Abstract
Neurocysticercosis (NCC), an infection of the brain by Taenia solium (Ts) cysts, is the most common cause of adult-onset epilepsy in developing countries. Serological testing consists primarily of varying methods to detect antibodies in body fluids and more recently antigen (Ag) detection assays to identify individuals or animals with viable parasites. Antigen assays currently in use employ monoclonal antibodies (mAbs) raised against T. saginata, which have known cross reactivity to animal cestodes but are highly specific in human samples. We produced, characterized and tested 21 mAbs raised against T. solium whole cyst antigens, vesicular fluid or excretory secretory products. Reactivity of the TsmAbs against specific cyst structures was determined using immunofluorescence and immunohistochemistry on histological sections of Ts muscle cysts. Four TsmAbs reacted to vesicular space alone, 9 to the neck and cyst wall, one to the neck and vesicular space and 7 to the neck, cyst wall and vesicular space. An in-house ELISA assay to detect circulating Ts antigen, using the TsmAbs as capture antibodies and a rabbit polyclonal anti-Ts whole cyst antibody as a detector antibody demonstrated that eight of the 21 TsmAbs detected antigens in known NCC-positive human sera and three of these also in urine samples. Reactivity was expressed as normalized ratios of optical densities (OD positive control/OD negative control). Three TsmAbs had ratios >10 and five between 2–10. The TsmAbs have potential utility for the diagnosis and post-treatment monitoring of patients with viable NCC infections.
Keywords: Taenia solium, Monoclonal antibodies, capture ELISA, Neurocysticercosis, diagnosis
Graphical Abstract
1. Introduction
Neurocysticercosis (NCC), caused by Taenia solium cysts localized in the central nervous system, is the most common cause of adult-onset epilepsy in developing countries and therefore a serious public health problem in regions of the world where pigs are raised and sanitation is lacking. NCC is also frequently recognized in developed countries due to migration of infected individuals and to a lesser extent, in travelers infected in endemic regions (1–3).
NCC is a pleomorphic disease; symptoms and signs are very variable ranging from asymptomatic infections to life threatening massive involvement. Clinical manifestations are strongly influenced by the number, viability, location and growth potential of the parasite as well as the presence and degree of host inflammation directed to the parasite (1). Diagnosis requires a combination of information including exposure, neuroimaging and serology (4, 5). None of the individual tests with the exception of direct identification of the parasite, an unusual occurrence, yields an absolute diagnosis in all instances. Magnetic resonance imaging (MRI) and computerized tomography (CT) imaging of the brain are most helpful and may lead to a certain diagnosis in some situations but their high cost makes them inaccessible to most of those with the disease, who are generally of low socioeconomic status (5). Infection is determined mainly by detection of antibodies directed against different parasite antigens using ELISA (6) or the electro immune transfer blot (EITB). The latter employs lentil lectin-purified parasite glycoproteins (LLGP-EITB) (7) as antigen and presently this antibody detection assay is considered the serological reference standard test for diagnosis of NCC (8). However, the main limitation of antibody detection is the inability to distinguish active infections from previous exposure to the parasite.
The detection of circulating parasite antigen (Ag) has the advantage of confirming the presence of live parasites and represents a useful tool to monitor patients during treatment (9). The current ELISA based assay employs monoclonal antibodies (mAbs) raised against secretory products of T. saginata, which cross react with undetermined circulating antigens present in T. solium and other animal cestodes (10–13). These assays have moderate sensitivity (14, 15), show very little cross-reactivity with other human parasites, and have proved to be clinically useful. Species-specific mAbs against T. solium cysts are not commercially available.
In the current study we generated mAbs against different antigenic components of the T. solium cyst. These were then characterized and subsequently employed in an antigen capture assay to detect T. solium circulating antigens. To our knowledge this is the first report of the generation of species-specific mAbs to detect circulating T. solium Ags.
2. Materials and Methods
2.1. Antigens
Three kinds of antigens were prepared at the same time using cysts excised from one cysticercosis-infected pig, bought from a known endemic region in the Peruvian highlands, Huancayo. Cyst viability was evaluated by evagination induced with porcine bile: each time, 50 cysts were placed in a solution of bile-RPMI medium (1: 1) at 37° C; the number of evaginated cysts was recorded after 18 h. In all cases, 100% viability was obtained.
2.1.1. Whole parasite antigen (WA)
Cysts were washed three times in phosphate-buffered saline (PBS) containing 1mM of the protease inhibitor phenylmethylsulfonyl fluoride (PMSF; Sigma, St. Louis, MO), placed on sterile Whatman filter paper (Sigma) to remove excess of washing solution and then homogenized in a sterile glass homogenizer in 3 volumes of PBS. The homogenate was then sonicated (Misonix Sonicator 3000) at 60-s intervals for 3 minutes at 33 watts, on ice, and the supernatant was collected following centrifugation at 15000 rpm/45 min/4°C.
2.1.2
Vesicular fluid antigen (VF) was obtained by puncture of individual cysts using a 1-ml syringe. The fluid was centrifuged at 2500 rpm/10 min/4°C and the supernatant was collected.
2.1.3. Excretory/secretory antigen (ES)
About six hundred cysts were washed three times in culture medium, cRPMI: RPMI 1640 medium (Gibco) supplemented with 10 mM HEPES (Gibco), 2mM glutamine and an antibiotic solution containing 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B (all from Gibco-Invitrogen, Gaithersburg, MD). Washed cysts were placed in culture flasks (200 cyst/flask) and incubated in cRPMI for 48 h at 37°C and 5% CO2. Culture supernatants were collected following centrifugation at 2500 rpm/10 min/4°C.
In all cases, SigmaFAST protease inhibitor cocktail (Sigma, St. Louis, MO) containing 2 mM AEBSF, 1 μM phosphoramidon, 130 μM bestatin, 14 μM E-64, 1 μM leupeptin, 0.2 μM aprotinin, 10 μM pepstatin was added to antigen fractions and aliquots were stored at −70°C until use. Protease inhibitor was not added to antigens destined for animal immunization, which were filtered through 0.22-μm filters (Millipore) before storage at −70°C. Protein concentration was determined using a protein assay kit (Bio-Rad, Hercules, CA).
2.2. Rabbit immunization
Two New Zealand rabbits, kept in cages in a dedicated area as approved by the Ethics Committee at Universidad Peruana Cayetano Heredia (UPCH), were immunized with 1 ml of 0.5 mg/ml of WA extract using 500 μg of WA in 500 μl of saline emulsified in equal volume of Sigma adjuvant system (Sigma, St. Louis, CA), which was injected on day 0 as follows: 300 μl intradermally (50 μl in each of 6 sites), 300 μl intramuscularly (150 μl into each hind leg), 200 μl subcutaneously (100 μl in each of 2 sites on the back region) and 200 μl intraperitoneally. Boosts were given on days 24 and 43 with 0.25 mg/ml as above. Blood was collected by intracardiac puncture on day 50, after anesthesia with intravenous ketamine (50 mg/kg). Pre-immune sera were obtained from blood samples immediately before immunization.
2.3. Mouse immunization
Six week old female BALB/c mice, housed in the infected animal area as indicated in the reports for the Ethics Committee at UPCH, were immunized with 0.2 ml of WA, VF or ES parasite antigens at 0.5 mg/ml. Briefly, 100 μg in 100 μl of saline were emulsified in an equal volume of Sigma adjuvant system (Sigma, St. Louis, CA) and injected subcutaneously on day 0 (100 μl in each of 2 sites on the back region). The first booster with 20 μg/100 μl of saline emulsified in equal volume of adjuvant was injected intraperitoneally on day 21 and a second booster with 20 μg/0.2 ml dose without adjuvant was injected intravenously on day 35.
2.4. Hybridoma generation
Cell fusion was performed 5 days after the last boost. Briefly, mice were sacrificed by intraperitoneal inoculation of ketamine (Ket-A-100, AgroVet Market S. A.) at 0.10 mg/10 gr weight, their spleens were removed aseptically and carefully disaggregated using a 5-ml syringe; the cell suspension obtained was washed 3 times using PBS. The myeloma cell line SP2/0, gently donated by Dr. Siddhartha Mahanty from NIH, was used for the fusion process; these cells were expanded, pooled and washed three times on the day of the fusion. Cells were mixed at a ratio of 1 spleen cell per 2 myeloma cells and carefully fused using 1 ml polytehylene glycol, PEG 1500 (Sigma, St. Louis, CA) for 1 minute. Fused cells were resuspended in Advanced DMEM medium (Gibco), supplemented with 2mM glutamine and same antibiotic solution as above, incubated for 10 min at 37°C and then centrifuged at 800 rpm/10 min/RT (room temperature) to eliminate the PEG. The supernatant was discarded and the fusion cells were resuspended in 50 ml of hybridoma medium, hDMEM (Advanced DMEM medium with 2mM glutamine, antibiotic solution and 10% of Hyclone fetal bovine serum), placed in 75-ml culture flasks and incubated for 24 h at 37°C in 5% CO2. After that, HAT supplement (0.10 mM sodium hypoxanthine, 0.40 mM aminopterin and 0.016 mM thymidine, Life Technologies) was added to the suspension of fused cells to eliminate unfused or self-fused HGPRT positive–myeloma cells, then plated into 96-well culture plates and incubated at 37°C in 5% CO2. After 72 h, the medium was replaced with hDMEM with HT supplement (0.10 mM sodium hypoxanthine and 0.016 mM thymidine, Life Technologies). After 1–2 weeks, the supernatants of wells containing hybridomas were screened for mAb reactivity against the antigen mixtures used to immunize the mice.
2.5. Cloning of hybridoma cells
Hybridomas in positive wells were expanded in 24 well-plates and then cloned successively by limiting dilution using cloning dilutions at 50 cells/ml, followed by 5 cells/ml and finally 1 cell/ml, respectively, in hDMEM. 100 μl/well was put into 96-well plates and incubated at 37°C in 5% CO2. After 1 week the wells containing hybridomas were screened for reactive antibody by a direct ELISA test and the cells in positive wells selected for a next round of cloning. Class and subclass of antibody were determined from supernatants of clones obtained after the last round of cloning.
2.6. Direct ELISA for screening of hybridomas
Screening of hybridoma supernatants was done with a direct ELISA using the same antigen used in the immunization. Briefly, 100 μl/well of antigens (1 μg/ml WA, 0.8 μg/ml VF or 2 μg/ml ES) were used to coat 96 well flat-bottomed microtiter plates (Immulon 1 B, Thermo Scientific) using carbonate-bicarbonate buffer, pH 9.6 (0.05 M NaHCO3/Na2CO3, Sigma, St. Louis, CA) and left overnight at 4°C. Plates were blocked with 100 μl/well of PBS pH 7. 4, 0.1% Tween-20 and 5% non-fat milk for 1 h at RT and then washed 5 times using PBS pH 7.4, 0.01% Tween-20. Then, 60 μl/well of each supernatant (in duplicate) was added, incubated for 1 h at RT and then washed 5 times. One hundred μl/well of horseradish peroxidase-conjugated goat anti-mouse IgG/IgM (KPL, Washington, MD) at 1:5000 in PBS pH 7.4, 0.01% Tween-20 was then added, incubated for 1 h at RT and washed 5 times. Finally, 100 μl/well of of tetramethylbenzidine substrate solution (SureBlue TMB peroxidase substrate, KPL) was added and incubated for 30 min. The reactivity was detected at 650 nm using a VersaMax ELISA microplate reader (Molecular Devices). Pre-immune mouse serum and mouse serum after full immunization were used as negative and positive controls, respectively.
2.7. Direct ELISA to evaluate specificity of hybridomas
Specificity of each mAb in the hybridoma supernatants was evaluated using a direct ELISA against the following antigens from related parasites: Taenia hydatigena vesicular fluid, Echinococcus granulosus vesicular fluid, Fasciola hepatica excretion/secretion products, and T. saginata whole antigen. Briefly, 100 μl/well of each antigen at 2 μg/ml in a carbonate-bicarbonate buffer pH 9.6 were used to coat 96 well flat-bottomed microtiter plates (Immulon 1 B, Thermo Scientific) and left overnight at 4°C. The process followed was the same used for the screening of hybridomas. Positive controls for the assay were sera from a T. hydatigena-infected pig, and human sera from patients with echinococcosis, fascioliasis, and taeniasis by T. saginata; negative controls were sera from healthy humans and pigs.
2.8. Isotype determination
Isotype was determined using a commercial kit (Hybridoma Subisotyping Kit; Calbiochem). Briefly, 100 μl/well of polyclonal goat anti-mouse antibodies was coated onto 96-well flat-bottomed microtiter plates (Maxisorb, Nunc) using the supplied coating buffer, and left overnight at 4°C. Plates were blocked with 200 μl/well of blocking solution for 1 h at RT and then washed 3 times using the supplied washing buffer. 50 μl/well of each supernatant was placed in 7 wells (1 well for each isotype evaluated and 1 blank), incubated for 1 h at RT and washed 3 times. Two drops/well of each rabbit anti-mouse IgG1, IgG2a, IgG2b, IgG3, IgM, IgA and 100 μl/well of PBS as blank were added, incubated for 1 h at RT and washed 3 times. 100 μl/well of horseradish peroxidase-conjugated goat anti-rabbit antibody was added, incubated for 1 h at RT and washed 3 times. Finally, 100 μl/well of supplied TMB substrate solution was added, incubated for 10 min and the reaction was stopped with 50 μl/well of 1M phosphoric acid solution. Reactivity was detected at 450nm using a VersaMax ELISA microplate reader (Molecular Devices).
2.9. Immunolocalization
Immunofluorescence assay (IFA) was performed on formalin-fixed, paraffin-embedded sections of T. solium muscle cysts obtained from naturally infected pigs. Four-μm tissue sections were deparaffinized, rehydrated, blocked with 100 μl of PBS pH 7. 4, 0.05% tween-20 and 5% non-fat milk for 1 hour at RT in a humid chamber and washed 3 times with PBS pH 7.4, 0.05% tween-20. Hybridoma culture supernatants at 100 μl/sample were added and left overnight at 4°C in a humid chamber. After 3 washes, 100 μl/sample of a 1:100 dilution of goat anti-mouse IgG/IgM conjugated to Dylight™488 (KPL) and counterstained with 0.01% Evans Blue (Sigma) was added for a 1-h incubation at RT in a humid chamber. After 3 washes, slides were mounted with buffered glycerin and observed with an epifluorescence microscope (Zeiss, Germany) under excitation wavelength of 450–490 nm and emission at 515–565 nm. Pre-immune mouse serum and serum after full immunization were used as negative and positive controls, respectively.
2.10. Capture ELISA for detection of T. solium antigen
An in-house ELISA assay to capture T. solium circulating antigens was developed using the mAbs as capture antibody and a rabbit polyclonal anti-T. solium WA antibody for detection. The mAbs used in this assay were purified from culture supernatants by ammonium sulfate precipitation (16). Pools of positive serum and urine samples were prepared using similar volumes (4ml for serum and 10ml for urine) from each of ten defined samples, mixed in constant agitation for 30 min at 4° C, then aliquoted and stored at −80° C until use. Positive samples were from patients with viable NCC demonstrated by neuroimaging and confirmed by a strong antibody response on EITB. A similar process was used to prepare negative control pools from healthy volunteers from non-endemic regions (also seronegative on EITB).
2.10.1. Capture ELISA for detection of T. solium antigen in serum
Briefly, 100 μl/well of each mAb at 5 μg/ml were used to coat 96 well flat-bottomed microtiter plates (Immulon 4 HBX, Thermo Scientific) using carbonate-bicarbonate buffer pH 9.6 (0.05 M NaHCO3/Na2CO3, Sigma, St. Louis, CA) and left overnight at 4°C. Plates were blocked with 100 μl/well of PBS pH 7. 4, 0.05% Tween-20 and 5% non-fat milk for 30 min at 37°C and then washed 5 times using PBS pH 7.4, 0.05% Tween-20. Then, 100 μl/well of positive or negative pools as described, diluted 1:8 in PBS pH 7.4 (by duplicate) was added, incubated for 30 min at 37°C and then washed 5 times. One hundred μl/well of polyclonal rabbit anti-T. solium WA antibody diluted at 5 μg/ml in PBS pH 7. 4, 0.05% Tween-20 and 1% non-fat milk was added and incubated 30 min at 37°C, then washed 5 times. One hundred μl/well of horseradish peroxidase-conjugated goat anti-rabbit IgG (KPL) at 1:5,000 in PBS pH 7. 4, 0.05% Tween-20 and 1% non-fat milk were added and incubated 30 min at 37°C, then washed 5 times. Finally, 100 μl/well of of TMB substrate solution (SureBlue TMB peroxidase substrate, KPL) was added and incubated for 30 min. The reactivity was detected at 650 nm using a VersaMax ELISA microplate reader (Molecular Devices), and each result was expressed as the OD ratio between the positive and the negative pool.
2.10.2. Capture ELISA for detection of T. solium antigen in urine
Assay conditions were set differently assuming that antigens in urine could be more diluted, so urine samples were incubated overnight. To compensate for the extended sample incubation time, the fixation time of capture mAbs was reduced to 1 h. Significant differences in performance of antigen binding when fixed at 37°C for 1 hour or overnight at 4°C were previously ruled out (data not shown). Briefly, 100 μl/well of each mAb at 5 μg/ml were used to coat 96 well flat-bottomed microtiter plates (Immulon 4 HBX, Thermo Scientific) using carbonate-bicarbonate buffer pH 9.6 (0.05 M NaHCO3/Na2CO3, Sigma, St. Louis, CA) and left for 1 h at 37°C. Then, unbound antibodies were discarded and 100 μl/well of blocking solution, PBS pH 7. 4, 0.05% Tween-20 and 5% non-fat milk, was added and incubated for 1 h at 37°C and then washed 5 times using PBS pH 7.4, 0.05% Tween-20. Samples used in the assay were pools of undiluted human urine from patients with proven NCC or without NCC, previously pre-incubated with polyclonal rabbit anti-T. solium WA antibody at 7.5 μg/ml for 1 h at 37°C. One hundred μl/well of pre-incubated sample was added (in duplicate) and incubated for 1 h at 37°C. The solution containing unbound material and further 100 μl pre-incubated sample was added to each well and left overnight at 4°C. After 5 washes, 100 μl/well of horseradish peroxidase-conjugated goat anti-rabbit IgG (KPL) at 1:5,000 in PBS pH 7. 4, 0.05% Tween-20 and 1% non-fat milk was added and incubated 1 h at 37°C, then washed again 5 times. Finally, 100 μl/well of of TMB substrate solution (SureBlue TMB peroxidase substrate, KPL) was added and incubated for 30 min. Reactivity was detected at 650 nm using a VersaMax ELISA microplate reader (Molecular Devices), and each result was expressed as the OD ratio between the positive and the negative pool.
2.11. Ethical considerations
The study protocol was revised and approved by the Institutional Committee of Animal Ethics at UPCH (SIDISI 000057672, renovated yearly), which states details for the proper housing, feeding and handling of animals used in research.
3. Results
3.1. Generation of hybridomas
After six rounds of fusion of SP2/0 cells with spleen lymphocytes from mice immunized with different T. solium antigens, 21 stable mAb-producing hybridomas (TsmAbs) were generated. Of these, 13 were directed against WA (TsW1-TsW13), 5 against VF (TsV1-TsV5) and 3 against ES products (TsE1-TsE3). The class/subclass of each TsmAb was determined by ELISA resulting in 8 IgM, 9 IgG1, 1 IgG2a and 3 IgG3-reacting hybridomas, of which 6 IgM, 4 IgG1 and 3 IgG3 were WA-reactive; 4 IgG1 and 1 IgG2a were VF-reactive; and 2 IgM and 1 IgG1 were ES-reactive hybridomas (Table 1).
Table 1.
TsmAbs with isotype, reactivity to other helminth antigens and immunolocalization.
Class and sub-class of monoclonal antibodies produced against whole antigen (TsW), vesicular fluid (TsV) and excretion/secretion products (TsE) of Taenia solium cyst antigens. Shown are ELISA results of reactivity against their homologous antigens, Ts hAg; cross reactivity with T. hydatigena vesicular fluid, Th VF; E. granulosus vesicular fluid, Eg VF; F. hepatica excretion/secretion products, Fh ES; and T. saginata whole antigen, Tsag WA. Immunolocalization by IFA on structural components of the cyst is also presented. Abbreviations: cyst wall (CW), neck (N) and vesicular space (VS).
| Clone | Isotype | ELISA reactivity (OD nm)
|
Immunolocalization pattern by IFA | ||||
|---|---|---|---|---|---|---|---|
| Ts hAg | Th VF | Eg V | Fh ES | Tsag WA | |||
|
| |||||||
| TsW1 | IgG1 | 1.050 | 0.150 | 0.248 | 0.039 | 0.512 | N, VS, CW |
| TsW2 | IgG1 | 1.030 | 0.157 | 0.274 | 0.039 | 0.045 | N, VS, CW |
| TsW3 | IgG1 | 1.060 | 0.083 | 0.228 | 0.068 | 0.031 | N, VS, CW |
| TsW4 | IgG3 | 2.575 | 0.050 | 0.043 | 0.040 | 0.273 | N, CW |
| TsW5 | IgM | 3.100 | 0.072 | 0.076 | 0.042 | 1.419 | N, CW |
| TsW6 | IgM | 1.030 | 0.036 | 0.048 | 0.042 | 1.840 | N, CW |
| TsW7 | IgG3 | 2.308 | 0.048 | 0.042 | 0.038 | 0.334 | N, CW |
| TsW8 | IgM | 3.400 | 0.081 | 0.082 | 0.053 | 1.115 | N, CW |
| TsW9 | IgM | 0.600 | 0.087 | 0.230 | 0.116 | 0.055 | VS |
| TsW10 | IgG1 | 0.505 | 0.046 | 0.067 | 0.040 | 1.293 | VS |
| TsW11 | IgM | 3.300 | 0.078 | 0.056 | 0.037 | 1.230 | N, CW |
| TsW12 | IgG3 | 2.775 | 0.048 | 0.070 | 0.042 | 0.272 | N, CW |
| TsW13 | IgM | 0.900 | 0.037 | 0.045 | 0.039 | 2.050 | N, VS |
| TsV1 | \IgG1 | 1.325 | 0.037 | 0.035 | 0.034 | 0.139 | VS |
| TsV2 | IgG2a | 2.325 | 0.455 | 0.769 | 0.037 | 0.516 | N, VS, CW |
| TsV3 | IgG1 | 3.025 | 0.037 | 0.035 | 0.036 | 0.033 | N, VS, CW |
| TsV4 | IgG1 | 1.450 | 0.055 | 0.056 | 0.032 | 0.036 | VS |
| TsV5 | IgG1 | 0.645 | 0.035 | 0.046 | 0.033 | 0.085 | N, CW |
| TsE1 | IgM | 0.675 | 0.040 | 0.161 | 0.047 | 0.077 | N, VS, CW |
| TsE2 | IgM | 0.690 | 0.037 | 0.186 | 0.037 | 0.101 | N, VS, CW |
| TsE3 | IgG1 | 1.402 | 0.040 | 0.145 | 0.039 | 0.053 | N, CW |
3.2. Reactivity and specificity of the TsmAbs
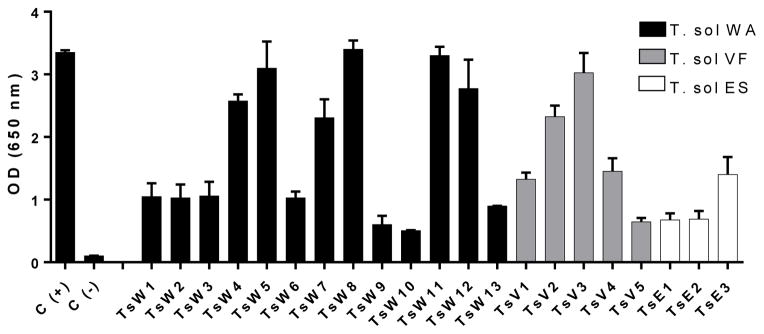
Supernatants of WA, VF and ES hybridomas with reactivity above 0.5 OD by direct ELISA against their homologous antigens were selected and cloned three successive times by limiting dilutions. Re-evaluation of the final supernatants of clones resulted in different levels of reactivity, with 15 showing high reactivity (OD > 1, including 8 with OD > 2; Table 1 and Figure 1). Through direct ELISA, none of the hybridomas were reactive against F. hepatica ES antigen, only one (TsV2) had very low reactivity against VF antigens of E. granulosus and T. hydatigena. Six (TsW5, TsW6, TsW8, TsW10, TsW11 and TsW13) were highly reactive against T. saginata whole antigen (OD>1, up to 2.050; Table 1 and Figure 2).
Figure 1. Reactivity of the monoclonal antibodies against homologous antigens.
Reactivity of hybridoma culture supernatants against whole cyst Ag (
 ), vesicular fluid Ag (
), vesicular fluid Ag (
 ), and excretion/secretion Ag (
), and excretion/secretion Ag (
 ), expressed as OD at 650 nm.
), expressed as OD at 650 nm.
Figure 2. Cross reactivity of the monoclonal antibodies against related parasite antigens.
Reactivity of hybridoma culture supernatants against Taenia hydatigena vesicular fluid Ag (
 ), Echinococcus granulosus vesicular fluid Ag (
), Echinococcus granulosus vesicular fluid Ag (
 ), Fasciola hepatica excretion/secretion Ag (
), Fasciola hepatica excretion/secretion Ag (
 ), and Taenia saginata whole Ag (
), and Taenia saginata whole Ag (
 ), expressed as OD at 650 nm.
), expressed as OD at 650 nm.
3.3. Immunolocalization
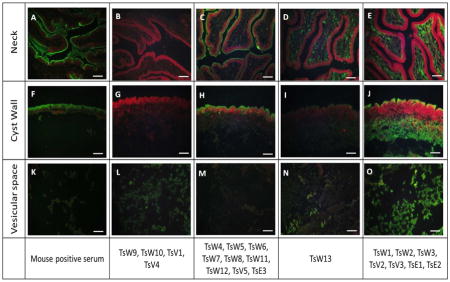
Immunofluorescence showed that the 21 selected TsmAbs reacted to one or more of the three main structural components of the cyst: neck (scolex), cyst wall and vesicular space. Pre-immune mouse serum did not react (not shown), while post immune serum reacted against all three components. Supernatants from selected hybridomas displayed four patterns of reactivity: Pattern 1: vesicular space alone (TsW9, TsW10, TsV1 and TsV4); Pattern 2: neck and cyst wall (TsW4, TsW5, TsW6, TsW7, TsW8, TsW11, TsW12, TsV5, TsE3); Pattern 3: neck and vesicular space (TsW13) and Pattern 4: neck, cyst wall and vesicular space (TsW1, TsW2 TsW3, TsV2, TsV3, TsE1 and TsE2) (Figure 3).
Figure 3. Immunolocalization of target Ags on histological sections of T. solium cysts by IFA.
Antigen recognition on T. solium cyst neck (A–E), cyst wall (F–J) and vesicular fluid (K–O). Positive control: post immunization mouse serum (A, F, K); negative control: pre-immune serum (not shown). Four main reactivity patterns observed on: vesicular space alone (B,G,L), neck and cyst wall (C,H,M), neck and vesicular space (D,I,N) and neck, cyst wall and vesicular space (E,J,O). Scale bar = 50 μm
3.4. Detection of circulating antigens
A capture ELISA assay using a TsmAb as a capture antibody and polyclonal rabbit antibody for detection allowed the analysis of T. solium circulating antigens in sera and urine pools from NCC patients. Eight TsmAbs (TsW4, TsW5, TsW6, TsW7, TsW8, TsW11, TsW12 and TsV3) were able to detect circulating Ag in human pooled sera with OD ratios above 2 (OD ratios=OD positive/OD negative control); three of these (TsW5, TsW8, and TsW11) had OD ratios above 10 (Figure 4). The same three TsmAbs were able to detect antigens in pooled human urine with OD ratios of ~10, TsW5 being the most reactive. The remaining TsmAbs did not detect circulating Ag in urine (Figure 5).
Figure 4. Antigen detection in human NCC sera.
T. solium Ags were detected in pools of NCC negative and NCC positive human sera, in duplicate, with a capture ELISA using purified TsmAbs as capture antibodies and polyclonal rabbit anti-T. solium WA antibody for detection. Results are expressed as a ratio: OD test sera/OD negative control at 650 nm.
Figure 5. Antigen detection in human urine from NCC patients.
T. solium Ags were detected in pools of negative and positive human urine, in duplicate, through capture ELISA as in Figure 3. Results are expressed as a ratio: OD positive control/OD negative control at 650 nm.
4. Discussion
Twenty-one newly generated mouse mAb to T. solium cysts (TsmAbs) were characterized and tested to determine their utility to detect T. solium antigens in serum and urine in an easily performed capture ELISA format. To our knowledge, the TsmAbs reported here are the first ones generated against T. solium cysts, which cause human neurocysticercosis. Possible advantages over previously reported and currently available antigen assays include: the development of assays specific to T. solium, which is essential to prevent detection of other cross-reacting antigens from pig cestodes; development of a more sensitive assay to allow detection of all viable T. solium parenchymal infections; discovery of unique antigens whose presence and levels correlate with clinically useful information such as a measure of acute cyst damage or parasite death; and development of easily obtained, low cost tests which would be particularly useful in poverty stricken regions where T. solium infections are common.
Monoclonal antibodies against T. crassiceps (17) and T. saginata (18) have been developed, as well as against T. solium eggs (19), to be used in the diagnosis of NCC. Our approach was to develop TsmAbs to various components and products of T. solium cysts, whole antigen (WA), excretion/secretion products (ES) and vesicular fluid (VF). Isotype determination showed 8/21 were IgM and 13/21 different subclasses of IgG; immunolocalization studies allowed grouping of antibodies into likely similar or identical reactivities as well as unique patterns whose reactivity may correlate with useful clinical information. In particular, TsmAbs associated with surface antigens, which would be those raised against WA, might be associated with species-specific determinants (15). Most of the highly reactive TsmAbs recognized surface structures of the neck and cyst wall. Thus far, these eight TsmAbs are T. solium specific. By direct ELISA they show no cross-reaction against E. granulosus VF, T. hydatigena VF and F. hepatica ES. Six of the produced mAbs reacted against whole T. saginata antigens; four of them from the eight highly reactive ones. A species-specific test should be generated using the clones that did not cross react.
Exploratory evaluation of a few individual CSF samples from patients with NCC has already been performed with encouraging results (data not shown). This initial characterization step will be followed by the development and optimization of an antigen detection assay using the TsmAb combinations performing better, which then will be tested for sensitivity and specificity using individual, well defined serum and CSF samples from different types of NCC. Individual sample assessment would demonstrate whether the detected antigens are consistently found in NCC cases.
Eight of 21 TsmAbs were able to detect cestode antigen present in pools of sera from infected patients using a capture antigen assay employing rabbit anti-T. solium WA antibody for detection after capture by TsmAbs. In addition, antigen can reliably be found in the urine of infected patients. In preliminary experiments various combinations of TsmAb could also be used to detect circulating antigens (20); early results suggest the sensitivity of these assays can be considerably enhanced.
5. Conclusion
In conclusion, our results show the successful generation of 21 mAbs against T. solium cysts, which showed no cross-reaction with related parasites, except for T. saginata in some cases; 8 TsmAbs are able to detect circulating antigens in human blood and urine samples. These TsmAb are very promising for future applications in the clinical management of NCC patients as well as to study the host-parasite relationship in this disease.
HIGHLIGHTS.
We generated twenty one hybridomas producing mAbs against T. solium cyst extracts.
A capture ELISA was designed using the TsmAbs and a rabbit polyclonal antibody.
Eight TsmAbs detected T. solium antigens in sera and 3 in urine of NCC patients.
The TsmAbs may be used for new diagnostic tests and to follow up treatment of NCC.
Acknowledgments
This work was supported partially by Innovate Peru, project FINCyT-IA-231-2013, by NIH/FIC Training grant TW001140, and by an intramural research program of the National Institutes of Allergy and Infectious Diseases.
Special thanks are due to Dr. Jaeson Calla-Choque for providing a whole antigen extract from T. saginata proglottids, and to Christian Pitot, MV, for his collaboration in procedures involving animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. The Lancet Neurology. 2014 Dec;13(12):1202–15. doi: 10.1016/S1474-4422(14)70094-8. Epub 2014/12/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Brutto OH, Garcia HH. Neurocysticercosis. Handbook of clinical neurology. 2013;114:313–25. doi: 10.1016/B978-0-444-53490-3.00025-X. Epub 2013/07/09. eng. [DOI] [PubMed] [Google Scholar]
- 3.Del Brutto OH. Neurocysticercosis: a review. The Scientific World Journal. 2012;2012:159821. doi: 10.1100/2012/159821. Epub 2012/02/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nature reviews Neurology. 2011 Oct;7(10):584–94. doi: 10.1038/nrneurol.2011.135. Epub 2011/09/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sotelo J. Clinical manifestations, diagnosis, and treatment of neurocysticercosis. Current neurology and neuroscience reports. 2011 Dec;11(6):529–35. doi: 10.1007/s11910-011-0226-7. Epub 2011/09/15. eng. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez S, Wilkins P, Dorny P. Immunological and molecular diagnosis of cysticercosis. Pathogens and global health. 2012 Sep;106(5):286–98. doi: 10.1179/2047773212Y.0000000048. Epub 2012/12/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) The Journal of infectious diseases. 1989 Jan;159(1):50–9. doi: 10.1093/infdis/159.1.50. Epub 1989/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 8.Del Brutto OH. Diagnostic criteria for neurocysticercosis, revisited. Pathogens and global health. 2012 Sep;106(5):299–304. doi: 10.1179/2047773212Y.0000000025. Epub 2012/12/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zumaquero-Rios JL, Garcia-Juarez J, de-la-Rosa-Arana JL, Marcet R, Sarracent-Perez J. Trichinella spiralis: monoclonal antibody against the muscular larvae for the detection of circulating and fecal antigens in experimentally infected rats. Experimental parasitology. 2012 Dec;132(4):444–9. doi: 10.1016/j.exppara.2012.09.016. Epub 2012/10/03. eng. [DOI] [PubMed] [Google Scholar]
- 10.Fleury A, Hernandez M, Avila M, Cardenas G, Bobes RJ, Huerta M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. Journal of neurology, neurosurgery, and psychiatry. 2007 Sep;78(9):970–4. doi: 10.1136/jnnp.2006.107243. Epub 2007/03/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleury A, Garcia E, Hernandez M, Carrillo R, Govezensky T, Fragoso G, et al. Neurocysticercosis: HP10 antigen detection is useful for the follow-up of the severe patients. PLoS neglected tropical diseases. 2013;7(3):e2096. doi: 10.1371/journal.pntd.0002096. Epub 2013/03/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorny P, Phiri IK, Vercruysse J, Gabriel S, Willingham AL, 3rd, Brandt J, et al. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. International journal for parasitology. 2004 Apr;34(5):569–76. doi: 10.1016/j.ijpara.2003.11.014. Epub 2004/04/06. eng. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez S, Dorny P, Tsang VC, Pretell EJ, Brandt J, Lescano AG, et al. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. The Journal of infectious diseases. 2009 May 1;199(9):1345–52. doi: 10.1086/597757. Epub 2009/04/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt JR, Geerts S, De Deken R, Kumar V, Ceulemans F, Brijs L, et al. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. International journal for parasitology. 1992 Jul;22(4):471–7. doi: 10.1016/0020-7519(92)90148-e. Epub 1992/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 15.Dorny P, Brandt J, Geerts S. Immunodiagnostic approaches for detecting Taenia solium. Trends in parasitology. 2004 Jun;20(6):259–60. doi: 10.1016/j.pt.2004.04.001. author reply 60-1. Epub 2004/05/19. eng. [DOI] [PubMed] [Google Scholar]
- 16.Mohanty JG, Elazhary Y. Purification of IgG from serum with caprylic acid and ammonium sulphate precipitation is not superior to ammonium sulphate precipitation alone. Comparative immunology, microbiology and infectious diseases. 1989;12(4):153–60. doi: 10.1016/0147-9571(89)90064-7. Epub 1989/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 17.Espindola NM, De Gaspari EN, Nakamura PM, Vaz AJ. Production of monoclonal antibodies anti-Taenia crassiceps cysticerci with cross-reactivity with Taenia solium antigens. Revista do Instituto de Medicina Tropical de Sao Paulo. 2000 May-Jun;42(3):175–7. doi: 10.1590/s0036-46652000000300013. Epub 2000/07/11. eng. [DOI] [PubMed] [Google Scholar]
- 18.Garcia HH, Harrison LJ, Parkhouse RM, Montenegro T, Martinez SM, Tsang VC, et al. A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. The Cysticercosis Working Group in Peru. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998 Jul-Aug;92(4):411–4. doi: 10.1016/s0035-9203(98)91069-0. Epub 1998/12/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montenegro TC, Miranda EA, Gilman R. Production of monoclonal antibodies for the identification of the eggs of Taenia solium. Annals of tropical medicine and parasitology. 1996 Apr;90(2):145–55. doi: 10.1080/00034983.1996.11813038. Epub 1996/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 20.Montenegro T, Gilman RH, Castillo R, Tsang V, Brandt J, Guevara A, et al. The diagnostic importance of species specific and cross-reactive components of Taenia solium, Echinococcus granulosus, and Hymenolepis nana. Revista do Instituto de Medicina Tropical de Sao Paulo. 1994 Jul-Aug;36(4):327–34. doi: 10.1590/s0036-46651994000400005. Epub 1994/07/01. eng. [DOI] [PubMed] [Google Scholar]