Abstract
Background
Constrictive pericarditis (CP) results in reduced pericardial compliance, ventricular interdependence, and right heart failure. Patients with untreated CP may develop liver fibrosis and ultimately cirrhosis due to chronic venous congestion. Chronic venous congestion ± fibrosis may lead to increased liver stiffness. Magnetic Resonance Elastography (MRE) was used to evaluate whether patients with CP have increased hepatic stiffness.
Methods
Prospectively, patients with suspected CP underwent 2D transthoracic echocardiography, cardiac MRI, and liver MRE. An automated method was used to draw regions of interest (ROI) on the stiffness maps to calculate the mean liver stiffness in kilopascals (kPa). A t-test with α = 0.05 was performed between stiffness values of patients with positive and negative CP findings based on previously published echocardiography criteria.
Results
Nineteen patients met inclusion criteria with a mean ± SD age of 51 ± 16years. Nine patients (47%) had CP. Mean liver stiffness trended higher in patients with CP compared to those without CP (4.04 kPa vs. 2.46; p=0.045). Liver stiffness correlated with MRI septal bounce (p=0.04), inferior vena cava size (p=0.003), echo abnormal septal motion (p=0.04), and echo mitral inflow variation >25% (p=0.02). Only MRI septal bounce predicted CP by echocardiography (p<0.001).
Conclusion
CP was associated with increased liver stiffness. The increased stiffness is most likely secondary to chronic hepatic venous congestion and/or fibrosis. MRE may be useful for noninvasive liver stiffness assessment in CP.
Introduction
Constrictive pericarditis (CP) results in reduced pericardial compliance, ventricular interdependence, and right heart failure with variable liver congestion (1,2). With loss of pericardial elasticity, right atrial pressure/central venous pressure increases often resulting in elevated jugular venous pressure and signs/symptoms of right heart failure with ascites, hepatomegaly, lower extremity edema, and dyspnea (1). The association of liver abnormalities in the setting of cardiac dysfunction is well recognized (2-8). However, liver function test abnormalities are often variable in patients with cardiac disease (3,8) but are adverse prognostic indicators when elevated (9,10) and appear to correlate with degree of left ventricular dysfunction(6) and disease chronicity (3). Myers et al described liver histologic changes in patients with acute, chronic, and acute on chronic cardiovascular disease (heart failure, pericardial disease, acute myocardial infarction, rheumatic heart disease, and cor pulmonale) including 24 patients with pericardial disease (3). The histological changes include centrilobular and sinusoidal dilatation, centrilobular necrosis, inflammation, with fibrotic architecture in up to 77% of patients with chronic and acute on chronic disease. Of 59 patients with advanced heart failure awaiting cardiac transplant or left ventricular assist device, 80% had hepatic fibrosis including 37% (n=22) with stage 3-4 fibrosis (4). Patients with CP may develop elevated serum liver enzyme levels, hepatic fibrosis, and subsequent liver failure due to chronic venous congestion if left untreated (11-15).
Historically, definitive diagnosis of liver disease required a liver biopsy and its attendant potential complications. Magnetic resonance elastography (MRE) is a noninvasive method to quantitatively measure liver stiffness utilizing a phase-contrast technique based on the speed of externally-induced acoustic waves propagating in tissues (16). Prior studies have investigated the relationship between liver stiffness and heart failure with ultrasound based elastography techniques (2,9,17-19), hence we evaluated liver stiffness as measured by MRE in patients with CP. We hypothesized that chronic venous congestion in the setting of CP with or without hepatic fibrosis may lead to increased liver stiffness as measured by MRE.
Methods
This HIPAA compliant study was approved by the Mayo Clinic Institutional Review Board (#13-003532). All patients provided informed consent for the study and for the use of demographic and clinical data for research purposes. We prospectively evaluated 20 consecutive adult patients (≥ 18 years) referred for CP who underwent cardiac magnetic resonance imaging and transthoracic echocardiography at the Mayo Clinic Rochester, between 10/1/2013-12/31/2014. One patient with hemochromatosis was excluded because of failure of reliable liver stiffness evaluation with MRE.
Cardiac Magnetic Resonance Imaging and Magnetic Resonance Elastography
All patients underwent cardiac magnetic resonance imaging MRI as part of an initial diagnostic evaluation at the Mayo Clinic Pericardial Disease Clinic. The cardiac MRI protocol for pericardial disease was performed on a 1.5T clinical scanner (General Electric, Waukesha, WI) and included: steady state free precession fiesta imaging for assessing early diastolic bounce, short axis free breathing sequence for assessment of respirophasic changes, double inversion recovery for pericardial thickness, and delayed enhancement imaging for pericardial and/or myocardial enhancement. All cardiac MRI studies were comprehensively reviewed by a level III CMRI-trained cardiologist (ERF) with experience in diagnosing CP.
Liver MRE was performed on all patients to evaluate for liver stiffness using a standard gradient recalled echo-based MRE protocol. A scout image was obtained at end expiration. The MRE passive driver was placed over the right lower chest and upper abdomen in mid clavicular line overlying the widest cross-section of liver at the level of the xiphisternum. Liver sequences included gradient recalled echo MRE -4 slices, and axial post contrast T1-weighted liver acquisition with volume acquisition (LAVA) sequence as previously described (20). All MRE data was processed using an automated method to draw artifact-free ROIs and calculate hepatic stiffnesses with stiffness reported as mean kiloPascals (kPa) ± standard deviation (20,21). A Normal reference range for healthy non congested liver is 2.09-2.21 ± 0.22-0.26 kPa (22,23) with levels above 2.5kPa interpreted as elevated as per institutional guidelines.
Transthoracic Echocardiography
All patients underwent transthoracic two-dimensional and tissue Doppler echocardiography as part of an initial diagnostic evaluation for possible constrictive pericarditis using a standard protocol including a nasal respirometer-based evaluation. Imaging windows included parasternal long and short axis, apical, and subcostal positions. Right atrial pressure (RAP) estimation was based on inferior vena cava size and distensibility as previously described (24). Right ventricular systolic pressure was estimated based on the modified Bernoulli equation (4v2 + estimated RAP, where v = tricuspid regurgitant velocity) per American Society of Echocardiography guidelines (25). All echocardiography studies were reviewed by a COCATS level III-trained cardiologist (ERF). Standard diagnostic echocardiographic criteria for constrictive pericarditis were utilized as described previously (26). Patients met criteria for CP if they had respirophasic ventricular septal shift in combination with either mitral valve medial annulus e’ ≥ 0.09 m/sec or hepatic vein expiratory diastolic flow reversal ratio ≥ 0.79. Respirophasic ventricular septal shift was defined as a shift in the ventricular septum towards the right ventricle during the expiratory phase and a shift towards the left ventricle during the inspiratory phase. Hepatic vein expiratory diastolic flow reversal was defined as: the ratio of diastolic reversal velocity divided by forward velocity during the expiratory phase.
Statistical Analysis
All statistical analyses were performed using JMP, version 10 (SAS, Cary, NC). Continuous variables are presented as mean ± standard deviation. Categorical variables are presented as frequencies and percentages. For all analyses, a 2-sided p<0.05 was considered to be significant. We tested the associations of all the variables with the echocardiography-based diagnosis of constrictive pericarditis. For the continuous variables, Wilcoxon Rank-Sum test was performed. For categorical variables, the Fisher's Exact test was performed due to the small sample size. Univariate logistic regression modeling was also conducted to predict echocardiography-diagnosed CP using MRI variables of interest. Odds ratios and 95% confidence intervals are reported. The likelihood ratio p-value is also reported for these analyses. Univariate linear regression modeling was conducted to predict liver stiffness. Multivariate logistical regression was performed in a stepwise fashion to adjust for auto liver stiffness.
Results
Patient Characteristics
Over the study period, 19 patients with a mean age of 51 ± 16 years underwent cardiac MRI, liver MRE, and transthoracic 2D Doppler echocardiography for evaluation of suspected constrictive pericarditis. Patient characteristics are displayed in Table 1. Nine patients (47%) met diagnostic echocardiographic criteria for constrictive pericarditis. Table 2 illustrates clinical and laboratory data in patients with and without constriction. Patients with constriction had similar liver enzyme profiles and inflammatory markers compared to patients without constriction. Of the nine patients who met criteria for CP, eight (80%) had evidence of acute or chronic recurrent pericarditis with pericardial enhancement on cardiac MRI indicating either pericardial inflammation or fibrosis. Table 3 displays echocardiographic and cardiac MRI characteristics of patients with an without CP. Liver stiffness was significantly higher in patients with CP (4.04 ± 1.83 kPa vs. 2.46 ± 0.56 kPa; p=0.045). Figure 1 illustrates liver MRE images in a patient with CP and comparatively in a patient without constrictive pericarditis. Figure 2 illustrates elevated liver stiffness in a patient with CP but morphologically normal liver comparing it with another patient without CP and normal liver and normal liver stiffness. Table 4 displays the unadjusted univariate associations with liver stiffness. Increased liver stiffness was related to MRI pericardial thickening (p=0.046), MRI septal bounce (p<0.04), MRI IVC size per mm (p=0.003), echo hepatic vein diastolic expiratory flow reversal ratio ≥ 0.79 (p=0.045), and echo respirophasic mitral inflow velocity variation > 25% (p=0.02). Multivariate analysis demonstrated no significant variables after adjusting for IVC size.
Table 1.
Characteristics of patients with suspected constrictive pericarditis.
| N=19 | |
|---|---|
| Clinical: Sex, % male | 10 (53) |
| Age, years (mean ± SD) | 51 ± 16 |
| Body mass index, kg/m2 (mean ± SD) | 31.0 ± 8.2 |
| Laboratory Data, mean ± SD | |
| Apartate aminotransferase, U/L, n=15 | 26 ± 8 |
| Alanine aminotransferase, U/L, n=13 | 33 ± 15 |
| Total bilirubin, mg/dl, n=14 | 0.6 ± 0.3 |
| Direct bilirubin, mg/dl, n=13 | 0.2 ± 0.2 |
| Alkaline phosphatase, U/L, n=14 | 90 ± 36 |
| Creatinine, mg/dl | 0.9 ± 0.1 |
| C-reactive protein, mg/L, n=14 (median (25;75 IQ) | 4.3 (1.3;21.0) |
| ESR, mm/1h, n=18, (median (25;75 IQ) | 11 (5;15) |
| Magnetic Resonance Elastography | |
| Automatic Liver Stiffness (kPa) | 3.21 ± 1.52 |
IQ, interquartile; ESR, erythrocyte sedimentation rate;
Table 2.
Clinical characteristics of patients with and without constrictive pericarditis.
| Constriction (N=9) | No Constriction (N=10) | P value | |
|---|---|---|---|
| Clinical: Sex, % male | 6 (67) | 4 (40) | 0.37 |
| Age, years (mean ± SD) | 50 ± 13 | 53 ± 19 | 0.54 |
| Body mass index, kg/m2 (mean ± SD) | 31.8 ± 6.5 | 30.3 ± 9.9 | 0.65 |
| Laboratory Data, mean ± SD | |||
| Apartate aminotransferase, U/L, | 28 ± 6 | 24 ± 10 | 0.17 |
| Alanine aminotransferase, U/L, | 34 ± 8 | 32 ± 21 | 0.35 |
| Total bilirubin, mg/dl, | 0.7 ± 0.4 | 0.5 ± 0.1 | 0.21 |
| Direct bilirubin, mg/dl, | 0.3 ± 0.3 | 0.1 ± 0.0 | 0.08 |
| Alkaline phosphatase, U/L, | 97 ± 37 | 83 ± 37 | 0.37 |
| Creatinine, mg/dl | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.97 |
| CRP, mg/L, (median (25;75 IQ) | 4.7 (1.0;14.7)* | 3.8 (1.5;74.5)^ | 0.44 |
| ESR, mm/1h (median (25;75 IQ) | 13 (3;15) | 9 (5;24.5)^^ | 0.42 |
| Pericardiectomy (%) | 2 (22) | 1 (10) | 0.58 |
Values are expressed as mean ± standard deviation unless otherwise stated; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate;
N=7;
N=7;
N=9;
Table 3.
Magnetic resonance elastography, 2D and tissue Doppler echocardiography, and cardiac magnetic resonance imaging data in patients with and without constrictive pericarditis.
| Constriction (N=9) | No Constriction (N=10) | P value | |
|---|---|---|---|
| Magnetic Resonance Elastography | |||
| Automatic Liver Stiffness (kPa) | 4.04 ± 1.83 | 2.46 ± 0.56 | 0.045 |
| Echocardiography | |||
| Abnormal Septal Motion (%) | 9 (100) | 2 (20) | 0.0007 |
| HV Diastolic Expiratory Reversal Ratio ≥ 0.79 (%) | 4# (67) | 0 (0) | 0.29 |
| HV Diastolic Expiratory Reversal Ratio (mean±SD) | 1.2 ± 0.74 | 0.51 ± 0.21 | 0.01 |
| Medial Mitral Annulus e’ velocity > 9 m/sec (%) | 6 (67) | 4 (40) | 0.37 |
| Medial Mitral Annulus e’ velocity (m/sec) | 0.12 ± 0.04 | 0.08 ± 0.03 | 0.04 |
| Lateral Mitral Annulus e’ Velocity (m/sec) | 0.12 ± 0.03 | 0.11 ± 0.03 | 0.41 |
| Medial e’ > Lateral e’ (%) | 4 (44) | 0 (0) | 0.03 |
| Mitral Inflow Velocity Variation > 25% (%) | 6 (67) | 2 (20) | 0.07 |
| Inferior Vena Cava Plethora > 21 mm (%) | 6 (67) | 0 (0) | 0.003 |
| Estimated Right Atrial Pressure (mmHg) | 12 ± 6 | 5 ± 0 | 0.001 |
| Pericardial Effusion (%) | 3 (33) | 2 (20) | 0.63 |
| LV Ejection Fraction (%;mean±SD) | 58 ± 8 | 64 ± 5 | 0.09 |
| LV Cardiac Index (l/min/m2) | 3.14 ± 0.64 | 3.00 ± 0.83 | 0.54 |
| LV Stroke Volume (ml) | 82 ± 18 | 81 ± 22 | 0.93 |
| LV Stroke Volume Index (ml/m2) | 39 ± 6 | 43 ± 23 | 0.90 |
| Cardiac MRI | |||
| Pericardial Thickening (%) | 7 (78) | 6 (60) | 0.63 |
| Pericardial Thickness (mm) | 5 ± 2 | 5 ± 3 | 0.53 |
| Pericardial Effusion (%) | 5 (56) | 6 (60) | 1.0 |
| Pericardial Delayed Enhancement (%) | 8 (89) | 8 (80) | 1.0 |
| Early Diastolic Septal Bounce (%) | 9 (100) | 4 (40) | 0.01 |
| Interventricular Dependence (%) | 8 (89) | 5 (50) | 0.14 |
| Inferior Vena Cava Size (mm) | 26 ± 3 | 22 ± 3 | 0.03 |
| Inferior Vena Cava Plethora | 8 (89) | 6 (60) | 0.30 |
| LVEDV (ml) | 130 ± 55 | 105 ± 30** | 0.54 |
| LVEDV Index (ml/m2) | 61 ± 18 | 54 ± 14** | 0.62 |
| LV Stroke Volume (ml) | 77 ± 22 | 68 ± 15** | 0.54 |
| LV Stroke Volume Index (ml/m2) | 37 ± 7 | 35 ± 9** | 0.82 |
| LV Ejection Fraction (%) | 63 ± 12 | 66 ± 9** | 0.93 |
| LV End Diastolic Mass (g/m2) | 68 ± 24 | 57 ± 16** | 0.45 |
| RVEDV (ml) | 141 ± 55 | 120 ± 27** | 0.89 |
| RVEDV Index (ml/m2) | 69 ± 18 | 63 ± 14** | 0.74 |
| RV Stroke Volume (ml) | 72 ± 17 | 66 ± 14** | 0.85 |
| RV Stroke Volume Index (ml/m2) | 36 ± 6* | 35 ± 8** | 0.88 |
| RV Ejection Fraction | 55 ± 8 | 55 ± 3** | 0.63 |
Values are expressed as mean ± standard deviation unless otherwise stated; HV, Hepatic Vein; LV, Left Ventricular; MRI, magnetic resonance imaging; LVEDV, Left Ventricular End Diastolic Volume; RVEDV, Right Ventricular End Diastolic Volume; RV, Right Ventricular;
N=6;
N=8;
N=9;
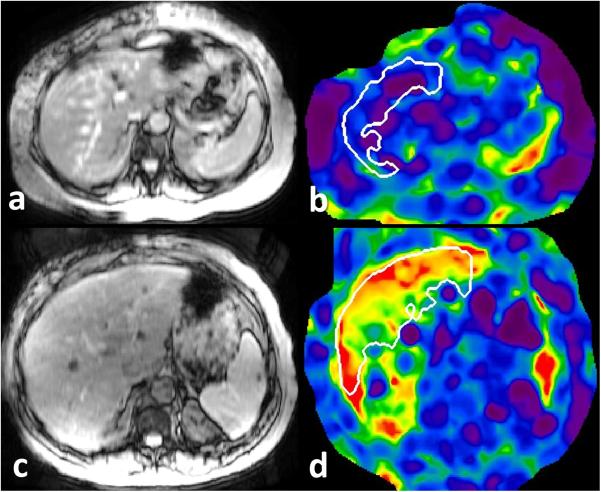
Figure 1.
MRE of liver in patients with suspected constrictive pericarditis. Top row: magnitude MRE image (a) and corresponding stiffness map (b) of liver in a 59 year old male with echo negative for CP and estimated right atrial pressure of 5mm Hg has a normal liver stiffness of 1.98kPa. Bottom row: magnitude (c) and stiffness map (d) in a 61 year old male with echo diagnostic of CP and estimated right atrial pressure of 14mmHg has elevated liver stiffness of 5.4kPa (normal cut-off value is 2.5kPa). The white outlined region represents region of interest drawn using automated algorithm avoiding liver edges and large vessels.
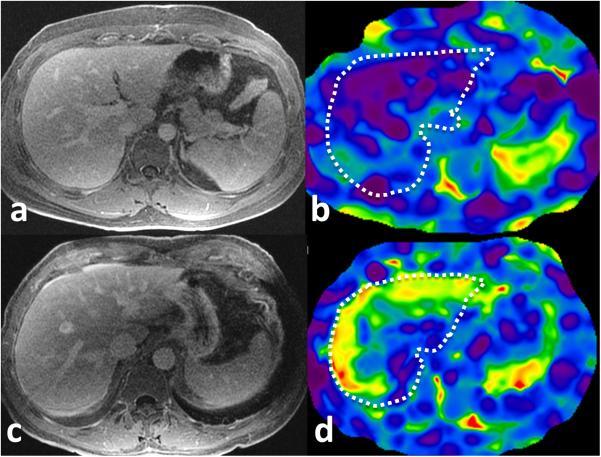
Figure 2.
MRE of liver in patients with suspected constrictive pericarditis. Top row: post gadolinium enhanced delayed T1-weighted axial image (a) and corresponding level stiffness map (b) of liver in a 26 year male with echo negative for CP and estimated right atrial pressure of 5mm Hg. The liver has normal morphology and a normal mean liver stiffness of 1.99kPa. Bottom row: post gadolinium enhanced delayed T1-weighted axial image (c) and stiffness map (d) in a 65 year old male with echo diagnostic of CP and estimated right atrial pressure of 20mmHg has elevated liver stiffness of 4.4kPa. Note the normal morphology of liver but prominent infewrior vena cava. The white dotted outline represents liver on the stiffness maps.
Table 4.
Unadjusted univariate association with liver stiffness.
| Variable | Estimate (SE) | P Value |
|---|---|---|
| MRI Pericardial Thickening | +1.47 (0.69) | 0.046 |
| MRI Pericardial Thickness (per 1 mm) | +0.27 (0.13) | 0.05 |
| MRI Delayed Pericardial Enhancement | −0.14 (0.49) | 0.77 |
| MRI Pericardial Effusion | +0.16 (0.36) | 0.66 |
| MRI Septal Bounce | +0.75 (0.34) | 0.04 |
| MRI Interventricular Dependence | +0.68 (0.35) | 0.07 |
| MRI IVC Size (per 1 mm) | +0.29 (0.08) | 0.003 |
| MRI IVC Plethora (> 21mm) | +0.28 (0.40) | 0.5 |
| Echo HV Diastolic Expiratory Reversal Ratio ≥ 0.79 | +1.39 (0.63) | 0.045 |
| Echo HV Diastolic Expiratory Reversal Ratio | +0.84 (0.50) | 0.12 |
| Echo Abnormal Septal Motion | +0.70 (0.32) | 0.04 |
| Echo Medial Mitral Annulus e’ velocity (m/sec) | +3.96 (9.13) | 0.67 |
| Echo Medial Mitral Annulus e’ velocity > 9 m/sec | +0.18 (0.71) | 0.80 |
| Echo Medial e’ > Lateral e’ | +1.12 (0.84) | 0.2 |
| Echo Mitral Inflow Velocity Variation > 25% | +1.57 (0.62) | 0.02 |
| Echo Estimated Right Atrial Pressure (mmHg) | +0.11 (0.06) | 0.11 |
| Echo Inferior Vena Cava Plethora | +0.61 (0.36) | 0.11 |
SE, Standard Error; MRI, Magnetic Resonance Imaging; IVC, Inferior Vena Cava; Echo, Echocardiography; HV, Hepatic Vein;
Cardiac MRI, and Transthoracic 2D Echocardiography
Comparative variables for liver MRE, transthoracic echocardiography, and cardiac MRI are presented in Table 3 for patients with and without constriction. On echocardiography (Table 3), patients with CP were more likely to have medial mitral annulus e’ greater than lateral e’ (p = 0.03), have a higher hepatic vein diastolic expiratory flow reversal ratio (p=0.01), have inferior vena cave plethora (p=0.003), have higher right atrial pressure (p=0.001). Echocardiographic variables that did not reach significance between groups included medial mitral annulus e’ velocity greater than 0.09 m/sec, mitral valve lateral annulus systolic velocity, and respirophasic mitral inflow variation > 25%, and pericardial effusion presence. Left ventricular volumes and function were not significantly different between groups.
On cardiac MRI, patients with CP had early diastolic septal bounce (p=0.01) and larger inferior vena cava diameter (p=0.03) compared to patients without CP. However, there was no difference between groups for interventricular dependence, pericardial delayed enhancement, or increased pericardial thickness cardiac MRI. With univariate analysis of cardiac MRI variables (Table 5), only septal bounce (p=0.04) only septal bounce was a predictor of CP as diagnosed by echocardiographic criteria. Pericardial thickness, delayed pericardial enhancement, and inferior vena cava plethora did not predict patients with constriction. With multivariate analysis, MRI variables were not significantly different when adjusting for liver stiffness. Cardiac volumes and function on cardiac MRI were not statistically different between groups. Of 10 patients without CP, eight were diagnosed with recurrent pericarditis. Two patients with CP underwent pericardiectomy after failure of medical treatment. One patient with recurrent relapsing pericarditis without CP underwent pericardiectomy for ongoing pain. All patients in both groups were alive as of 5/1/2015.
Table 5.
Unadjusted univariate predictors of constrictive pericarditis.
| Variable | Odds Ratio | P Value |
|---|---|---|
| Auto Liver Stiffness (per 1 kPa) | 3.14 (1.24;15.64) | 0.06 |
| MRI Pericardial Thickening | 2.33 (0.33;21.55) | 0.40 |
| MRI Pericardial Thickness (per 1 mm) | 1.94 (0.04;144.44) | 0.73 |
| MRI Delayed Pericardial Enhancement | 2.00 (0.16;48.3) | 0.59 |
| MRI Pericardial Effusion | 0.83 (0.13;5.30) | 0.84 |
| MRI Septal Bounce | * | < 0.001 |
| MRI Interventricular Dependence | 8.00 (0.93;179.04) | 0.06 |
| MRI IVC Size (per 1 mm) | 1.56 (1.08;2.75) | 0.05 |
| MRI IVC Plethora (> 21mm) | 5.33 (0.59;119.71) | 0.14 |
| MRI LV End Diastolic Volume Index | 1.03 (0.97;1.11) | 0.38 |
| LV Ejection Fraction | 0.97 (0.87;1.07) | 0.58 |
MRI, Magnetic Resonance Imaging; IVC, Inferior Vena Cava; LV, Left Ventricular;
Odds ratio not reported due to low sample size in each group;
Discussion
Patients with constrictive pericarditis appear to have increased liver stiffness as assessed by MRE compared to a cohort of patients without CP. The liver stiffness in patients with CP is nearly two-times higher compared to previously reported normal healthy individuals (4.04 ± 1.83 kPa in CP patients vs. 2.21 ± 0.12 kPa in healthy controls) (22,23,27-31) and is comparable to patients with chronic liver disease (4.28 ± 0.33 kPa).(28,32-38) In the current study, increased liver stiffness was associated with septal bounce on MRI, larger inferior vena cava, Doppler echo evidence of hepatic vein diastolic expiratory flow reversal ratio ≥ 0.79, and respirophasic variation of the mitral inflow velocity > 25% which are findings that may be seen in constriction among other cardiovascular disease and reflect the hemodynamic changes that occur with constriction. (24,26) Increased liver stiffness is a surrogate of either congestive hepatopathy or hepatic fibrosis from chronic congestion which is challenging to distinguish between without tissue for histologic analysis. Liver stiffness was associated with increased pericardial thickening, septal bounce on MRI, abnormal septal motion on echocardiography, hepatic vein diastolic expiratory reversal ratio ≥ 0.79, and respirophasic mitral inflow velocity variation which are considered surrogate indicators of constriction. Although liver stiffness as a standalone variable was not predictive of constrictive pericarditis likely because of the underpowered sample size, the trend of increased stiffness is promising for this method to be useful in detecting patients with CP.
On MRI, pericardial enhancement and pericardial effusion was present in an equal proportion of patients with and without CP. There were also no between group differences in pericardial thickness, interventricular dependence on free-breathing images, or cardiac volumes and function. When compared to patients who did not have CP, patients with constriction had significantly larger IVC diameter and early diastolic bounce on cardiac MRI. These cardiac MRI findings are similar to Young et al who also demonstrated IVC plethora and abnormal septal motion in a similar percentage of patients with CP confirmed after pericardiectomy (39) and Zurick et al who described a septal bounce and IVC plethora in all 25 patients with CP.(40) However, in the current study, neither septal bounce on MRI nor IVC plethora in multivariate analysis demonstrated predictive accuracy of CP in the present study likely because of the underpowered small sample size.
The inferior vena cava appears to be a visual barometer reflecting venous pressure changes that occur as a chronic response to constriction but also along with the hepatic veins can provide clues to hemodynamic changes that occur in the setting of constriction. By echocardiography, patients with constriction had inferior vena cava plethora as well as elevated right atrial pressure compared to patients without constriction. Elevated right atrial pressure is not unexpected especially considering that it is estimated based on IVC size and distensibility. Hepatic vein diastolic expiratory flow reversals on Doppler echocardiographic imaging are pathognomonic for either constrictive pericarditis (including effusive-constrictive pericarditis) or cardiac tamponade. The hepatic vein reversal ratio ≥ 0.79 provides a cutoff at which to consider constriction but should be taken into consideration with ventricular septal motion and the mitral e’ velocity. If the Doppler findings remain present following pericardiocentesis, then constriction should be considered likely. Both cardiac MRI and transthoracic echocardiography are complementary imaging tests that provide synergistic information to the clinician when evaluating pericardial disease. Cardiac MRI can provide meaningful information on ongoing pericardial inflammation or fibrosis as well as evaluate for other etiologies such as cardiomyopathies and ischemic heart disease. The addition of liver MRE can also provide insight into liver changes that can happen as a result of exposure to chronic elevated venous pressure as can occur with constriction and in the future may aid in the diagnosis of CP.
Our study has several limitations. First, no histologic correlation was performed as liver biopsies were not performed in these patients. Clinically, the majority of patients had normal liver function tests and therefore these subjects did not undergo any invasive liver biopsies. The increased stiffness may be due to congestion or fibrosis or both. Future studies with histologic correlation would be useful to address this. The present study was underpowered to detect MRI variables predictive of constrictive pericarditis as diagnosed by echocardiography. However, even with low numbers in each cohort, we were able to demonstrate a difference in liver stiffness between groups, demonstrated that patients with constriction have increased IVC diameter, and have abnormal septal motion on MRI. Some investigators have proposed that liver elastography may overestimate liver stiffness due to congestive heart failure (18) and only demonstrates modest change in response to short-term diuresis.(2) Despite potential limitations, the finding that liver stiffness is increased in CP suggests liver MRE may hold promise as an adjunctive imaging modality when incorporated with the cardiac MRI and transthoracic echocardiography. Elevated liver stiffness with MRE may be useful as additional evidence for CP with MRI and/or echocardiography findings of CP. MRE would be particularly useful in patients who have elevated liver stiffness but normal liver function tests and no morphological changes in liver, thereby raising suspicion for significant effect of CP on liver. These patients may be monitored for development of liver fibrosis.
The current study demonstrates increased liver stiffness in patients with CP and confirms previously described MRI findings of constriction. Future research will focus on changes in liver stiffness that occurs following pericardiectomy in patients with constriction. Liver MRE is a safe noninvasive reproducible imaging modality that warrants further investigation in the setting of constrictive pericarditis.
Acknowledgements
None.
Funding Sources: NIH EB01981
Footnotes
Disclosures: None
References
- 1.Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100(13):1380–1386. doi: 10.1161/01.cir.100.13.1380. [DOI] [PubMed] [Google Scholar]
- 2.Hopper I, Kemp W, Porapakkham P, et al. Impact of heart failure and changes to volume status on liver stiffness: non-invasive assessment using transient elastography. Eur J Heart Fail. 2012;14(6):621–627. doi: 10.1093/eurjhf/hfs044. [DOI] [PubMed] [Google Scholar]
- 3.Myers RP, Cerini R, Sayegh R, et al. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology. 2003;37(2):393–400. doi: 10.1053/jhep.2003.50062. [DOI] [PubMed] [Google Scholar]
- 4.Gelow JM, Desai AS, Hochberg CP, Glickman JN, Givertz MM, Fang JC. Clinical predictors of hepatic fibrosis in chronic advanced heart failure. Circ Heart Fail. 2010;3(1):59–64. doi: 10.1161/CIRCHEARTFAILURE.109.872556. [DOI] [PubMed] [Google Scholar]
- 5.Richman SM, Delman AJ, Grob D. Alterations in indices of liver function in congestive heart failure with particular reference to serum enzymes. Am J Med. 1961;30:211–225. doi: 10.1016/0002-9343(61)90093-6. [DOI] [PubMed] [Google Scholar]
- 6.Kubo SH, Walter BA, John DH, Clark M, Cody RJ. Liver function abnormalities in chronic heart failure. Influence of systemic hemodynamics. Arch Intern Med. 1987;147(7):1227–1230. [PubMed] [Google Scholar]
- 7.Sherlock S. The liver in heart failure; relation of anatomical, functional, and circulatory changes. Br Heart J. 1951;13(3):273–293. doi: 10.1136/hrt.13.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. 2010;16(1):84–90. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. 2009;11(2):170–177. doi: 10.1093/eurjhf/hfn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batin P, Wickens M, McEntegart D, Fullwood L, Cowley AJ. The importance of abnormalities of liver function tests in predicting mortality in chronic heart failure. Eur Heart J. 1995;16(11):1613–1618. doi: 10.1093/oxfordjournals.eurheartj.a060785. [DOI] [PubMed] [Google Scholar]
- 11.Idriss FS, Nikaido H, Muster AJ. Constrictive pericarditis simulating liver disease in children. Arch Surg. 1974;109(2):223–226. doi: 10.1001/archsurg.1974.01360020085016. [DOI] [PubMed] [Google Scholar]
- 12.Tang TT, Davis S, Keelen MH, Jr., Lepley D, Jr., Babbitt DP. Clinical-pathological conference. Hepatomegaly and recurrent ascites in an 11-year-old boy. J Pediatr. 1977;91(6):1015–1020. doi: 10.1016/s0022-3476(77)80919-0. [DOI] [PubMed] [Google Scholar]
- 13.Solano FX, Jr., Young E, Talamo TS, Dekker A. Constrictive pericarditis mimicking Budd-Chiari syndrome. Am J Med. 1986;80(1):113–115. doi: 10.1016/0002-9343(86)90058-6. [DOI] [PubMed] [Google Scholar]
- 14.Kirsch M, Fleshler B. Deceptive liver histology delays diagnosis of cardiac ascites. South Med J. 1992;85(11):1151–1152. doi: 10.1097/00007611-199211000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Sheth AA, Lim JK. Liver disease from asymptomatic constrictive pericarditis. J Clin Gastroenterol. 2008;42(8):956–958. doi: 10.1097/MCG.0b013e318031915c. [DOI] [PubMed] [Google Scholar]
- 16.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37(3):544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colli A, Pozzoni P, Berzuini A, et al. Decompensated chronic heart failure: increased liver stiffness measured by means of transient elastography. Radiology. 2010;257(3):872–878. doi: 10.1148/radiol.10100013. [DOI] [PubMed] [Google Scholar]
- 18.Millonig G, Friedrich S, Adolf S, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52(2):206–210. doi: 10.1016/j.jhep.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Dogan Y, Soylu A, Kilickesmez O, et al. The value of hepatic diffusion-weighted MR imaging in demonstrating hepatic congestion secondary to pulmonary hypertension. Cardiovasc Ultrasound. 2010;8:28. doi: 10.1186/1476-7120-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dzyubak B, Glaser K, Yin M, et al. Automated liver stiffness measurements with magnetic resonance elastography. J Magn Reson Imaging. 2013;38(2):371–379. doi: 10.1002/jmri.23980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzyubak B, Venkatesh SK, Manduca A, Glaser KJ, Ehman RL. Automated liver elasticity calculation for MR elastography. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.25072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesh SK, Wang G, Teo LL, Ang BW. Magnetic resonance elastography of liver in healthy Asians: normal liver stiffness quantification and reproducibility assessment. J Magn Reson Imaging. 2014;39(1):1–8. doi: 10.1002/jmri.24084. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Guo Q, Xia F, Sun J, Gao Y. Short- and midterm repeatability of magnetic resonance elastography in healthy volunteers at 3.0 T. Magn Reson Imaging. 2014;32(6):665–670. doi: 10.1016/j.mri.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Fenstad ER, Le RJ, Sinak LJ, et al. Pericardial effusions in pulmonary arterial hypertension: characteristics, prognosis, and role of drainage. Chest. 2013;144(5):1530–1538. doi: 10.1378/chest.12-3033. [DOI] [PubMed] [Google Scholar]
- 25.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-688. [DOI] [PubMed] [Google Scholar]
- 26.Welch TD, Ling LH, Espinosa RE, et al. Echocardiographic diagnosis of constrictive pericarditis: Mayo Clinic criteria. Circ Cardiovasc Imaging. 2014;7(3):526–534. doi: 10.1161/CIRCIMAGING.113.001613. [DOI] [PubMed] [Google Scholar]
- 27.Lee DH, Lee JM, Han JK, Choi BI. MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging. 2013;38(5):1215–1223. doi: 10.1002/jmri.23958. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Lee JM, Lee JE, et al. MR elastography for noninvasive assessment of hepatic fibrosis: reproducibility of the examination and reproducibility and repeatability of the liver stiffness value measurement. J Magn Reson Imaging. 2014;39(2):326–331. doi: 10.1002/jmri.24147. [DOI] [PubMed] [Google Scholar]
- 29.Shire NJ, Yin M, Chen J, et al. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. J Magn Reson Imaging. 2011;34(4):947–955. doi: 10.1002/jmri.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hines CD, Bley TA, Lindstrom MJ, Reeder SB. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging. 2010;31(3):725–731. doi: 10.1002/jmri.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mannelli L, Godfrey E, Graves MJ, et al. Magnetic resonance elastography: feasibility of liver stiffness measurements in healthy volunteers at 3T. Clin Radiol. 2012;67(3):258–262. doi: 10.1016/j.crad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesh SK, Yin M, Takahashi N, Glockner JF, Talwalkar JA, Ehman RL. Non-invasive detection of liver fibrosis: MR imaging features vs. MR elastography. Abdom Imaging. 2015;40(4):766–775. doi: 10.1007/s00261-015-0347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batheja M, Vargas H, Silva AM, et al. Magnetic resonance elastography (MRE) in assessing hepatic fibrosis: performance in a cohort of patients with histological data. Abdom Imaging. 2015;40(4):760–765. doi: 10.1007/s00261-014-0321-8. [DOI] [PubMed] [Google Scholar]
- 34.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135(1):32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 35.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5(10):1207–1213. e1202. doi: 10.1016/j.cgh.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60(6):1920–1928. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatesh SK, Xu S, Tai D, Yu H, Wee A. Correlation of MR elastography with morphometric quantification of liver fibrosis (Fibro-C-Index) in chronic hepatitis B. Magn Reson Med. 2014;72(4):1123–1129. doi: 10.1002/mrm.25002. [DOI] [PubMed] [Google Scholar]
- 38.Venkatesh SK, Wang G, Lim SG, Wee A. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol. 2014;24(1):70–78. doi: 10.1007/s00330-013-2978-8. [DOI] [PubMed] [Google Scholar]
- 39.Young PM, Glockner JF, Williamson EE, et al. MR imaging findings in 76 consecutive surgically proven cases of pericardial disease with CT and pathologic correlation. Int J Cardiovasc Imaging. 2012;28(5):1099–1109. doi: 10.1007/s10554-011-9916-0. [DOI] [PubMed] [Google Scholar]
- 40.Zurick AO, Bolen MA, Kwon DH, et al. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy: a case series with histopathological correlation. JACC Cardiovasc Imaging. 2011;4(11):1180–1191. doi: 10.1016/j.jcmg.2011.08.011. [DOI] [PubMed] [Google Scholar]