Abstract
The neurotransmitter serotonin (5HT) acting via 5HT1a receptors (5HT1aR) is a potent determinant of respiratory rhythm variability. Here, we address the 5HT1aR-dependent control of respiratory rhythm variability in C57BL6/J mice. Using the in situ perfused preparation, we compared the effects of systemic versus focal blockade of 5HT1aRs. Blocking 5HT1aRs in the Kölliker-Fuse nucleus (KFn) increased the occurrence of spontaneous apneas and accounted for the systemic effects of 5HT1aR antagonists. Further, 5HT1aRs of the KFn stabilized the respiratory rhythm’s response to arterial chemoreflex perturbations; reducing the recovering time, e.g., the latency to return to the baseline pattern. Together, these results suggest that the KFn regulates both intrinsic and sensory determinants of respiratory rhythm variability.
Keywords: Serotonin (5-HT), control of breathing, variability, Kölliker-Fuse nuclei (KFN), gain control, Rett Syndrome, Mecp2-deficient mice
1. Introduction
At the Oxford Conference on Breathing and Modelling, we presented recent modeling and experimental work suggesting that the Kölliker-Fuse nuclei (KFn), a component of the ponto-medullary respiratory pattern generator (Dutschmann and Dick 2012), regulates the gain of both intrinsic and extrinsic sources of respiratory rhythm variability. Intrinsic sources are channel- and synaptic-noise within rhythm generating nuclei whereas the extrinsic sources are afferent feedbacks arising from sensory sources such as pulmonary stretch receptors (Dhingra et al. In review). In addition, variability of respiration is linked to the postnatal maturation of the KFn (Dutschmann et al. 2008) and disrupting the maturation processes of the KFn can enhance respiratory variability and cause pathologic breathing irregularities observed in neurodevelopmental disorders such as Rett Syndrome (Stettner et al. 2007; Dhingra et al. 2013). Recent evidence suggests that increased breathing variability is associated with reduced GABAergic innervation of the KFn (Abdala et al. 2016).
The underlying synaptic mechanisms that generate intrinsic and extrinsic variability of the respiratory rhythm depend on the serotoninergic 1a receptor (5HT1aR), which modulates the respiratory control network. The 5HT1aR is an inhibitory receptor that decreases respiratory neuron excitability via Gi/o-dependent mechanisms (see Richter et al. 2003). Systemic administration of a 5HT1aR agonist reduced the occurrence spontaneous central apneas, and therefore intrinsic respiratory rhythm variability, in C57BL6/J and Mecp2-/y mice (Yamauchi et al. 2008). Moreover, Pet-1 and Lmx1b knock-out mice which lack serotonergic neurons show increased respiratory rhythm variability in early postnatal life (Erickson et al. 2007). Further, 5HT-dependent respiratory rhythm variability may depend selectively on 5HT1aR-dependent neurotransmission because mice lacking the 5HT1aR also have increased in breathing variability at baseline despite an increase in serotonin release (Barrett et al. 2012).
Here, we test whether systemic or focal application of the selective 5HT1aR antagonist WAY100635 increases the variability of the baseline respiratory rhythm, and delays the recovery of the baseline pattern after an arterial chemoreflex-mediated perturbation of the respiratory rhythm.
2. Methods
Experimental protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee, and were performed with strict adherence to all American Association of Accreditation of Laboratory Animal Care and National Institutes of Health guidelines.
2.1 In situ perfused brainstem preparation
Experiments were performed in female, adult (3 mo. postnatal) wild-type mice maintained on a background (C57BL6/J x 129Sv x Balb/c, n=8) to minimize genotype-dependent effects. The in situ perfused preparation was prepared as described previously (Dhingra et al. 2013). Briefly, mice were deeply anesthetized with isofluorane (1.5–3%, Piramal Healthcare, Andra Pradesh, IN). Once the animal failed to respond to a noxious paw pinch, the animal was transected below the diaphragm and submerged in ice-cold ACSF. The skull was quickly removed for precollicular decerebration, and cerebellectomy. The lungs were removed, and the right phrenic, hypoglossal and vagal nerves were dissected for recording efferent activity. The descending aorta was also isolated for cannulation. The preparation was then transferred to the recording chamber. The descending aorta was cannulated, and the preparation was immediately perfused with an artificial cerebrospinal solution (aCSF), which had a pH 7.4 (in mM: 125 NaCl, 3 KCl, 1.25 KH2PO4, 2.5 CaCl2, 1.25 MgSO4, 25 NaHCO3, 10 D-Glucose and 1.25% (w/v) Ficoll) maintained at 31°C via a recirculating heat exchanger and bubbled with a gas mixture containing 94% O2 and 6% CO2. Within minutes of aCSF perfusion, the preparation showed respiratory movements. The activity of the phrenic, vagal and hypoglossal nerves were recorded using on bipolar suction electrodes. Nerve activity was amplified (20000x, Grass P511, West Warwick, RI, USA), filtered (0.003 – 3 kHz), digitized (10 kHz, Power1401, CED, Cambridge, UK) and stored on a computer using Spike2 software (CED, Cambridge, UK). Finally, the preparation was paralyzed via a bolus of vecuronium bromide (1mg/250mL perfusate) to minimize motion artifacts. Typical flow rate and perfusion pressure were 18–21 mL/min and 40–60 mmHg, respectively.
2.2 Experimental protocol
Once a stable respiratory rhythm was established, baseline respiratory output was recorded for 5 min. The chemoreflex was evoked via a bolus of NaCN (0.1mL, 0.03% (w/v)). In the first protocol (n=3), we applied the 5HT1aR antagonist WAY100635 systemically in the perfusate (10 μM, Sigma Aldrich, St. Louis, MO, USA). Respiratory output was again recorded for 5 min to assess respiratory effects of 5HT1aR blockade. The chemoreflex was evoked 10 min after WAY100635 administration to assess whether 5HT1aR blockade modulated the respiratory chemosensitivity.
In the second protocol (n=5), we microinjected WAY100635 into the dorsolateral (dl) pons using triple-barreled microinjection pipettes. Triple barreled pipettes were pulled using a vertical puller (Narishige International USA, Amityville, NY, USA) from standard glass capillaries (1.2/0.68 mm ID/OD, World Precision Instruments, Sarasota, FL, USA). Drugs were delivered via a pressure microinjection system (Veradyne). Injected volumes were calibrated using a reticule to monitor the movement of the meniscus within the pipette. Respiratory related areas in the dl pons were identified by mapping with glutamate microinjections (50nL, 10mM). Sites where glutamate microinjection evoked a transient slowing of the respiratory pattern were selected for subsequent microinjection of WAY100635 (100 nL, 10 mM, Sigma Aldrich) and Alexa 488-conjugated microspheres (Invitrogen, Grand Island, NY, USA). The same procedure was repeated on the contralateral side to achieve bilateral administration of the drug in the dl pons. Effective sites were typically found at the following coordinates: AP: 0.2–0.5 mm caudal to the inferior colliculus, ML: midline±1.6–2.2 mm, Depth: 1.5–2 mm below surface.
2.3 Histology
At the end of the experiment, the brainstem was removed, and postfixed overnight in 4% PFA/PBS. The tissue was transferred into a 20% sucrose/PBS solution for cryoprotection. The fixed tissue was then sectioned on a freezing microtome (50 μm, SM2010R, Leica Biosystems, Buffalo Grove, IN, USA). Pontine sections were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI), and imaged (Eclipse 80i, Nikon Instruments, Melville, NY, USA; Retiga 200R, QImaging, Surrey, BC, CAN) for post-hoc identification of the injection sites.
2.4 Data analysis
Phrenic nerve activity (PNA) was used as an index of fictive respiratory output. From 5 min epochs recorded before and after administration of WAY100635, we measured the mean duration of inspiration (TI) and expiration (TE), and plotted the instantaneous respiratory period (TTOT) to identify central apneas, and quantify their frequency. A central apnea was defined as a respiratory period more than twice the mean respiratory period in the epoch. The apnea index reflects the frequency of these events. The apnea index was measured from epochs that did not include spontaneous swallows, which were characterized simultaneous brief bursts in hypoglossal and vagal nerve activities. From chemoreflex responses, we measured the peak and minimum respiratory frequencies during the trial. Additionally, we measured the latency for recovery from chemosensory perturbation as the time from the onset of the chemoreflex to the first cycle with a duration comparable to the respective baseline duration.
All data are presented as means ± SEM. Statistical comparisons were made with a one-way ANOVA with Bonferroni correction for multiple comparisons.
3. Results
To assess the role of brainstem- versus dl pontine-5HT1aRs on intrinsic respiratory variability, we analyzed the respiratory pattern and variability before and after systemic or focal blockade of 5HT1aRs with WAY100635 in situ (Fig. 1).
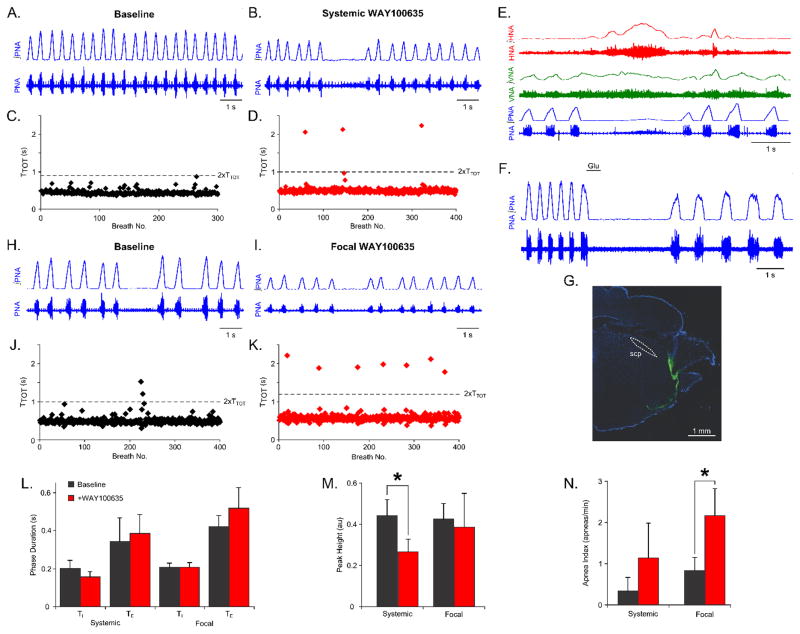
Figure 1. Blockade of 5HT1aRs increases the frequency of spontaneous apneas.
(A,B) Representative traces of phrenic nerve activity (PNA) are shown during a baseline epoch (A) and an epoch after systemic administration of the 5HT1aR antagonist WAY100635 (B). (C,D) Plots of the instantaneous respiratory period (TTOT) are shown during a baseline epoch (C), and an epoch after systemic administration of WAY100635 (D). (E) Representative recordings of phrenic (PNA), vagal (VNA), and hypoglossal (HNA) nerve activity are shown during spontaneous apnea. (F,G) The dorsolateral (dl) pons was identified by mapping focal responses to glutamate microinjection (50 nL, 10mM). WAY100635 was microinjected at sites where glutamate evoked a prolongation of expiratory duration. Fluorescent beads were microinjected for post-hoc histochemical characterization of the injection sites. A representative injection site is shown in (G). (H,I) Representative traces of PNA are shown during a baseline epoch (H), and an epoch after focal administration of WAY100635 in the dl pons (I). (J,K) Plots of the instantaneous respiratory period (TTOT) are shown during a baseline epoch (J), and an epoch after focal administration of WAY100635 in the dl pons (K). (L) Neither systemic nor focal administration of WAY100635 in the dl pons evoked any significant change in respiratory phase durations. (M) Systemic, but not focal administration of WAY100635 evoked a significant reduction in the amplitude of phrenic nerve activity (p<0.05). (N) Focal administration of WAY100635 evoked a significant increase in the frequency of spontaneous apneas (p<0.05).
Representative traces of PNA are shown before and after systemic administration of WAY100635 in Figure 1A & B. Systemic blockade of 5HT1aRs did not evoke any change in the respiratory period (TTOT) or the durations of inspiration or expiration (Fig. 1L). However, systemic administration reduced the amplitude of PNA (baseline, 0.44 ± 0.08 AU versus after systemic WAY100635, 0.27 ± 0.06 AU, p<0.05, Fig. 1M). Moreover, systemic administration of WAY100635 tended to increase respiratory rhythm variability. Representative plots of the respiratory period before and after systemic 5HT1aR blockade is shown in Figure 1C and D. Enhanced respiratory variability was observed as an increase in the frequency of spontaneous central apneas (baseline, 0.35 ± 0.35 apneas/min versus after systemic WAY100635, 1.15 ± 0.86 apneas/min, Fig. 1N). A representative central apneic event is presented in Figure 1E. As described by Abdala et al. (2010), central apneas consisted of prolonged bursts of hypoglossal and vagal nerve activity, and was also associated with low-amplitude activity in PNA. The pattern of respiratory motor outputs during central apneas after systemic administration of WAY100635 was qualitatively the same as that during baseline.
To assess whether dl pontine 5HT1aRs were responsible for enhanced respiratory rhythm variability, we microinjected WAY100635 focally in the dl pons. The dl pons was identified by mapping the response to focal glutamate application (Fig. 1F). At targeted sites, glutamate evoked a prolongation in the duration of expiration. A representative section showing a dl pontine injection site marked with fluorescein-conjugated dextran is presented in Figure 1G.
Representative traces of PNA before and after focal WAY100635 microinjection are shown in Figures 1H&I. Similar to systemic administration, dl pontine microinjection of WAY100635 did not evoke any changes in respiratory period or phase durations (Fig. 1L). Unlike systemic administration, the reduction in PNA amplitude was not significant for the group (Fig. 1M). Together, these data suggest that 5HT1aR-dependent regulation of respiratory rhythm variability depends on the activity of these receptors selectively in the dl pons, whereas the 5HT1aR-dependent regulation of fictive inspiratory volume depend on the activation of these receptors in other, likely medullary, respiratory compartments.
Like systemic administration, focal blockade of 5HT1aRs in the dl pons enhanced respiratory rhythm variability, but to a greater extent. Representative plots of the respiratory period before and after focal administration of WAY100635 are shown in Figures 1J&K. In contrast to systemic administration of the drug, focal WAY100635 in the dl pons significantly increased the frequency of spontaneous central apneas (baseline, 0.84 ± 0.34 apneas/min versus after focal WAY100635, 2.17 ± 0.67 apneas/min, p<0.05, Fig. 1N). Like systemic administration, the pattern of respiratory motor outputs during central apneas was unchanged.
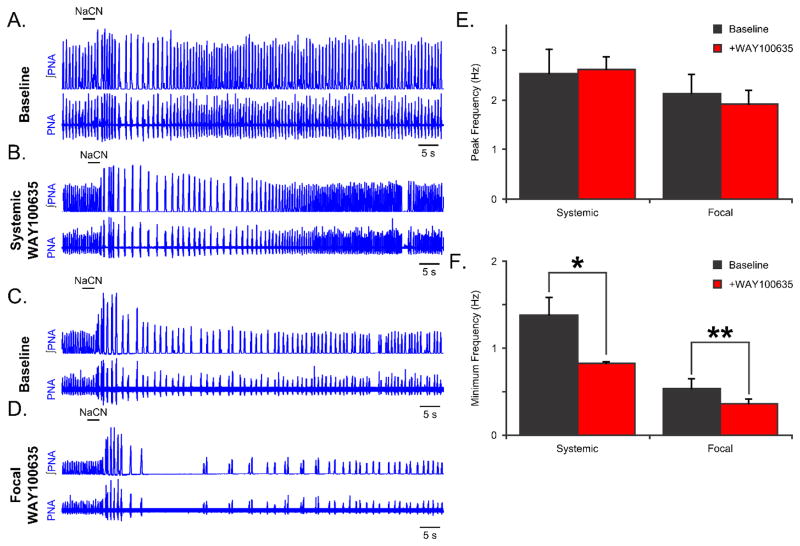
To assess whether 5HT1aRs modulate the respiratory oscillation stability in response to sensory perturbations, we evoked the chemosensory response by bolus administration of NaCN before and after blockade of 5HT1aRs (Fig. 2). Representative traces of PNA before and after systemic administration of WAY10035 are shown in Figure 2A & B. Systemic blockade of 5HT1aRs did not alter the peak frequency of the chemosensory reflex (Fig. 2E). However, blockade of 5HT1aRs prolonged the time to recovery (the return to baseline frequency) after chemosensory perturbation (25.6 ± 6.1 s baseline, versus 67.1 ± 16.6 s with systemic WAY100635, p<0.05). Also systemic WAY100635 reduced the lowest respiratory frequency following a brief bolus of NaCN (baseline, 1.38 ± 0.22 Hz versus after systemic WAY100635, 0.83 ± 0.04 Hz, p<0.05, Fig. 2F).
Figure 2. Blockade of 5HT1aRs prolongs the recovery from chemosensory perturbations.
(A,B) Representative traces of phrenic nerve activity (PNA) are shown during a chemosensory perturbation (0.1mL, 0.03% NaCN) before (A) and after (B) systemic administration of the 5HT1aR antagonist WAY100635. (C,D) Representative traces of PNA are shown during a chemosensory perturbation (0.1mL, 0.03% NaCN) before (A) and after (B) focal administration of WAY100635 in the dorsolateral (dl) pons. (E) Neither systemic, nor focal administration of WAY100635 changed the peak frequency response to chemosensory perturbation. (F) Both systemic and focal administration of WAY100635 prolonged the recovery from chemosensory perturbation.
To determine the overlap between the dl pontine-specific and the systemic response to 5HT1aR blockade, we also assessed the effect of chemosensory perturbation before and after focal administration of WAY100635. Representative traces of PNA before and after dl pontine 5HT1aR blockade are shown in Figures 2C & D. Like systemic administration of the drug, we did not observe any alteration in the peak respiratory frequency evoked by a bolus of NaCN. However, focal administration of WAY100635 prolonged the time to recovery from chemosensory perturbation (25.6 ± 6.1 s baseline to 98.6 ± 9.6 s after systemic WAY100635, p<0.001). This was also reflected by a lower respiratory frequency following NaCN administration (0.55 ± 0.13 Hz decreased to 0.38 ± 0.07 Hz, after focal WAY100635, p<0.05, Fig. 2F). Together, these data suggest that 5HT1aR-dependent synaptic transmission in the dl pons modulates the stability of the respiratory network to chemosensory perturbations.
4. Discussion
By comparing the effects of 5HT1aR blockade via systemic or focal administration in the KFn area, we were able to test the specific role of 5HT1aR-dependent neurotransmission in the dl pons. We have shown that pontine 5HT1aRs regulate the frequency of spontaneous apneas, and hence the intrinsic respiratory rhythm variability. Moreover, in examining the arterial chemoreflex before and after the application of a 5HT1aR antagonist, we found that KFn 5HT1aRs also determine the respiratory network’s stability to extrinsic chemosensory perturbations. Together, these results support the working hypothesis that dl pontine respiratory network compartments determine the variability of the respiratory rhythm.
4.1. The role of the KFn area in the mediation of breathing variability
Growing scientific evidence suggests that the pontine KFn area plays a major role in the generation of breathing variability. A well-described function of KFn neurons is the mediation of the inspiratory off-switch (IOS) via its descending connectivity with the medullary respiratory column (Dutschmann and Dick 2012). Thus, pathological (see 4.2 for details) increases in the excitability of KFn can be predicted to produce prolonged IOS resulting in apnea and/or breath-holding, which consequently will increase breath-by-breath variability. The observed increase in apneic events after disinhibition of the KFn circuitry following pharmacological blockade of inhibitory 5HT1aRs, which were reported to be expressed densely in the KFn (M. Dutschmann et al. 2009), are consistent with this hypothesis. Moreover, this is the first study showing that modulation of serotonergic neurotransmission in the KFn can trigger an increase in breathing variability. The present data also suggest that reported decreases in respiratory variability after systemically applied 5HT1aR agonists such as 8-OH-DPAT (Stettner et al. 2008; Yamauchi et al. 2008) could be largely attributed to the drug’s action within the KFn circuitry.
4.2 Sensory input as source of breathing variability
A major source of breathing variability is arising from sensory inputs of the airways and arterial chemoreceptors that are relayed within the nucleus of the solitary tract (nTS). The operation of such sensory feedback loops is important for adapting the breathing pattern to varying homeostatic and metabolic demands (Dutschmann and Dick 2012). Periodic sensory feedback of pulmonary stretch receptors can entrain (periodic forcing) the respiratory rhythm. In particular the pulmonary stretch receptor feedback is a powerful modulator of KFn activity (Dick et al. 2008). Experimental data and modeling suggest that interaction and plasticity of the central (KFn) and periodic sensory (stretch receptors) mechanisms amplify respiratory rhythm variability (Dutschmann et al. 2008; Dhingra et al. In review).
On the other hand, not all sensory feedbacks are periodic, which is likely the case for arterial chemoreflex inputs to the ponto-medullary respiratory network. In the present work, we observed that 5HT1aR-dependent neurotransmission in the KFn area modulates the respiratory rhythm variability evoked by a transient chemosensory perturbation. Moreover, this observation was consistent with recent reports that the KFn area plays a selective role in the response to prolonged hypoxia (Bonis et al. 2010; Song et al. 2011). Therefore, the interaction between intrinsic and extrinsic sources of breathing variability can be generalized to involve non-periodic (tonic) feedback loops such as the arterial chemoreceptor reflex.
Together, these results suggest that 5HT acting at 5HT1aR in the KFn may modulate both the intrinsic and extrinsic sources of respiratory rhythm variability. The KFn mediated modulation of extrinsic (sensory) sources of breathing variability is strongly linked to with a descending gain-control of the sensory relay mechanisms in the NTS (Dutschmann et al. 2008; Dhingra et al. In review).
4.3. Clinical relevance for breathing variability
It is important to consider that there is a continuum between physiologic and pathologic breathing variability. Potentially, the best definition for pathologic breathing variability is that it causes oxygen desaturation. Currently, the contribution of KFn-mediated breathing variability is best investigated in the neurodevelopmental disorder Rett-syndrome. Several reports show that pathologic increases in breath-by-breath variability caused by apnea and breath-holding is associated with hyperexcitability of the KFn (Stettner et al. 2007; Abdala et al. 2016). Beneficial effects of targeting 5HT1aR to stabilize the neurogenic breathing disorders in Rett syndrome have been demonstrated, and the present study further supports that the positive drug effects can be attributed to action on the KFn circuitry. Other relevant actions of 5HT1aR in the KFn area can be linked to opioid poisoning. A recent study showed that microinjection of μ-opioid receptor agonists into the KFn triggers severe breathing instability (Levitt et al. 2015). Opioid-induced breathing instability and arrest are suppressed by application of 5-HT1aR agonists (Sahibzada et al. 2000). Finally, other clinically-relevant respiratory dysfunction that can be associated with the KFn and thus have the 5HT1aRs as a potential therapeutic target are neurodegenerative diseases linked with tauopathy, sudden infant death and sleep apnea syndromes (Dutschmann and Dick. 2010). Together, this work supports the investigation and utilization of 5HT1aR-dependent pharmacotherapies as a selective means to regulate KFn-dependent control of the respiratory rhythm.
Acknowledgments
Supported by HL087377 (TED), HL004913 (TED&RRD), the Future Fellowship from Australian Research Council (MD) and T32HL-007913 (RRD).
References
- Abdala AP, Toward MA, Dutschmann M, Bissonnette JM, Paton JFR. Deficiency of GABAergic synaptic inhibition in the Kölliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett Syndrome. J Physiol. 2016;594:223–237. doi: 10.1113/JP270966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala Ana PL, Dutschmann Mathias, Bissonnette John M, Paton Julian FR. Correction of Respiratory Disorders in a Mouse Model of Rett Syndrome. Proc Nat Acad Sci. 2010;107:18208–18213. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KT, Kinney HC, Aihua L, Daubenspeck JA, Leiter JC, Nattie EE. Subtle alterations in breathing and heart rate control in the 5-HT1A receptor knockout mouse in early postnatal development. J Appl Physiol. 2012;113:1585–1593. doi: 10.1152/japplphysiol.00939.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. The pontine respiratory group, particularly the Kölliker-Fuse nucleus, mediates phases of the hypoxic ventilatory response in unanesthetized goats. J Appl Physiol. 2010;108:1321–1335. doi: 10.1152/japplphysiol.00935.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra RR, Dutschmann M, Dick TE, Galán RF. On how periodic sensory feedback increases stochastic variability in the respiratory rhythm. 2016 In review. [Google Scholar]
- Dhingra RR, Zhu Y, Jacono FJ, Katz DM, Galán RF, Dick TE. Decreased Hering-Breuer input-output entrainment in a mouse model of Rett Syndrome. Front Neural Circuits. 2013;7:42. doi: 10.3389/fncir.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol. 2008;586:4265–4282. doi: 10.1113/jphysiol.2008.152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol. 2012;2:2443–2469. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Reuter J, Zhang W, Gestreau C, Stettner GM, Kron M. Postnatal emergence of synaptic plasticity associated with dynamic adaptation of the respiratory motor pattern. Respir Physiol Neurobiol. 2008;164:72–79. doi: 10.1016/j.resp.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris E. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007;159:85–101. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, Abdala AP, Paton JFR, Bissonnette JM, Williams JT. μ Opioid receptor activation hyperpolarizes respiratory-controlling Kölliker–Fuse neurons and suppresses post-inspiratory drive. J Physiol. 2015;593:4453–4469. doi: 10.1113/JP270822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: Guardians of stable breathing. Trends Mole Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, Gillis RA. Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine 1A receptors. J Pharm Exper Ther. 2000;292:704–713. [PubMed] [Google Scholar]
- Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kölliker-Fuse/parabrachial complex in dorsolateral pons. Neurosci. 2011;175:145–153. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M. Breathing dysfunctions associated with control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Hilaire G, Dutschmann M. 8-OH-DPAT suppresses spontaneous central apneas in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008;161:10–15. doi: 10.1016/j.resp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol. 2008;162:117–125. doi: 10.1016/j.resp.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]