physiol Endocrinol Metab. 2013;305(6):E745–750. doi: 10.1152/ajpendo.00289.2013. [DOI] [PubMed] [Google Scholar]
- 70.Clerk LH, Vincent MA, Barrett E, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293:E1804–E1809. doi: 10.1152/ajpendo.00498.2007. [DOI] [PubMed] [Google Scholar]
- 71.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab. 2002;282(3):E714–720. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- 72.Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997;46(9):1381–1388. doi: 10.2337/diab.46.9.1381. [DOI] [PubMed] [Google Scholar]
- 73.St-Pierre P, Genders AJ, Keske MA, Richards SM, Rattigan S. Loss of insulin-mediated microvascular perfusion in skeletal muscle is associated with the development of insulin resistance. Diabetes Obes Metab. 2010;12(9):798–805. doi: 10.1111/j.1463-1326.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- 74.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55(5):1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 75.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care. 2009;32(9):1672–1677. doi: 10.2337/dc09-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290(6):E1191–1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- 77.Ross RM, Kolka CM, Rattigan S, Clark MG. Acute blockade by endothelin-1 of haemodynamic insulin action in rats. Diabetologia. 2007;50(2):443–451. doi: 10.1007/s00125-006-0525-8. [DOI] [PubMed] [Google Scholar]
- 78.Rattigan S, Dora KA, Colquhoun EQ, Clark MG. Serotonin-mediated acute insulin resistance in the perfused rat hindlimb but not in incubated muscle: a role for the vascular system. Life Sci. 1993;53(20):1545–1555. doi: 10.1016/0024-3205(93)90563-i. [DOI] [PubMed] [Google Scholar]
- 79.Mizugaki M, Ishizawa F, Yamazaki T, Hishinuma T. Epigallocatechin gallate increase the prostacyclin production of bovine aortic endothelial cells. Prostaglandins Other Lipid Mediat. 2000;62(2):157–164. doi: 10.1016/s0090-6980(00)00060-5. [DOI] [PubMed] [Google Scholar]
- 80.Li RW, Douglas TD, Maiyoh GK, Adeli K, Theriault AG. Green tea leaf extract improves lipid and glucose homeostasis in a fructose-fed insulin-resistant hamster model. J Ethnopharmacol. 2006;104(1–2):24–31. doi: 10.1016/j.jep.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 81.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138(9):1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Axling U, Olsson C, Xu J, Fernandez C, Larsson S, Strom K, Ahrne S, Holm C, Molin G, Berger K. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 2012;9(1):105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004;4:18. doi: 10.1186/1471-2210-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr. 2006;136(10):2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 85.Ortsater H, Grankvist N, Wolfram S, Kuehn N, Sjoholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr Metab (Lond) 2012;9:11. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 87.Basu A, Du M, Sanchez K, Leyva MJ, Betts NM, Blevins S, Wu M, Aston CE, Lyons TJ. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition. 2011;27(2):206–213. doi: 10.1016/j.nut.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, Bluck L, Coward A, Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr. 2009;101(6):886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown AL, Lane J, Holyoak C, Nicol B, Mayes AE, Dadd T. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr. 2011;106(12):1880–1889. doi: 10.1017/S0007114511002376. [DOI] [PubMed] [Google Scholar]
- 90.Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome–a randomized placebo-controlled trial. J Soc Gynecol Investig. 2006;13(1):63–68. doi: 10.1016/j.jsgi.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 91.Diepvens K, Kovacs EM, Vogels N, Westerterp-Plantenga MS. Metabolic effects of green tea and of phases of weight loss. Physiol Behav. 2006;87(1):185–191. doi: 10.1016/j.physbeh.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 92.Frank J, George TW, Lodge JK, Rodriguez-Mateos AM, Spencer JP, Minihane AM, Rimbach G. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. 2009;139(1):58–62. doi: 10.3945/jn.108.096412. [DOI] [PubMed] [Google Scholar]
- 93.Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, Howe PR. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr. 2007;26(4):396S–402S. doi: 10.1080/07315724.2007.10719628. [DOI] [PubMed] [Google Scholar]
- 94.Hursel R, Westerterp-Plantenga MS. Green tea catechin plus caffeine supplementation to a high-protein diet has no additional effect on body weight maintenance after weight loss. Am J Clin Nutr. 2009;89(3):822–830. doi: 10.3945/ajcn.2008.27043. [DOI] [PubMed] [Google Scholar]
- 95.Fukino Y, Shimbo M, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J Nutr Sci Vitaminol (Tokyo) 2005;51(5):335–342. doi: 10.3177/jnsv.51.335. [DOI] [PubMed] [Google Scholar]
- 96.Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. 2008;27(3):363–370. doi: 10.1016/j.clnu.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 97.Hsu CH, Liao YL, Lin SC, Tsai TH, Huang CJ, Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev. 2011;16(2):157–163. [PubMed] [Google Scholar]
- 98.Josic J, Olsson AT, Wickeberg J, Lindstedt S, Hlebowicz J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: a randomized controlled trial. Nutr J. 2010;9:63. doi: 10.1186/1475-2891-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kovacs EM, Lejeune MP, Nijs I, Westerterp-Plantenga MS. Effects of green tea on weight maintenance after body-weight loss. Br J Nutr. 2004;91(3):431–437. doi: 10.1079/BJN20041061. [DOI] [PubMed] [Google Scholar]
- 100.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15(6):1473–1483. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 101.Nagao T, Meguro S, Hase T, Otsuka K, Komikado M, Tokimitsu I, Yamamoto T, Yamamoto K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring) 2009;17(2):310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 102.Stendell-Hollis NR, Thomson CA, Thompson PA, Bea JW, Cussler EC, Hakim IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. J Hum Nutr Diet. 2010;23(6):590–600. doi: 10.1111/j.1365-277X.2010.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. 2005;13(7):1195–1204. doi: 10.1038/oby.2005.142. [DOI] [PubMed] [Google Scholar]
- 104.Ryu OH, Lee J, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Effects of green tea consumption on inflammation, insulin resistance and pulse wave velocity in type 2 diabetes patients. Diabetes Res Clin Pract. 2006;71(3):356–358. doi: 10.1016/j.diabres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Venables MC, Hulston CJ, Cox HR, Jeukendrup AE. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr. 2008;87(3):778–784. doi: 10.1093/ajcn/87.3.778. [DOI] [PubMed] [Google Scholar]
- 106.Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. 2012;149(3):315–322. doi: 10.1007/s12011-012-9448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32(6):421–427. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Shimada K, Kawarabayashi T, Tanaka A, Fukuda D, Nakamura Y, Yoshiyama M, Takeuchi K, Sawaki T, Hosoda K, Yoshikawa J. Oolong tea increases plasma adiponectin levels and low-density lipoprotein particle size in patients with coronary artery disease. Diabetes Res Clin Pract. 2004;65(3):227–234. doi: 10.1016/j.diabres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 109.Hosoda K, Wang MF, Liao ML, Chuang CK, Iha M, Clevidence B, Yamamoto S. Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care. 2003;26(6):1714–1718. doi: 10.2337/diacare.26.6.1714. [DOI] [PubMed] [Google Scholar]
- 110.Muniyappa R, Karne RJ, Hall G, Crandon SK, Bronstein JA, Ver MR, Hortin GL, Quon MJ. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes. 2006;55(11):3142–3150. doi: 10.2337/db06-0714. [DOI] [PubMed] [Google Scholar]
- 111.Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr. 2008;88(6):1685–1696. doi: 10.3945/ajcn.2008.26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen H, Karne RJ, Hall G, Campia U, Panza JA, Cannon RO, 3rd, Wang Y, Katz A, Levine M, Quon MJ. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol. 2006;290(1):H137–145. doi: 10.1152/ajpheart.00768.2005. [DOI] [PubMed] [Google Scholar]
- 113.Shao L, Liu K, Huang F, Guo X, Wang M, Liu B. Opposite effects of quercetin, luteolin, and epigallocatechin gallate on insulin sensitivity under normal and inflammatory conditions in mice. Inflammation. 2013;36(1):1–14. doi: 10.1007/s10753-012-9514-x. [DOI] [PubMed] [Google Scholar]


